Back to Journals » Drug Design, Development and Therapy » Volume 16
Pharmacokinetic Study of Nalbuphine in Surgical Patients Undergoing General Anesthesia with Varying Degrees of Liver Dysfunction
Authors Gao XN , Nie XY , Gao JL , Heng TF , Zhang YQ, Hua L, Sun YQ, Feng ZY , Wang MX, Jia L
Received 20 April 2022
Accepted for publication 9 July 2022
Published 26 July 2022 Volume 2022:16 Pages 2383—2393
DOI https://doi.org/10.2147/DDDT.S371596
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Xiao-nan Gao,1 Xu-yang Nie,1 Jing-lin Gao,1 Tian-fang Heng,2 Yu-qi Zhang,2 Li Hua,1 Ya-qi Sun,1 Zhang-ying Feng,1 Ming-xia Wang,1 Li Jia2
1Department of Clinical Pharmacology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 2Department of Anesthesiology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China
Correspondence: Ming-xia Wang, Department of Clinical Pharmacology, The Fourth Hospital of Hebei Medical University, 12 Jiankang Road, Shijiazhuang, People’s Republic of China, Tel +86 311-66696233, Email [email protected] Li Jia, Department of Anesthesiology, The Fourth Hospital of Hebei Medical University, 12 Jiankang Road, Shijiazhuang, People’s Republic of China, Email [email protected]
Purpose: This study aimed to characterize the pharmacokinetics of nalbuphine in patients undergoing general anesthesia with varying degrees of liver dysfunction.
Patients and Methods: Twenty-four patients were enrolled and divided into three cohorts based on liver function: normal liver function (n = 13), mild liver dysfunction (n = 5), and moderate/severe liver dysfunction (n = 6). During the induction of anesthesia, they received 15 mg of nalbuphine intravenously. Venous blood samples were collected from each patient. The plasma concentration of nalbuphine was determined using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). The pharmacokinetic parameters of nalbuphine were calculated by non-compartmental analysis (NCA) using Phoenix WinNonlin software.
Results: Compared with the normal liver function group, the plasma elimination half-life (T1/2) of nalbuphine was increased by approximately 33% in the moderate/severe liver dysfunction group (2.66 h vs 3.54 h, P< 0.05), and the volume of distribution (Vd) increased by approximately 85% (100.08 L vs 184.95 L, P< 0.05). Multivariate analysis revealed that weight and platelet were associated with clearance (CL); total bilirubin as an independent factor was associated with T1/2, and weight associated with area under the curve (AUC(0→∞)) independently.
Conclusion: The T1/2, mean residence time, and Vd of nalbuphine in patients with moderate/severe liver dysfunction were prolonged or increased significantly compared with those in the normal liver function group. These data suggest that it may need to be used with caution when nalbuphine is administered to patients with moderate or severe liver dysfunction.
Keywords: nalbuphine, intravenous, liver dysfunction, UPLC-MS/MS, pharmacokinetics
Introduction
Nalbuphine is a semisynthetic opioid analgesic that was first synthesized in 1965 and has been used clinically for more than 40 years. The analgesic effect of nalbuphine involves activating the κ receptor and antagonizing part of the μ receptor.1 In addition to maintaining or enhancing opiate-based analgesia, it also mitigates the common problems associated with receptor-mediated adverse effects.2–4 The analgesic effect is similar to morphine, 3 times of pentazocine and 6 times of codeine. It is worth noting that respiratory inhibition of nalbuphine has a “capping effect”. When the dose is greater than 0.3–0.5 mg/kg, the respiratory inhibition no longer increases with the increase in dose.5 It has been widely used during induction and maintenance of general anesthesia under close supervision.6
Oral administration of nalbuphine results in significant first-pass effects and low bioavailability.7 Therefore, it is usually administered intravenously in clinical practice, which takes effect quickly and maintains activity for approximately 3–6 h with a half-life of 2–5 h.8 Nalbuphine is extensively metabolized by Uridine 5’-diphospho-glucuronosyltransferase (UGT) 2B7, UGT1A3, cytochrome P450 (CYP) 2C9 and CYP2C19 in the liver, yielding two hydroxylated derivatives and two conjugated metabolites.9,10 The metabolites are mainly excreted into the feces, and approximately 7% of the unbound nalbuphine is excreted in the urine.1
Liver impairment is a major global problem affecting human health.11 China is one of the countries with a high incidence, millions of patients with liver dysfunction undergo surgery annually.12 Therefore, patients with diverse liver dysfunction are common in clinical practice in China.13 Various physiological functions of the liver may be affected by liver dysfunction, such as material metabolism, bile synthesis and secretion, detoxification, and immune response.14 The liver is the major site of drug metabolism; hence, liver dysfunction is mainly associated with considerable pharmacokinetic (PK) and pharmacodynamic changes in anesthetic drugs. It may affect hepatic blood flow, metabolic enzyme activity and of drug binding to plasma proteins, thus affecting drug metabolism.15
Existing literature on the PK study of nalbuphine mostly focused on healthy volunteers or patients who underwent different types of surgery.7,8,10 To the best of our knowledge, no studies have specifically addressed whether hepatic impairment affects the pharmacokinetics of nalbuphine. Thus, our study aimed to investigate the PK characteristics of nalbuphine in patients with liver dysfunction who underwent abdominal surgery to provide theoretical support for clinical medication.
Materials and Methods
Design and Participants
This study was approved by the Committee on Ethics, at the Fourth Hospital of Hebei Medical University, Shijiazhuang, China (No. 2019121) and was conducted there itself. Written consent was obtained from all patients. This study was conducted in accordance with ethical principles in the Declaration of Helsinki. Our study consisted of 27 patients who were scheduled to undergo hepatobiliary surgery between August 2021 and December 2021 and had an American Society of Anesthesiologists Physical Status of 1 or 2. All of the patients’ body weights were within 30% of their ideal body weights. Exclusion criteria involved patients who: 1) were allergic to nalbuphine, 2) had long-term opioid medications, 3) were pregnant, 4) had excessive intraoperative bleeding, 5) had known or suspected cardiopulmonary or renal disease, and 6) had a history of chronic pain.
Based on the preoperative levels of total bilirubin (TBIL) and aspartate transaminase (AST), the patients were divided into three groups: normal liver function, mild liver dysfunction and moderate/severe liver dysfunction (Table 1 for classification basis).
 |
Table 1 ODWG Hepatic Function Criteria |
Conduct of Anesthesia
Before surgery, all patients were routinely fasted and water-deprived for 6–8 h. Radial artery puncture was performed under local anesthesia of lidocaine after entering the operating room to monitor the mean arterial pressure during operation. Then, left peripheral vein was opened for drug injection, and right peripheral vein was opened for blood collection. They were monitored using electrocardiogram, pulse, pulse oxygen saturation (SpO2), blood pressure (BP), and bispectral index (BIS). Each patient was preoxygenated with 100% oxygen through a facemask. Anesthesia was induced with intravenous (IV) injection of 15 mg nalbuphine (Yichang Humanwell Pharmaceutical, Hubei, China), 0.05 mg/kg midazolam (Jiangsu Nhwa Pharmaceutical Co., Ltd., Jiangsu, China), 0.03 mg/kg etomidate (Jiangsu Nhwa Pharmaceutical Co., Ltd., Jiangsu, China), and 0.2 µg/kg sufentanil (Yichang Humanwell Pharmaceutical, Hubei, China), followed by cisatracurium (Jiangsu Hengrui Medicine Co., Ltd, Jiangsu, China) 0.2 mg/kg IV injection. After the patients had lost consciousness completely, direct laryngoscopic endotracheal intubation was performed. Anesthesia was maintained with remifentanil (Yichang Humanwell Pharmaceutical, Hubei, China), and intermittent bolus doses of cisatracurium and sevoflurane (Maruishi Pharmaceutical Co., Ltd., Japan) adjusted to maintain the depth of anesthesia and muscle relaxation throughout the operation.
Blood Sample Collection
Venous blood samples (2 mL) were drawn from a vein in the contralateral arm and placed in a tube containing K2-ethylene-diamine-tetra-acetic acid as an anticoagulant before nalbuphine IV injection and at 0.05, 0.08, 0.17, 0.33, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, and 12 h later. The obtained samples were subsequently centrifuged (2,000×g at 4°C for 10 min), and the separated plasma samples were stored at −80°C in sample and backup tubes (no less than 500 μL respectively) pending analysis.
Determination of Drug Concentration
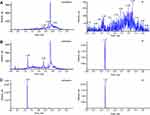
Plasma nalbuphine concentrations were determined using validated ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). The UPLC-MS/MS system consists of an ExionLC liquid chromatograph (AB SCIEX, USA) and a Triple Quad 5500 mass spectrometer (AB SCIEX, USA). For pretreatment, 50 μL of plasma sample mixed with 50 μL water was precipitated using 200 μL acetonitrile precipitant containing an internal standard (nalmefene), vortex-mixed for 3 min, and centrifuged for 10 min at 12,500×g. Subsequently, 150 μL of the supernatant was transferred into 150 μL of water, followed by vortex-mixing for 1 min and centrifugation at 12,500×g for 3 min. Finally, the clear supernatant was transferred to auto-sampler glass vials. The separation was performed on a Kinetex phenyl-hexyl column (50 mm×2.1 mm, 1.7 μm) (Phenomenex, USA). The elution mobile phase was a mixture of water containing 0.1% formic acid with 3mM ammonium acetate (mobile phase A) and acetonitrile (mobile phase B). The gradient elution was as follows: 95–20% A (0–2.8 min), 20–95% A (2.8–3.5 min), 95–95% A (3.5–4.5 min). The autosampler was set to 4 °C, and a 3 μL sample was injected at a flow rate of 0.5 mL/min into UPLC-MS/MS system. AB Sciex Q-TRAP 5500 mass spectrometer was characterized by electrospray ionization for positive ions in multiple reaction monitoring (MRM) mode. The quantitative ion pairs were m/z 358.4→340.1 for nalbuphine and m/z 340.0→268.3 for nalmefene. The typical MRM spectra of blank plasma (A), blank plasma spiked with nalbuphine and IS (B), and plasma sample after IV (C) are displayed in Figure 1. The method was validated in terms of specificity, matrix effect, linearity, recovery, accuracy, precision and stability. The calibration curves showed good linearity (r2>0.99) over concentration range of 0.1–500 ng/mL. The intra-and inter-batch precisions were within 10.67%, and accuracy ranged from 94.07% to 105.34%. The recovery and matrix effect were 94.52%–106.30% and 95.70%–103.80%, respectively.
Pharmacokinetic Analysis
The PK parameters were analyzed based on a noncompartmental analysis (NCA) using WinNonlin software 8.3. The analyte concentrations below the limit of quantification were set to zero. Nalbuphine concentrations were obtained from the participants, plasma drug concentration-time data were fitted to determine the area under the curve (AUC(0→t) and AUC(0→∞)), the elimination half-life (T1/2), the clearance (CL), the mean residence time (MRT(0→t) and MRT(0→∞)) and the apparent volume of distribution (Vd). The values for the highest plasma drug concentration (Cmax) of nalbuphine and the time to reach Cmax (Tmax) were obtained from the observed data using the concentration–time curve.
Statistics
GraphPad Prism 8.0.1 was used for drawing plasma concentration–time curves. Statistical analysis was all conducted using SPSS 25.0, and findings were considered statistically significant if P-value was less than 0.05. All quantitative data were tested for normality by Shapiro–Wilk test of SPSS software. According to their distribution, the quantitative data were expressed as mean ± standard deviation (SD) or median (interquartile range, IQR). The normally distributed data were assessed by one-way ANOVA analysis of variance followed by Dunnett t-test or least significant difference t-test while other quantitative data were analyzed by the Kruskal–Wallis test.
Univariate associations between basic information of patients and main PK parameters of nalbuphine were performed to obtain Pearson’s correlation coefficients (r). Subsequently, clinical factors with P values <0.05 in the univariate analysis were examined in a multivariate analysis using multiple linear regression analysis.
Results
Patients
We screened 27 patients between August 2021 and December 2021: two patients with excessive loss of blood collection points and one with severe massive hemorrhage were excluded, and 24 patients were finally enrolled. There were 11 males and 13 females aged 24 to 76 years, with a bodyweight of 48–82 kg. Patients were assigned to three groups based on their hepatic function. Thirteen patients had normal liver function, while the remaining patients had some degree of liver dysfunction (five and six patients had mild and moderate/severe dysfunction, respectively).
Patient characteristics were described and compared among all groups. Patients’ demographics and preoperative laboratory values for different liver functions revealed that the levels of alanine transaminase (ALT) and AST in the mild and moderate/severe liver dysfunction groups were significantly higher than in the normal liver function group. TBIL, direct bilirubin (DBIL) and alkaline phosphatase (ALP) levels were significantly higher in moderate/severe liver dysfunction group than in the normal liver function group (P<0.05). The baseline demographic and clinical characteristics of participants were summarized in Tables 2, 3, and 4.
 |
Table 2 Demographic Information in Patients with Various Degrees of Hepatic Impairment |
 |
Table 3 Operation-Related Information in Patients with Various Degrees of Hepatic Impairment |
 |
Table 4 Perioperative Laboratory Biochemical Index Inpatients with Various Degrees of Hepatic Impairment |
Pharmacokinetics
The mean plasma concentration–time profiles are shown in Figure 2. Compared with mild and moderate/severe liver dysfunction groups, the concentrations declined quickly in normal liver function group. Variability in CL and MRT(0→12h) values were similar across the cohorts, and the extent of exposure as indicated by the AUC(0–∞) and Cmax, was similar among the different groups. However, T1/2, Vd and MRT(0→∞) demonstrated a significant difference between the normal liver function group and the moderate/severe liver dysfunction group. The median T1/2 and Vd of nalbuphine in patients with moderate/severe liver dysfunction were approximately 1.5–2 times that of patients with normal liver function. Table 5 summarizes the PK data of nalbuphine from 24 patients.
 |
Table 5 PK Parameters in Patients with Various Degrees of Hepatic Impairment |
 |
Figure 2 Plasma concentration-time profile ((A) linear scale and (B) semi-log scale) in volunteers (n =24) after a single intravenous injection of 15 mg nalbuphine. |
Univariate Analyses Between PK Parameters and Clinical Factors
Univariate correlation analysis revealed a significant negative correlation between AUC of nalbuphine and weight, ALT, ALP and platelet (PLT) (P<0.05), respectively. Weight and PLT positively correlated with nalbuphine CL (P<0.05). The T1/2 positively correlated with age of patients and TBIL and negatively correlated with albumin (ALB) (P<0.05). The Vd increased as ALT, TBIL, ALP and fibrinogen (FIB) levels increased (P< 0.05) (Table 6).
 |
Table 6 Univariate Analyses Between Influencing Factors and PK Parameters |
Multivariate Analysis Using Multiple Linear Regression Analysis
Multiple linear regression analysis was performed to adjust for significantly related factors in the univariate analyses (Table 7). The results revealed that weight was an independent clinical factor associated with AUC after a single dose of nalbuphine was administration intravenous. TBIL was an independent clinical factor associated with T1/2. Weight and PLT significantly affected CL.
 |
Table 7 Results of Multiple Linear Regression Analysis for Clinical Factors Related to PK Parameters |
Discussion
Much information and experience support that nalbuphine as an efficacious and well-tolerated analgesic treatment in the population. Liver dysfunction is expected to impact the metabolism by reducing hepatic blood flow, metabolic enzyme activity, and drug binding to plasma protein.15 It is significant to understand the PK differences of nalbuphine in populations with various degrees of hepatic impairment. Therefore, we evaluated PK characteristics of an IV injection of 15 mg nalbuphine in patients with normal hepatic function and different levels of hepatic impairment.
The PK parameters of nalbuphine may be influenced by body weight, as plasma exposure to nalbuphine is higher in lighter participants. However, PK parameters were not adjusted for body weight because the median body weight was similar among the three groups (P<0.05). No statistical difference in Cmax and AUC were observed between the different groups, suggesting that nalbuphine plasma exposure may not be affected by hepatic impairment.
Compared with normal liver function group, there was an apparent increase of T1/2 and Vd in the moderate/severe liver dysfunction group, which increased by approximately 40% and 80%, respectively. Multivariate analysis showed that patients with high TBIL levels tended to have longer T1/2 of nalbuphine. It has been reported that the PK parameters of the same drug may differ in patients with different liver diseases.17 For example, there were significant alterations in PK parameters of etomidate in patients with cirrhosis,18 but were not changed in patients with obstructive jaundice.19 According to clinical diagnosis, these six patients with moderate/severe liver dysfunction were diagnosed with obstructive jaundice.
Obstructive jaundice is defined as the retention of bile and its components after intrahepatic or extrahepatic bile duct obstruction, mainly seen in malignant diseases such as carcinoma of pancreas and hilar cholangiocarcinoma.20 It can lead to the increase of serum bilirubin (hyperbilirubinemia), aggravate hepatocyte injury and liver dysfunction, and decrease the activity and expression of liver metabolic enzymes to varying degrees, thus affecting drug metabolism.21 It can also cause changes in hepatic blood flow. Kanda et al22,23 discovered that total hepatic blood flow decreased significantly in dogs with obstructive jaundice, and the blood flow of the superior mesenteric artery and portal vein decreased significantly in patients with obstructive jaundice. In the later stage of obstruction, as biliary obstruction aggravates inflammatory response, the liver microcirculation is also inhibited.24 Therefore, we speculated that the changes in hepatic blood flow and microenvironment caused by obstructive jaundice were the keys to the PK changes of nalbuphine in patients with moderate/severe liver dysfunction in this study, which would slow down its metabolism and prolong T1/2.
Liver is the main organ for the synthesis of ALB. Liver dysfunction leads to a decrease in the content of synthesized ALB and causes the accumulation of endogenous substances such as bilirubin and free fatty acids to compete with drugs for protein-binding sites.25 It could further reduce the plasma protein binding rate of drugs and increase the concentration of unbound drugs. In this study, patients with moderate/severe hepatic impairment had lower ALB, and TBIL was 4–8 times higher than in other groups. Free drug is the fraction available for distribution and clearance; hence, the higher proportion of unbound nalbuphine could distribute to peripheral tissues to a greater extent, explaining the larger Vd.26 Therefore, we hypothesized that this increase of Vd in moderate/severe liver dysfunction group may be related, in part, to an increased free fraction of nalbuphine resulting from low plasma ALB and high TBIL.
The estimated hepatic extraction ratio of nalbuphine is 0.5–0.7, which undergoes a vital hepatic metabolism.27 It was reported that changes in organ weight and blood flow were primarily responsible for the age-related changes in hepatic clearance, and hepatic clearance decreased by 0.80% per year with aging.28 We used univariate analyses to assess the associations between age and nalbuphine PK parameters and discovered that T1/2 prolonged with age in this study. Jaillon et al27 studied patients of different ages and demonstrated that the T1/2 of nalbuphine was significantly longer in the elderly than that in the young, and CL was reduced. Our results are consistent with those reported, indicating age is also an important factor affecting the pharmacokinetics of nalbuphine.
All patients underwent general anesthesia for surgery in this study. The median CL and Vd of nalbuphine in patients with normal liver function were 27.4 L/h and 100.08 L, respectively. However, Cai et al29 and He et al8 observed CL and Vd to be 60–90 L/h and 202–326 L, respectively, in healthy patients; this is higher than those observed in our study. It was not surprising considering the hemodynamic and body fluid changes associated with anesthesia and surgery. We conjectured the difference in the parameters might be due to surgical versus nonsurgical populations, as surgeries were potentially associated with blood loss, hypotension, and changes in liver perfusion.30 Using dopamine, norepinephrine and other vasoactive drugs might increase the cardiac output of patients and affect the CL and hepatic blood flow, resulting in a variance in the plasma concentration of nalbuphine.31 However, due to the different use times of patients, it was hard to predict the degree of effect on PK parameters. Whether other combined anesthetic drugs such as sufentanil, propofol and dexmedetomidine would affect the distribution and metabolism of nalbuphine was still unclear, which needs to be further explored.
For its anesthetic effects and safety results, we only recorded adverse reactions to endotracheal intubation after anesthesia induction. The results showed that only one case of mild choking cough and one case mild muscle tremor. However, due to the limited in sample size, the above adverse reactions may not be representative. In the future, we will be able to enlarge the sample size and optimize the study design to record in detail the vital signs, hemodynamic indicators and stress response before and after endotracheal intubation to explore the relationship between anesthetic effects and PK characteristic of nalbuphine in patients with hepatic disease.
Conclusion
Our study demonstrated for the first time that the pharmacokinetics of nalbuphine was affected by moderate/severe liver dysfunction. We discovered that the T1/2, MRT(0→∞) and Vd of nalbuphine in patients with moderate/severe liver dysfunction were prolonged or increased significantly compared with those in the normal liver function group. Based on these findings, it may need to be used carefully when nalbuphine is administered to patients with moderate or severe liver dysfunction.
Ethics and Consent
Ethical approval was provided by the Ethical Committee of the Fourth Hospital of Hebei Medical University, Shijiazhuang, China. All patients provided informed consent and all procedures were conducted according to the Declaration of Helsinki.
Acknowledgments
This work was supported by National Science and Technology Major Project for Major New Drugs Innovation and Development 2020 in China (No. 2020ZX09201006) and Hebei Medical Science Research Project (No. 20200105).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Schmidt WK, Tam SW, Shotzberger GS, et al. Nalbuphine. Drug Alcohol Depend. 1985;14:339–362. doi:10.1016/0376-8716(85)90066-3
2. Walker EA, Young AM. Discriminative-stimulus effects of the low efficacy mu agonist nalbuphine. J Pharmacol Exp Ther. 1993;267:322–330.
3. Yeh YC, Lin TF, Chang HC, et al. Combination of low-dose nalbuphine and morphine in patient-controlled analgesia decreases incidence of opioid-related side effects. J Formos Med Assoc. 2009;108:548–553. doi:10.1016/S0929-6646(09)60372-7
4. Raghav R, Jain R, Dhawan A, et al. Chronic co-administration of nalbuphine attenuates the development of opioid dependence. Pharmacol Biochem Behav. 2018;175:130–138. doi:10.1016/j.pbb.2018.10.001
5. Brunton L. Goodman and Gilman’s Pharmacological Basis of Therapeutics.
6. Davis MP, Fernandez C, Regel S, et al. Does nalbuphine have a niche in managing pain? J Opioid Manag. 2018;14:143–151. doi:10.5055/jom.2018.0441
7. Lo MW, Schary WL, Whitney CC. The disposition and bioavailability of intravenous and oral nalbuphine in healthy volunteers. J Clin Pharmacol. 1987;27:866–873. doi:10.1002/j.1552-4604.1987.tb05581.x
8. He K, Ji W, Zhao H, et al. Pharmacokinetic comparison of nalbuphine with single injection and patient-controlled analgesia mimic method in healthy Chinese volunteers. J Clin Pharm Ther. 2021;46:1166–1172. doi:10.1111/jcpt.13421
9. Liang RJ, Shih YN, Chen YL, et al. A dual system platform for drug metabolism: nalbuphine as a model compound. Eur J Pharm Sci. 2020;141:105093. doi:10.1016/j.ejps.2019.105093
10. Wang HJ, Hsiong CH, Chang WL, et al. New finding of nalbuphine metabolites in men: NMR spectroscopy and UPLC–MS/MS spectrometry assays in a pilot human study. Metabolomics. 2014;10(4):709–718. doi:10.1007/s11306-013-0605-y
11. Marcellin P, Kutala BK. Liver diseases: a major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018;38(Suppl 1):2–6. doi:10.1111/liv.13682
12. Wang JY. Clinical utility of entecavir for chronic hepatitis B in Chinese patients. Drug Des Devel Ther. 2013;8:13–24. doi:10.2147/DDDT.S41423
13. Yao G. Entecavir is a potent anti-HBV drug superior to lamivudine: experience from clinical trials in China. J Antimicrob Chemother. 2007;60:201–205. doi:10.1093/jac/dkm175
14. El-Khateeb E, Darwich AS, Achour B, et al. Review article: time to revisit child-Pugh score as the basis for predicting drug clearance in hepatic impairment. Aliment Pharmacol Ther. 2021;54:388–401. doi:10.1111/apt.16489
15. Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147–1161. doi:10.1007/s00228-008-0553-z
16. Takebe N, Beumer JH, Kummar S, et al. A Phase I pharmacokinetic study of belinostat in patients with advanced cancers and varying degrees of liver dysfunction. Br J Clin Pharmacol. 2019;85:2499–2511. doi:10.1111/bcp.14054
17. Palatini P, De MS. Pharmacokinetic drug interactions in liver disease: an update. World J Gastroenterol. 2016;22:1260–1278. doi:10.3748/wjg.v22.i3.1260
18. Bonnardot JP, Levron JC, Deslauriers M, et al. Pharmacokinetics of continuous infusion of etomidate in cirrhotic patients. Ann Fr Anesth Reanim. 1991;10:443–449. doi:10.1016/S0750-7658(05)80847-0
19. Song JC, Meng XY, Yang H, et al. Obstructive jaundice doesn’t change the population pharmacokinetics of etomidate in patients who underwent bile duct surgery. Curr Drug Deliv. 2021;19(5):635–641.
20. Li JX, Zhuo SJ, Chen BH, et al. Clinical efficacy of laparoscopic modified loop cholecystojejunostomy for the treatment of malignant obstructive jaundice. J Int Med Res. 2020;48:300060519866285. doi:10.1177/0300060519866285
21. Liu W, Yang B, Ji JW, et al. The effect of obstructive jaundice on the sensitivity of intravenous anesthetic of remimazolam: study protocol for a controlled multicenter trial. Trials. 2022;23:23. doi:10.1186/s13063-021-05987-y
22. Kanda H, Nimura Y, Yasui A, et al. Hepatic blood flow after acute biliary obstruction and drainage in conscious dogs. Hepatogastroenterology. 1996;43:235–240.
23. Kanda H, Nimura Y, Yasui A, et al. Recovery of portal blood flow after percutaneous transhepatic biliary drainage in patients with obstructive jaundice. Surg Today. 1997;27:120–123. doi:10.1007/BF02385899
24. Ito Y, Machen NW, Urbaschek R, et al. Biliary obstruction exacerbates the hepatic microvascular inflammatory response to endotoxin. Shock. 2000;14:599–604. doi:10.1097/00024382-200014060-00005
25. Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52:1–8. doi:10.1007/s40262-012-0018-5
26. Ulldemolins M, Roberts JA, Wallis SC, et al. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: special emphasis on unbound pharmacokinetics. J Antimicrob Chemother. 2010;65:1771–1778. doi:10.1093/jac/dkq184
27. Jaillon P, Gardin ME, Lecocq B, et al. Pharmacokinetics of nalbuphine in infants, young healthy volunteers, and elderly patients. Clin Pharmacol Ther. 1989;46:226–233. doi:10.1038/clpt.1989.130
28. Soejima K, Sato H, Hisaka A. Age-related change in hepatic clearance inferred from multiple population pharmacokinetic studies: comparison with renal clearance and their associations with organ weight and blood flow. Clin Pharmacokinet. 2022;61:295–305. doi:10.1007/s40262-021-01069-z
29. Cai LJ, Zhang J, Wang XM, et al. Validated LC-MS/MS assay for the quantitative determination of nalbuphine in human plasma and its application to a pharmacokinetic study. Biomed Chromatogr. 2011;25:1308–1314. doi:10.1002/bmc.1601
30. Wrzosek A, Jakowicka-Wordliczek J, Zajaczkowska R, et al. Perioperative restrictive versus goal-directed fluid therapy for adults undergoing major non-cardiac surgery. Cochrane Database Syst Rev. 2019;12:CD012767. doi:10.1002/14651858.CD012767.pub2
31. Deng C, Bellomo R, Myles P. Systematic review and meta-analysis of the perioperative use of vasoactive drugs on postoperative outcomes after major abdominal surgery. Br J Anaesth. 2020;124:513–524.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.