Back to Journals » Drug Design, Development and Therapy » Volume 11
Pharmacokinetic and safety evaluation of MB12066, an NQO1 substrate
Authors Lee HW , Seong SJ, Ohk B , Kang WY , Gwon MR, Kim BK , Kim HJ, Yoon YR
Received 22 May 2017
Accepted for publication 10 August 2017
Published 13 September 2017 Volume 2017:11 Pages 2719—2725
DOI https://doi.org/10.2147/DDDT.S142339
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Hae Won Lee,1,* Sook Jin Seong,1,* Boram Ohk,1,2 Woo Youl Kang,1,2 Mi-Ri Gwon,1 Bo Kyung Kim,1,2 Hyun-Ju Kim,3 Young-Ran Yoon1,2
1Clinical Trial Center, Kyungpook National University Hospital, 2Department of Biomedical Science, BK21 Plus KNU Bio-Medical Convergence Program for Creative Talent, Kyungpook National University Graduate School, 3Cell and Matrix Research Institute, Daegu, Republic of Korea
*These authors contributed equally to this work
Objective: This study evaluated the pharmacokinetics (PKs) and safety of a newly developed β-lapachone (MB12066) tablet, a natural NAD(P)H:quinone oxidoreductase 1 (NQO1) substrate, in healthy male volunteers.
Methods: In a randomized, double-blind, multiple-dose, two-treatment study, 100 mg MB12066 or placebo was given twice daily for 8 days to groups of eight or three fasted healthy male subjects, respectively, followed by serial blood sampling. Plasma concentrations for β-lapachone were determined using liquid chromatography–tandem mass spectrometry. PK parameters were obtained with non-compartmental analysis. Tolerability was assessed based on physical examinations, vital signs, clinical laboratory tests, and electrocardiograms.
Results: Following a single 100 mg MB12066 oral dose, maximum plasma concentration (Cmax) of β-lapachone was 3.56±1.55 ng/mL, and the median (range) time to reach Cmax was 3 h (2–5 h). After the 8 days of 100 mg twice daily repeated dosing was completed, mean terminal half-life was determined to be 18.16±3.14 h, and the mean area under the plasma concentration vs time curve at steady state was 50.44±29.68 ng·h/mL. Accumulation index was 2.72±0.37. No serious adverse events (AEs) were reported, and all reported intensities of AEs were mild.
Conclusion: The results demonstrated that MB12066 was safe and well tolerated in healthy volunteers and that there were no serious AEs. Accumulation in plasma with twice-daily administration was associated with a 2.72 accumulation ratio.
Keywords: β-lapachone, healthy volunteers, MB12066, pharmacokinetics, safety
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease.1 Nonalcoholic steatohepatitis (NASH), a severe form of NAFLD, may progress to cirrhosis and hepatocellular carcinoma.1–3 NAFLD, commonly associated with insulin resistance, obesity, dyslipidemia, type 2 diabetes mellitus, and cardiovascular disease, is considered a hepatic manifestation of the metabolic syndrome.3,4 The present standard therapy in patients with NAFLD focuses on weight loss through dietary modification and lifestyle change. Although vitamin E and thiazolidinedione pioglitazone use shows some beneficial effects in selected cases, currently no pharmacologic treatment has been established for NAFLD.4–6
β-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione; Figure 1), originally isolated from the South American lapacho tree, is a well-known NAD(P)H:quinone oxidoreductase 1 (NQO1) substrate (diphtheria toxin diaphorase).7–9 β-lapachone is an antioxidant flavoprotein that catalyzes the two-electron reduction of various quinones using NAD(P)H as an electron donor, and it has been reported to have anticancer, anti-inflammatory, antibacterial, antifungal, antiviral, anti-healing, and antihypertensive properties.7–15 Furthermore, results from several recent studies in rodent models that evaluated β-lapachone’s therapeutic potential suggest that β-lapachone administration ameliorated metabolic syndrome-related symptoms, including obesity, glucose intolerance, dyslipidemia, and fatty liver, through pharmacologic stimulation of NQO1-dependent cytosolic NADH oxidation.16–18 Synthetic β-lapachone, MB12066, has been recently developed by KT&G Life Sciences Corporation (Suwon, Republic of Korea).
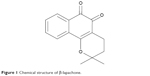  | Figure 1 Chemical structure of β-lapachone. |
In a preclinical pharmacokinetic (PK) study, time to reach maximum plasma concentration (tmax) for β-lapachone was 6 h and absolute oral bioavailability was 15.5%, when administered orally and intravenously to rats.13 Some factors that might have contributed to the low oral bioavailability and delayed tmax in that study included first-pass β-lapachone metabolism in the liver, small and large intestinal tracts, and their low aqueous solubility.19 Mean elimination half-lives (t½) were 2.5–11.4 h, depending on the administration route.19,20 β-lapachone is mainly eliminated via NQO1 and subsequent UDP-glucuronosyltransferase (UGT)-catalyzed metabolism.21 After [14C]MB12066 oral administration to rats, β-lapachone was rapidly and extensively distributed to tissues, and most of the radioactive dose (94%) was excreted (8 h post dose) via bile (unpublished data; KT&G Life Sciences Corporation).
According to the investigator’s brochure, MB12066 was determined to be safe and well tolerated in a single-dose Phase I study. Peak β-lapachone plasma concentrations have been observed to be between 2.5 and 5 h after dosing in healthy volunteers, and mean t½ was about 10.4–24.6 h (unpublished data; KT&G Life Sciences Corporation). β-lapachone urinary excretion in the parent form was ≤1%, and mean renal clearance was 12.8–18.1 L/h. Systemic exposure showed dose proportionality after single dosing between 30 and 200 mg.
The present study was conducted to evaluate MB12066’s PKs and safety following oral administration in healthy Korean volunteers.
Methods
Study subjects
Healthy Korean male volunteers, aged 20–45 years, with a body weight of 60–90 kg and body mass index (BMI) between 18.5 and 25, were eligible for the study if they had no clinically significant findings on medical history, physical examination, routine clinical laboratory tests (blood hematology, biochemistry, urinalysis), serology tests (hepatitis B surface antigens, anti-hepatitis C virus antibody, anti-HIV antibody, and Venereal Disease Research Laboratory [VDRL]), and 12-lead electrocardiography conducted within 3 weeks prior to the study.
Subjects were excluded from the study if they met any one of the following criteria: 1) history of allergy or hypersensitivity to any drug; 2) history or evidence of hepatic (including viral hepatitis), renal, pulmonary, endocrine, central nervous system, immune, hematologic, psychiatric, cardiovascular, or malignant diseases; 3) history of gastrointestinal disease or gastrointestinal surgery that might affect study drug absorption (excluding simple appendectomy or herniorrhaphy); 4) hemoglobin level <12 g/dL; 5) fasting blood sugar <70 or >126 mg/dL; 6) systolic BP of ≥140 or ≤90 mmHg, diastolic BP of ≥90 or ≤40 mmHg, or pulse rate of >100/min, measured after 5 min resting in a seated position; 7) history of drug abuse; 8) use of any prescription medication or herbal remedies within 14 days or use of any over-the-counter remedies or vitamins within 7 days prior to the study drug administration; 9) participation in another study with an investigational or approved drug ≤8 weeks prior to dosing; 10) donation or loss of ≥400 mL blood <8 weeks prior to screening; 11) alcohol consumption (>21 units/week) or unwillingness to abstain from drinking during the study period; 12) tobacco product use ≤3 months prior to study drug administration; 13) excessive intake of caffeine, caffeine-containing food, grapefruit, grapefruit juice, or grapefruit-containing food; 14) inability to take a standard meal that was provided in the study center; 15) previous participation in such a study; and 16) subjects who were not eligible to participate at the discretion of the study investigator, based on clinical laboratory tests.
The study protocol was approved by the Ministry of Food and Drug Safety and the institutional review board of the Kyungpook National University Hospital (KNUH), Daegu, Republic of Korea (ClinicalTrials.gov identifier: NCT02338856), in accordance with the ethical standards for studies in humans from the Declaration of Helsinki and its amendments and applicable Good Clinical Practice guidelines. Before participating in this study, all subjects received detailed written and oral study information and signed a written informed consent document before screening.
Study design
This randomized, double-blind, multiple-dose, two-treatment study was conducted at the KNUH Clinical Trial Center. A code for randomization (Microsoft Excel 2010; Microsoft Corporation, Redmond, WA, USA) was used to randomly assign the 11 subjects to one of the two different treatment groups. Eight subjects received MB12066 (lot no M 13001; expiration date, January 2015; KT&G Life Sciences Corporation), and three subjects received placebo (lot no P 13001; expiration date, January 2015; KT&G Life Sciences Corporation).
The study consisted of a screening period (days −21 to day −2), baseline evaluation (day −1), admission (days −1 and 7), multiple-dose treatment period (days 1–8, 100 mg MB12066 or placebo administered twice orally after an overnight fast for ≥10 h), discharge (days 2 and 9), and safety follow-up on days 14–18. Subjects were admitted to the study center at 8 pm on days −1 and 7. After an overnight fast for 10 h, subjects received the first or the last (at 8 am on day 8) oral dose of MB12066 100 mg or placebo 100 mg with 150 mL of water at 8 am in the morning of days 1 and 8, under the supervision of the investigator. Additional water intake was permitted 2 h after dosing, and food intake was not allowed for 4 h with standardized lunch and evening meals served at 4 and 10 h after the first or last dose. Subjects were hospitalized from 12 h before the first or last dose to 24 h after dose. On days 2–7, all subjects came to the study center and received an oral dose of the study medication every 12 h.
To determine β-lapachone plasma concentration, serial blood samples (5 mL each) were collected from an indwelling intravenous catheter into a sodium heparin-containing tube just before and at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, and 24 h after dosing on day 1, just before dosing on day 5, and also before and at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 16, 24, 36, 48, and 60 h after dosing on day 8. The tubes with blood samples were shaken lightly and immediately chilled on ice for ≤5 min before plasma separation via centrifugation (3,200 rpm for 7 min at 4°C). Plasma samples (150 μL) were transferred to Eppendorf tubes and stored at −70°C until analysis by the KNUH’s analytical laboratory.
β-lapachone concentration analysis
β-lapachone plasma concentrations were determined using high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC–MS/MS). Briefly, 100 μL plasma was pipetted into a polypropylene tube, and 200 μL β-lapachone-d6 (5 ng/mL in acetonitrile) was added as an internal standard. The mixture was then vortexed for 5 min and centrifuged (13,200 rpm, 10 min). A 3 μL aliquot of this solution was injected for analysis. Samples were analyzed by HPLC–MS/MS using an Agilent 1200 series HPLC system (Agilent Technologies, Santa Clara, CA, USA) coupled to an API 5000 mass spectrometer (AB Sciex, Framingham, MA, USA). Chromatographic separation was performed using a Zorbax XDB-C18 column (2.1×150 mm internal diameter, 3.5 μm particle size) at a flow rate of 0.2 mL/min. The mobile phase consisted of a 30:70 (v/v) mixture of 0.1% formic acid in 5 mM ammonium acetate and acetonitrile. The multiple reaction monitoring transitions for quantification were as follows: m/z 243.11→187.03 for β-lapachone and m/z 249.05→159.07 for β-lapachone-d6. Linear calibration curves were established between 0.5 and 200 ng/mL for β-lapachone (r≥0.9950). Intraday % coefficients of variation (CVs) were 3.4, 3.0, 2.1, and 3.6 for 0.5, 1.5, 16, and 160 ng/mL of β-lapachone, respectively. At the same respective concentrations, interday %CVs were 4.0, 4.4, 3.6, and 3.6, respectively. The overall accuracy ranged from 98.8% to 106.0% (intraday) and from 96.2% to 104.3% (interday). The lower limit of quantification was 0.5 ng/mL.
PK analysis
β-lapachone PK parameters in plasma were determined by non-compartmental methods, using WinNonlin Pro 5.3 software (Pharsight Corporation, Mountain View, CA, USA), based on individual subject β-lapachone plasma concentrations, using actual sampling times after the first or last 100 mg maintenance dose administration. Maximum plasma concentration (Cmax), minimum plasma concentration (Cmin), and time to reach Cmax (tmax) were estimated directly from the observed plasma concentration–time data curve. Terminal elimination rate constant (ke) was determined by linear regression on the final data points (at least three) of log-linear decline. β-lapachone apparent elimination half-life (t½) was calculated as 0.693/ke. The area under the plasma concentration–time curve over the dosing interval after multiple-dose administration (AUCτ) was calculated using the linear trapezoidal method. All estimated PK parameters were summarized descriptively as mean ± standard deviation (SD) values.
Safety assessment
MB12066 safety was assessed throughout the study period by recording clinical and laboratory adverse events (AEs), which were collected and compared to evaluate differences between β-lapachone pre- and post-dosing. All subjective symptoms reported by subjects and objective signs observed by clinical investigators were collected and assessed. Vital signs (blood pressure, pulse rate) were assessed at screening, on days 1 and 8 (before and at 4 and 24 h after administration of the first and the last dose), and at the follow-up visit. Body temperature was measured at screening and at the follow-up visit. A full physical examination was performed at screening, on days 1 and 8 (before dosing), days 2 and 9 (before discharge), and at the follow-up visit. The following laboratory tests were conducted at screening and follow-up visits at an accredited laboratory (Department of Laboratory Medicine, KNUH, Daegu, Republic of Korea): blood hematology (hemoglobin, hematocrit, red blood cell, platelet, and white blood cells with neutrophil, lymphocyte, monocyte, eosinophil, and basophil counts), urinalysis (specific gravity, pH, protein, glucose, ketone, bilirubin, occult blood, urobilinogen, nitrite, and microscopic examination [red and white blood cells]), and serum chemistry (fasting glucose, blood urea nitrogen, creatinine, total cholesterol, triglyceride, low-density lipoprotein [LDL] cholesterol, high-density lipoprotein [HDL] cholesterol, total protein, albumin, total bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, γ-glutamyltransferase, and lactate dehydrogenase). A 12-lead ECG was conducted at screening and at the follow-up visit.
Treatment-emergent adverse events (TEAEs) were defined as those that occurred on or after administration of the first dose. TEAEs were evaluated by study physicians in terms of intensity (mild, moderate, and severe), duration, severity, outcome, and relationship to the study drug administered.
Statistical analyses
Because this study was designed to explore and describe β-lapachone PK after oral 100 mg MB12066 tablet administration, a sample size calculation to robustly test our statistical hypothesis was not performed. Based on earlier studies and practical considerations, the total number of subjects enrolled was 11 (8 active medication and 3 placebo).
The results are expressed as mean ± SD values unless otherwise indicated, and a p-value <0.05 was considered as statistically significant. PK parameters between single- and multiple-dose administrations were compared using the Mann–Whitney U test or independent t-test. All statistical tests were performed using SPSS software (version 18.0 for Windows; SPSS Inc., Chicago, IL, USA).
Results
Subjects
Of the 11 healthy Korean male subjects enrolled in this study (age, 27.8±5.6 years [range, 22–39 years]; weight, 68.7±7.8 kg [range, 60.2–81.4 kg]; height, 175.2±8.1 cm [range, 161.6–187.0 cm]), eight received MB12066 and three received placebo. All subjects completed the study without any major protocol violation and were included in the safety analysis. Data from eight subjects were included in the PK analysis.
PK properties
Figure 2 shows the mean plasma β-lapachone concentration–time profiles (Figure 2A) after single dose and 8-day multiple dose twice daily oral administration of MB12066 and (Figure 2B) details of time (168–228 h after the last dose). The main PK parameters for β-lapachone are summarized in Table 1.
Following a single oral 100 mg MB12066 dose, the Cmax value for β-lapachone was 3.56±1.55 ng/mL and the median (range) tmax was 3 h (2–5 h). Following 100 mg twice daily repeated dosing for 8 days, the median (range) tmax was 5 h (1–5 h), which was not statistically significantly different from single-dose administration (p=0.234). The mean t½ was 18.16±3.14 h, and the mean AUC0–τ was 50.44±29.68 ng·h/mL. The accumulation index was 2.72±0.37, indicating that there was accumulation after multiple doses of 100 mg MB12066 administered twice daily.
Safety
Safety assessments were performed on the 11 subjects who received β-lapachone or placebo at least once. Overall, four (36.7%) subjects experienced a total of four TEAEs during the study period. Two events (dyspepsia and diarrhea) were considered to be related to the medication. All AEs were transient and mild in intensity. All AEs resolved spontaneously with no specific treatment. None of the TEAEs were considered to be serious or unexpected.
Discussion
This Phase I study evaluated MB12066 PK and safety in healthy volunteers. MB12066 is a synthetic version of β-lapachone. Based on a literature review (PubMed key terms: β-lapachone, PK, healthy volunteer [August 2016]), there were no research reports that addressed β-lapachone PK in healthy volunteers.
The mean ratio of AUC0–t (area under the plasma concentration vs time curve from 0 to the last quantifiable time point)/AUC0–∞ (area under the plasma concentration vs time curve from 0 to infinity) after repeated dosing for 8 days was 90.8% in our study, indicating that the sampling schedule was adequate to provide a reliable estimate of exposure extent (eg, at least 80% AUC0–∞).
Following single-dose 100 mg MB12066 administration, the Cmax and AUC0–t values for β-lapachone (3.56±1.55 ng/mL and 16.16±4.93 ng·h/mL, respectively) from the present study were not compatible with those reported in the investigator’s brochure (1.56±0.50 ng/mL and 19.74±11.92 ng·h/mL, respectively). There may be several reasons for these differences; the sampling times in the study from the investigator’s brochure were different (just before and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 16, 24, 36, 48, and 72 h after dosing). Furthermore, as the second dose was administered 12 h after the first dose on day 1 in our study, the last time point for AUC0–t was 12 h after the first dose. Accordingly, the AUC0–t and t½ values could not be exactly calculated after a single dose. Following multiple-dose MB12066 (100 mg twice daily) administration for 8 days in this study, Cmax and AUC0–t values for β-lapachone were significantly higher than those after a single dose (14.96±15.56 ng/mL and 85.97±32.59 ng·h/mL, respectively; p=0.21 and p<0.001, respectively). Furthermore, the accumulation index for β-lapachone calculated in this study was 2.72±0.37, indicating that there was considerable accumulation in plasma with twice-daily administration.
In the present study, the median tmax values ranged between 3 and 5 h post dose, compatible with those reported in the investigator’s brochure (2.5–5.0 h). The mean t½ value following multiple dosing in this study was 18.16±3.14 h. The reported t½ values in the preclinical study and the investigator’s brochure after oral administration with 10–400 mg of MB12066 in healthy volunteers showed similar t½ values (10.4–24.6 h) as seen in our study.19
In our study, interindividual variability seen in the PK values for β-lapachone was considerable. For single- and multiple-dose administrations, the CV% for Cmax values was 43.6% and 104.9%, respectively. The CV% for AUC ranged from 30.5% to 58.8%. According to the investigator’s brochure, the CV% for Cmax and AUC0–t, when MB12066 was administered to 50 healthy volunteers in a randomized, placebo-controlled, single-dose, dose-escalation clinical trial, ranged from 31.9% to 99.5% and from 30.2% to 106.9%, respectively (unpublished data; KT&G Life Sciences Corporation). As β-lapachone is predominantly metabolized by NQO1-mediated quinone reduction and subsequent UGT-catalyzed glucuronidation, NQO1 or UGT polymorphisms or mutations could have had a plausible impact on the large interindividual variability seen.21,22 Among 22 single-nucleotide polymorphisms (SNPs) in the NQO1 gene, the NQO1*2/*2 homozygote frequency with negligible NQO1 enzymatic activity ranged between 4.4% and 20.3% and NQO1*1/*2 heterozygote frequency with activities between that in the wild-type NQO1*1/*1 and mutant NQO1*2/*2 homozygotes ranged from 33.8% to 52.2%.23–26
According to the investigator’s brochure, 15 of 50 subjects who received the study drug experienced 34 AEs. Of these 34 AEs, 20 events were considered to be related to the medication and mild in severity. AEs that corresponded to more than one event included diarrhea, abdominal pain, and dizziness. MB12066 was well tolerated in normal healthy volunteers enrolled in this study, and the observed AEs that occurred following multiple-dose administration were not severe or serious.
There were several limitations in this study. First, even though a small number of subjects per treatment group are generally acceptable for Phase I clinical trials, the sample size of this study was relatively small. Second, the results were obtained from healthy male subjects. The PK of MB12066 might differ depending on the patient population or in relation to gender or age. Therefore, further studies are needed to investigate MB12066’s PK and tolerability in other population subgroups.
Conclusion
These results demonstrate that MB12066 was safe and well tolerated in healthy male volunteers during this study with no serious adverse effects. In addition, the accumulation in plasma with twice-daily administration was associated with an accumulation ratio of 2.72.
Acknowledgments
This study was supported by the grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI15C0001, HI14C2750); the Bio & Medical Technology Development Program of the National Research Foundation (NRF), funded by the Ministry of Science, ICT & Future Planning, Republic of Korea (NRF-2013M3A9B6046416); and the Industrial Core Technology Development Program (10051129, development of the system for ADME assessment using radiolabeled compounds), funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea). This study was funded by KT&G Life Sciences Corporation, Suwon, Republic of Korea.
Disclosure
The authors report no conflicts of interest in this work.
References
Masuoka HC, Chalasani N. Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann N Y Acad Sci. 2013;1281:106–122. | ||
Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–1523. | ||
Lomonaco R, Sunny NE, Bril F, Cusi K. Nonalcoholic fatty liver disease: current issues and novel treatment approached. Drugs. 2013;73(1):1–14. | ||
Hardy T, Anstee QM, Day CP. Nonalcoholic fatty liver disease: new treatments. Curr Opin Gastroenterol. 2015;31(3):175–183. | ||
Malhotra N, Beaton MD. Management of non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;7(30):2962–2967. | ||
Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Eng J Med. 2010;362(18):1675–1685. | ||
Schaffner-Sabba K, Schmidt-Ruppin KH, Wehrli W, Schuerch AR, Wasley JW. beta-Lapachone: synthesis of derivatives and activities in tumor models. J Med Chem. 1984;27(8):990–994. | ||
Pink JJ, Planchon SM, Tagliarino C, Varnes ME, Siegel D, Boothman DA. NAD(P)H:quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J Biol Chem. 2000;275(8):5416–5424. | ||
Albena AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1(NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys. 2010;501(1):116–123. | ||
Lee EJ, Ko HM, Jeong YH, Park EM, Kim HS. β-Lapachone suppresses neuroinflammation by modulating the expression of cytokines and matrix metalloproteinases in activated microglia. J Neuroinflammation. 2015;12:133. | ||
Jeon YJ, Bang W, Choi YH, Shim JH, Chae JI. Beta-lapachone suppresses non-small cell lung cancer proliferation through the regulation of specificity protein 1. Biol Pharm Bull. 2015;38(9):1302–1308. | ||
Bang W, Jeon YJ, Cho JH, et al. β-lapachone suppresses the proliferation of human malignant melanoma cells by targeting specificity protein 1. Oncol Rep. 2016;35(2):1109–1116. | ||
Kung HN, Yang MJ, Chang CF, Chau YP, Lu KS. In vitro and in vivo wound healing-promoting activities of beta-lapachone. Am J Physiol Cell Physiol. 2008;295(4):C931–C943. | ||
Kim YH, Hwang JH, Noh JR, et al. Activation of NAD(P)H:quinone oxidoreductase ameliorates spontaneous hypertension in an animal model via modulation of eNOS activity. Cardiovasc Res. 2011;91(3):519–527. | ||
Kim YH, Hwang JH, Kim KS, et al. NAD(P)H:quinone oxidoreductase 1 activation reduces blood pressure through regulation of endothelial nitric oxide synthase acetylation in spontaneously hypertensive rats. Am J Hypertens. 2015;28(1):50–57. | ||
Hwang JH, Kim DW, Jo EJ, et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58(4):965–974. | ||
Shin S, Park J, Li Y, et al. β-Lapachone alleviates alcoholic fatty liver disease in rats. Cell Signal. 2014;26(2):295–305. | ||
Choi WH, Ahn J, Jung CH, Jang YJ, Ha TY. β-Lapachone prevents diet-induced obesity by increasing energy expenditure and stimulating the browning of white adipose tissue via downregulation of miR-382 expression. Diabetes. 2016;65(9):2490–2501. | ||
Kim I, Kim H, Ro J, et al. Preclinical pharmacokinetic evaluation of β-lapachone: characteristics of oral bioavailability and first-pass metabolism in rats. Biomol Ther (Seoul). 2015;23(3):296–300. | ||
Savage RE, Hall T, Bresciano K, Bailey J, Starace M, Chan TC. Development and validation of a liquid chromatography-tandem mass spectrometry method for the determination of ARQ 501 (beta-lapachone) in plasma and tumors from nu/nu mouse xenografts. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;872(1–2):148–153. | ||
Liu H, Li Q, Cheng X, Wang H, Wang G, Hao H. UDP-glucuronosyltransferase 1A determinates intracellular accumulation and anti-cancer effect of β-lapachone in human colon cancer cells. PLoS One. 2015;10(2):e0117051. | ||
Cheng X, Liu F, Yan T, et al. Metabolic profile, enzyme kinetics, and reaction phenotyping of β-Lapachone metabolism in human liver and intestine in vitro. Mol Pharm. 2012;9(12):3476–3485. | ||
Nebert DW, Roe AL, Vandale SE, Bingham E, Oakley GG. NAD(P)H:quinone oxidoreductase (NQO1) polymorphism, exposure to benzene, and predisposition to disease: a HuGE review. Genet Med. 2002;4(2):62–70. | ||
Martinez-Hernández A, Córdova EJ, Rosillo-Salazar O, et al. Association of HMOX1 and NQO1 polymorphisms with metabolic syndrome components. PLoS One. 2015;10(5):e0123313. | ||
Zheng B, Wang Z, Chai R. NQO1 C609T polymorphism and colorectal cancer susceptibility: a meta-analysis. Arch Med Sci. 2014;10(4):651–660. | ||
Kelsey KT, Ross D, Traver RD, et al. Ethnic variation in the prevalence of a common NAD(P)H quinone oxidoreductase polymorphism and its implications for anti-cancer chemotherapy. Br J Cancer. 1997;76(7):852–854. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


