Back to Journals » Drug Design, Development and Therapy » Volume 15
Pharmaceutical Development of 5-Fluorouracil-Eluting Stents for the Potential Treatment of Gastrointestinal Cancers and Related Obstructions
Authors Arafat M , Song Y , Brewer K, Fouladian P, Parikh A, Albrecht H, Blencowe A, Garg S
Received 27 December 2020
Accepted for publication 23 March 2021
Published 9 April 2021 Volume 2021:15 Pages 1495—1507
DOI https://doi.org/10.2147/DDDT.S299401
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Mohammad Arafat,1 Yunmei Song,1 Kyle Brewer,2 Paris Fouladian,1 Ankit Parikh,1 Hugo Albrecht,3 Anton Blencowe,2 Sanjay Garg1
1Pharmaceutical Innovation and Development (PIDG) Group, Clinical and Health Sciences, University of South Australia, Adelaide, SA, 5000, Australia; 2Applied Chemistry and Translational Biomaterials (ACTB) Group, Clinical and Health Sciences, University of South Australia, Adelaide, SA, 5000, Australia; 3Drug Discovery and Development Group, Clinical and Health Sciences, University of South Australia, Adelaide, SA, 5000, Australia
Correspondence: Sanjay Garg
Pharmaceutical Innovation and Development (PIDG) Group, Clinical and Health Sciences, University of South Australia, Adelaide, SA, 5000, Australia
Email [email protected]
Anton Blencowe
Applied Chemistry and Translational Biomaterials (ACTB) Group, Clinical and Health Sciences, University of South Australia, Adelaide, SA, 5000, Australia
Email [email protected]
Background: Drug-eluting gastrointestinal (GI) stents are emerging as promising platforms for the treatment of GI cancers and provide the combined advantages of mechanical support to prevent lumen occlusion and as a reservoir for localized drug delivery to tumors. Therefore, in this work we present a detailed quality assurance study of 5-fluorouracil (5FU) drug-eluting stents (DESs) as potential candidates for the treatment of obstructive GI cancers.
Methods: The 5FU DESs were fabricated via a simple two-step sequential dip-coating process of commercial GI self-expanding nitinol stents with a 5FU-loaded polyurethane basecoat and a drug-free protective poly(ethylene-co-vinyl acetate) topcoat. The drug loading, content uniformity and drug stability were determined using a validated high-performance liquid chromatography (HPLC) method, which is also recommended in the United States Pharmacopeia. In vitro drug release studies were performed in phosphate buffered saline to determine the drug releasing properties of the two 5FU-loaded stents. Gas chromatography (GC) and HPLC were employed to determine total residual tetrahydrofuran and N,N-dimethylformamide in the stents remaining from the manufacturing process. Sterilization of the stents was performed using gamma radiation and stability testing was carried out for 3 months.
Results: The drug loading analysis revealed excellent uniformity in the distribution of 5FU between and within individual stents. Determination of drug stability in the biorelevant release media confirmed that 5FU remains stable over 100 d. In vitro drug release studies from the stents revealed sustained release of 5FU across two different time scales (161 and 30 d), and mathematical modeling of drug release profiles revealed a diffusion-controlled mechanism for the sustained 5FU release. GC and HPLC analysis revealed that the daily residual solvent leached from the stents was below the United States (US) Food and Drug Administration (FDA) guidelines, and therefore, unlikely to cause localized/systemic toxicities. Sterilization of the stents with gamma radiation and accelerated stability tests over a period of 3 months revealed no significant effect on the stability or in vitro release of 5FU.
Conclusion: Our results demonstrate that the 5FU DESs meet relevant quality standards and display favourable drug release characteristics for the potential treatment of GI cancers and related obstructions.
Keywords: drug-eluting stent, 5-fluorouracil, gastrointestinal cancer, self-expanding metal stents
Introduction
Conventional gastrointestinal (GI) stents have a proven track record of clinical safety and effectiveness in the localized management of stenoses and/or obstructions.1 Nevertheless, in-stent restenosis (ISR) is a common problem due to benign hyperplastic or malignant tissue growth within the stented region.1–3 ISR frequently results in a significant reduction in the effective duration of the stenting treatment, thus necessitating placement of a second stent or emergency surgical intervention.1–4 While the introduction of vascular drug-eluting stents (DESs) has drastically reduced the incidence of ISR among coronary artery disease (CAD) patients,2,5,6 the translation of GI DESs towards the clinic holds promise for the treatment of ISR and simultaneous reduction of tumor burden for GI cancers.
DESs are generally composed of three primary components: drug, polymeric drug-delivery coating/carrier, and the stent platform.3,7,8 To date, GI DESs have been investigated using contemporary chemotherapeutics in conjunction with different types of GI stents.9–13 These specialised GI stents have been designed to provide controlled and localized delivery of drugs at the stenting site, with the intent of maximising drug bioavailability within the local tumor tissue and minimising systemic toxicities. The selection of an appropriate anticancer drug and dose regime is crucial to ensure the clinical safety and effectiveness of DES-based chemotherapy, similar to that required for conventional systemic and non-systemic chemotherapies.3,14–16 While a large number of United States (US) Food and Drug Administration (FDA)-approved cancer drugs are widely used for the clinical treatment of GI cancers,14,17 5-fluorouracil (5FU) remains one of the most popular due to its high potency and broad anticancer activity. Commonly, 5FU is administered intravenously for the treatment of GI cancers as a result of poor oral bioavailability, although this can be associated with severe GI, hematologic, cardiac and dermatologic side effects.14,18–21 Few studies have investigated the potential of localized stent-based 5FU delivery to overcome the toxicities associated with systemic delivery.18–20,22–24 Several studies have evaluated the efficacy of 5FU-eluting esophageal stents in vivo and revealed significantly higher 5FU concentrations in the local esophageal tissue as compared to peripheral organs without any signs of tissue damage.19,23,24 In addition, Li et al prepared a series of biodegradable, 5FU-loaded polydioxanone stents and demonstrated that higher 5FU loadings correlated with superior anti-ISR and antitumor effects in a mice xenograft model.20 These prior studies support the feasibility of stent-based localized anticancer drug delivery with reduced systemic toxicities, and demonstrate that 5FU is a suitable candidate for drug-eluting GI stents.
Another critical component of DESs is the polymeric coating/carrier which should provide sufficient drug loading capacity8,25 and effective control of the drug release kinetics.26 To date, various biostable and biodegradable polymers have been investigated in combination with different self-expandable metal stents (SEMSs) for the fabrication of DES platforms.1,27,28 However, biostable polymers are preferable for GI DES, as biodegradable polymer coatings could result in early stent blockage due to malignant tumor growth, stricture recurrence, or stent migration.1,27 While biostable polyurethanes (PUs), polysiloxanes, and poly(ethylene-co-vinyl acetate) (PEVA) have been commonly used for DES applications,7,27,29 PUs offer excellent biocompatibility and mechanical properties, and can readily be combined with drugs.1,27 Nevertheless, the high aqueous solubility of 5FU (13.26 mg/mL)30 can potentially result in burst release profiles, and therefore, it is advantages to incorporate a drug-free protective layer to slow the diffusion and release kinetics of 5FU from coated stents.31
While previous studies have demonstrated the potential of stent-based localized 5FU delivery, there is very little information regarding the critical quality attributes that are essential for any DES platform from a quality control or regulatory perspective. Unfortunately, all available regulatory documents or literature detailing quality control requirements, tests/procedures, and recommendations are based on vascular coronary DESs2,8 and there is no such information specifically for non-vascular DESs. In our previous study, we demonstrated the potential advantages of dip-coating to rapidly and reproducibly produce DESs for the controlled delivery of 5FU for the treatment of GI cancers and related obstructions.32 Therefore, in the present study we aimed to utilise dip-coating for the fabrication of 5FU-loaded GI stents and assess their performance via a series of quality control checks in order to ensure both their quality and functionality in accordance with current FDA requirements. Here, we report the first comparative evaluation of the critical quality attributes of two 5FU DESs fabricated with the same polymer coating technique and formulation but using two different types of self-expanding nitinol stent platforms.
Materials and Methods
Materials
Clinically relevant, non-vascular self-expanding silicone (Si) membrane-covered (Niti-S™ S-type biliary stents; diameter = 10 mm) and bare (uncovered) nitinol stents (Niti-S™ D-type pyloric/duodenal/enteral colonic stents; diameter = 22 mm) were kindly supplied by Taewoong Medical Co., Ltd (Gimpo-si, Gyeonggi-do, South Korea) (Supplementary Information (SI), Figure S1). ChronoFlex AL, a biostable medical-grade aliphatic polycarbonate-based thermoplastic PU, with a Shore hardness of 80A was kindly provided by AdvanSource Biomaterials Corporation (Wilmington, MA, USA). Tetrahydrofuran (THF, ACS Reagent grade) was purchased from Chem-Supply Pty Ltd (Gillman, SA, Australia). 5FU was purchased from Hangzhou Dayang Chem Co., Ltd. (Hangzhou, Zhejiang, China). N,N-Dimethylformamide (DMF, high-performance liquid chromatography (HPLC) grade), PEVA (vinyl acetate (VA) 40 wt. %), and ammonium hydroxide (ammonia content 28 to 30%, ACS Reagent grade) were procured from Sigma-Aldrich Pty Ltd (North Ryde BC, NSW, Australia). Dichloromethane (DCM, HPLC grade) and silver aluminum foil self-sealing zip-lock bags were purchased from Merck KGaA (Darmstadt, Germany) and a local supplier, respectively. More detailed information on the materials used in the present study is provided in SI, Table S1. All reagents were of analytical grade and were used as received unless otherwise stated.
Methods
Fabrication of 5FU-Loaded GI Stents
Based on our previous study,32 DESs were fabricated via the two-step sequential dip-coating of commercial GI nitinol stents with a 5FU-loaded PU (ChronoFlex AL) basecoat and a drug-free protective PEVA topcoat (SI, Table S2). Briefly, two different coating solutions were prepared for the basecoat and topcoat application. Initially, 5FU (3.62 g) was dissolved in DMF (41.20 mL) with sonication (Model 5510E-DTH, Branson Ultrasonics Corporation, Danbury, CT, USA). Separately, ChronoFlex AL (52.50 g) was dissolved in THF (258.80 mL) with stirring and heating at 60 °C in a water bath. The temperature of the water bath was reduced to 40 °C and after 30 min the 5FU solution was slowly added dropwise. The resulting solution was sonicated for 1 h, heated at 40 °C in a water bath and then used immediately to coat two different types of commercially available GI stents (Si-covered and bare nitinol stents) (SI, Figure S1) via dip-coating with a Model TL0.01 desktop dip-coater (MTI Corporation, Richmond, CA, USA).32 The resulting Si-PUFU and Ba-PUFU base-coated nitinol stents were dried in an oven for 36 h at 60 °C, and weighed to determine the mass of the basecoat (after THF/DMF evaporation), which theoretically consisted of 6.5% w/w of 5FU. The drug-free protective topcoat solution was prepared by dissolving PEVA (78 g) in DCM (300 mL) with sonication at 37 °C for 4 h and stored at ambient temperature for > 12 h until further use. The PEVA solution was dip-coated onto the Si-PUFU and Ba-PUFU stents and air-dried for 24 h in a fume cupboard (Model Dynaflow 1500GRP, Melrose Park, NSW, Australia) to afford Si-PUFU-PEVA and Ba-PUFU-PEVA stents, which were weighed to determine the mass of the topcoat. A flow chart illustrating the key steps of the DES fabrication process can be found in SI, Figure S2.
HPLC Determination of 5FU
5FU was quantified using the reversed-phase HPLC method described in the United States Pharmacopeia-National Formulary (USP-NF).33 The detailed HPLC test system configuration and method parameters are summarised in SI, Table S3.
Determination of Drug Loading and Content Uniformity
Drug content evaluation was performed on the Si-PUFU and Ba-PUFU stents to determine the true amount of 5FU present in the active PU basecoat layer, as well as the uniformity and distribution of drug within the stent samples. To prepare samples for drug extraction, the Si-PUFU and Ba-PUFU stents were cut into small pieces weighing 14.93 ± 0.38 mg and 9.85 ± 0.67 mg, respectively (n = 6 for both base-coated stent types). The pieces were added separately to DCM (0.60 or 0.90 mL) in plastic tubes and the polymer coatings were allowed to completely dissolve over 12 h (SI, Figure S3). Subsequently, 1 N ammonium hydroxide solution in water (1.40 or 2.10 mL) was added to each tube, mixed well, and then centrifuged for 15 min at 3000 rpm and 21 °C. The aqueous phase was collected from each tube and the 5FU was quantified via HPLC .33 The efficiency of the extraction process was evaluated using the same procedure, except that known concentrations (range: 584.0 to 2648.0 µg/mL) of pure 5FU drug were added to DCM instead of 5FU-loaded stent cut pieces.
5FU Stability Studies in Media
5FU stability was assessed in vitro in 10 mM phosphate buffer saline (PBS) at three different pH values (5.8, 6.6, and 7.4) over a period of 100 d. Initially, 5FU solutions (54.57 ± 0.79 µg/mL) were prepared in triplicate in sealed vials and shaken on a horizontal mixer (20 mm orbital diameter, 175 rpm) at 37 °C. At specific time intervals, 500 µL of the medium was removed from each container and the 5FU concentration was quantified via HPLC. To assess pH stability or the presence of any drug precipitation, the pH of solutions was recorded at day 0, 1 and 100, followed by microscopic imaging for crystallization using an Olympus BX41TF microscope (Olympus Corporation, Tokyo, Japan).
In vitro Release of 5FU from Coated Stents
Based on previously reported values for the pH of the colon (SI, Table S4) and release studies (SI, Table S5), 10 mM PBS at pH 7.4 was selected as a biorelevant medium for studying 5FU release from the coated stents. The full-length Si-PUFU-PEVA and Ba-PUFU-PEVA stents (n = 3 for each) were submerged – using a fabric mesh (SI, Figure S4) – in 20 and 40 mL of medium, respectively, in separate plastic containers, which was sufficient to maintain sink conditions; the saturation concentration of 5FU in PBS (10 mM, pH 7.4) was determined to be 7.65 ± 1.50 mg/mL (n = 4). The containers were sealed and placed on a horizontal shaker (20 mm orbital diameter, 175 rpm) at 37 °C. At specific time intervals, 1 mL of the release medium was withdrawn from each container for 5FU quantification via HPLC and replaced with 1 mL of fresh PBS.
Mathematical Modeling of Drug Release Profiles
To study the mechanism of 5FU release from the bilayer coated stents, the percentage of drug release data obtained from the in vitro release studies of the Si-PUFU-PEVA and Ba-PUFU-PEVA stents were evaluated as a function of time (days) and applied to various mathematical models, using the Microsoft Excel add-in software program DDSolver. The goodness of fit of the experimentally observed 5FU release (at least 80%) data to these models were evaluated and selected based on the three most popular statistical criteria; the adjusted coefficient of determination (R2 adjusted), the root mean squared error (RMSE), and the Akaike Information Criterion (AIC). The mathematical model that gave the highest R2 adjusted value and smaller RMSE and ACI values best described the experimental drug release data.8,34–36
Determination of Residual Solvents in Coated Stents
Determination of the total residual THF and DMF in the coated stents was conducted using gas chromatography (GC). For analysis of residual THF, Si-PUFU and Ba-PUFU stents were cut into pieces, accurately weighed (~ 10 mg) and then 300 µL of DMF was added to dissolve the PU coating (n = 3 for each type of stent). After 2 h, the insoluble metal stent mesh was removed, dried in vacuo (0.1 mbar, 23 °C) and weighed to determine the amount of basecoat dissolved in the solution. The concentration of THF in the solution was then determined using a Shimadzu GC-2010 gas chromatograph, equipped with a flame ionization detector (FID) and fitted with a Supelco SPB®-35 capillary column (30 m × 0.32 mm × 0.25 µm). Nitrogen was used as the carrier gas at a constant linear velocity of 56.5 cm/s, with injection port and detector temperatures of 200 and 250 °C, respectively. A 1 µL split injection (split ratio, 50:1) and a column temperature program of 40 °C (1 min hold time) to 200 °C (2 min hold time) at 30 °C/min were used. The concentration of residual DMF was determined using the same procedure, however, THF (300 µL) was used instead to dissolve the PU basecoat. Determination of the time-dependent release of residual DMF from the coated bilayer stents was conducted alongside the 5FU release study in PBS (vide supra), using a Shimadzu Prominence HPLC system fitted with an Apollo C8 column. The procedural details related to the HPLC analysis of residual DMF are presented in SI, Table S6.
Sterilization Conditions and Stent Testing
Gamma irradiation sterilization was conducted by Steritech Pty Ltd (Dandenong, Victoria, Australia) on full-length Si-PUFU-PEVA and Ba-PUFU-PEVA stents sealed in separate aluminum foil bags (SI, Figure S5). All samples were gamma-irradiated with a dose of 25 kGy at room temperature. The effect of gamma radiation on the stent performance was evaluated via in vitro drug release studies over a period of ~ 14 d, using the same experimental conditions as described previously (vide supra). The drug release profiles of the gamma-irradiated and non-irradiated (control) stents were quantitatively compared using the difference factor f1 and similarity factor f2, as recommended by the US FDA (for f1 and f2 equations, refer to SI, Figure S6). The f1 and f2 values for test stents versus control stents were calculated from the mean percentage drug release at each time point, with the application of DDSolver software package.8,35–38
Stability Studies
Si-PUFU-PEVA and Ba-PUFU-PEVA stents were cut into small pieces of approximately equal weight and individually sealed in aluminum foil bags. The bags were then stored at either 25 °C/60% relative humidity (RH) or 40 °C/75% RH in separate stability chambers (Binder GmbH, Tuttlingen, Germany) or at 4 °C. Samples were withdrawn after 1 and 3 months, visually inspected, and the 5FU was extracted and quantified via HPLC.
Results and Discussion
Fabrication of 5FU-Loaded GI Stents
Various drug-polymer coating techniques, including spray coating, dip-coating, hot-melt coating and electrospinning, have been utilised for DES fabrication. However, among the available techniques, dip-coating is commonly accepted as the most simple, rapid and economic method for coating stent devices.11–13,39–42 Therefore, 5FU-loaded nitinol stents were prepared by dip-coating the commercial GI nitinol stents (used for the treatment of GI cancers) sequentially with a drug-incorporated PU basecoat solution and a drug-free protective PEVA topcoat solution.32 While the PU base layer functions as the drug reservoir, the PEVA topcoat was intended to control the diffusion and premature burst release of the highly hydrophilic 5FU.31,43–45 Two types of GI nitinol stents (silicone membrane-covered and bare) were used (SI, Figure S1) in this study to investigate the applicability of the same drug-polymer formulations to different types of SEMS platforms via dip-coating, and thus compare potential differences in quality and drug release performance between the DESs. The fabricated dip-coated stents were referred to as Si-PUFU-PEVA and Ba-PUFU-PEVA for silicone membrane-covered and bare nitinol stents coated with a 5FU-loaded PU layer then 5FU-free PEVA layer, respectively.
Interestingly, up to now there are no reported examples of 5FU-loaded stents prepared via dip-coating, other than our recent study32 on dip-coated 5FU-loaded nitinol stents. This potentially highlights the challenges of combining the hydrophilic 5FU with hydrophobic polymers (for example, PUs) in suitable formulations for dip-coating. Hence, a biostable ChronoFlex AL (PU)-based basecoat formulation which would provide a high 5FU loading was initially developed and optimized with a mixed solvent system (DMF:THF, 1:6.3, v/v). Subsequently, the 5FU-incorporated PU basecoat was used for dip-coating the nitinol stent platforms, resulting in Si-PUFU and Ba-PUFU stents with a 5FU loading of 6.5% (w/w) (Figure 1). Following the PU-5FU base layer coating, a drug-free PEVA topcoat was dip-coated onto the base-coated Si-PUFU and Ba-PUFU stents using a 26% (w/v) DCM solution. Resultingly, Si-PUFU-PEVA and Ba-PUFU-PEVA stents were fabricated (Figure 1) – with excellent reproducibility using the optimized coating formulations and the dip-coating process – for further in vitro drug release and quality assessment testing.
Optimization of HPLC Method for 5FU Determination
In addition to the HPLC method described in the current edition of the USP-NF, there are a number of HPLC methods that have been reported in the literature over the past 40 years for the determination of 5FU content in a variety of liquid mediums and/or mixtures.19,22,33,46–49 Considering the well-established reliability of the USP-NF analytical HPLC method, it was chosen and applied in the present study for 5FU determinations.33 However, as acknowledged by the US FDA in 21 CFR 211.194(a)(2), it is not necessary to validate further the reliability of the analytical methods specified in USP-NF,48 and therefore a full validation was not undertaken. Rather, we verified the suitability of the same USP method in our laboratory under real operating conditions (SI, Table S3) to assess the two important method validation parameters; accuracy and linearity (SI, Table S7 and Figure S7).46,48,49
Accuracy is usually defined as the closeness of the measured (experimental) quantity value to the true (or accepted true) quantity value of an analyte.46,48,49 The mean of accuracy (as recovery%) and relative standard deviation percentage (RSD%) values at eleven different 5FU concentrations (0.5, 1, 2, 4, 6, 8, 10, 20, 30, 40, and 50 μg/mL) in water showed acceptable recovery results with RSD values < 2%. To check the linearity of the HPLC assay method, the calibration curve was constructed49,50 with the same eleven concentrations of 5FU in water versus average peak area data. The linearity of the curve was determined by utilising a straight-line fit equation and analysis of the coefficient of determination (R2) values.46,49 As shown in SI, Figure S7, the values of the slope, intercept, and R2 for 5FU calibration curve were found as 61,761, 10,235, and 1, respectively, indicating that the linearity of the calibration curve was excellent. In addition, validation of the “stability” parameter was achieved by measuring the stability of 5FU in aqueous 10 mM PBS solutions at three different pH values (5.8, 6.6, and 7.4) (vide infra).49 These results verify that the USP recommended HPLC method we used for 5FU analysis had an acceptable level of accuracy across the concentration range from 0.5 to 50 µg/mL 5FU, and thus the method was found to be suitable and reliable for 5FU assay in the present study.
Determination of Drug Loading and Content Uniformity of DESs
Drug loading and content uniformity of 5FU was determined using weighed Si-PUFU and Ba-PUFU stent pieces cut from along the length of individual stents. It was considered that the drug content from six sampled locations along the stent length would provide a reasonable approximation of the actual amount of 5FU present in the stents and its uniformity of distribution. The PU matrix for each stent piece was dissolved in DCM and the 5FU extracted into aqueous 1 N ammonium hydroxide prior to determination via HPLC (Table 1). The recovery efficiency (accuracy) of this process was validated with spiked 5FU samples (584.0 to 2648.0 µg/mL) (SI, Table S8) and the mean (± standard deviation (SD)) percentage recovery was found to be 99.4 ± 1.9, clearly indicating that the extraction procedure was suitable for the quantification of 5FU. For Si-PUFU and Ba-PUFU stent pieces the experimental 5FU loadings were observed to be almost identical to the theoretical 5FU loadings with low SD values and mean percentage recoveries of ~ 98 and 100%, respectively, confirming the uniform distribution of 5FU within the PU base layer regardless of the stent platform type.
 |
Table 1 Theoretical and Experimental Drug Loading Content for Dip-Coated Nitinol Stents (n = 6) |
5FU Stability Studies in PBS
In this study, to maximise the drug efficacy locally with significantly lower doses than used clinically in conventional systemic chemotherapy of GI cancers, we aimed to achieve a controlled 5FU release from the DESs over a course of several weeks to months. Thus, the stability of 5FU during its release from the Si-PUFU-PEVA and Ba-PUFU-PEVA stents is important to appropriately characterise the release kinetics and to avoid suboptimal dosing of 5FU within or adjacent to tumor tissues. Previously, in vitro stability studies of 5FU under different conditions have shown it to be stable in alkaline media but undergoes hydrolysis in acidic media.51–55 As the pH of the GI tract (for example, colon) varies in response to a wide variety of physiological/pathophysiological conditions,56–59 DESs for the treatment of GI cancers are likely to be exposed to variable pH levels, and therefore, we studied the in vitro stability of 5FU (54.57 ± 0.79 µg/mL) in 10 mM PBS at pH values of 5.8, 6.6, and 7.4 over a period of 100 d. The concentration of 5FU at each timepoint was measured via HPLC.
For all three pH conditions tested, there were no observable colour changes or turbidity, and no crystallization was visible microscopically at 40-fold magnification after 100 d of storage at 37 °C. The pH of the 5FU solutions did not show any significant changes between day 0 and day 100 (SI, Table S9). For all pH conditions tested, there was negligible variation in the concentration of 5FU as indicated by HPLC analysis (SI, Table S9). According to the US FDA, a drug substance is considered stable if the lower bound of the 95% confidence interval (CI) on the mean concentration remains above 90% of its initial concentration.52 Our results are fully compliant with the US FDA recommended stability requirements and prove that 5FU can be considered chemically stable for at least 100 d within the experimental limits of this study.
In vitro Release of 5FU from DESs
In vitro release of 5FU from the Si-PUFU-PEVA and Ba-PUFU-PEVA stents was conducted in PBS (10 mM, pH 7.4, 37 °C) and monitored at regular intervals via HPLC. To ensure sink conditions were maintained the total 5FU loading and 5FU solubility in the medium were determined (7.65 ± 1.50 mg/mL) and the volume of release media was calculated accordingly. The 5FU release from the Si-PUFU-PEVA and Ba-PUFU-PEVA stents presented as asymptotic profiles with distinctively different release periods (Figure 2), even though the same dip-coating formulation and process parameters were applied to fabricate both DESs. For the Si-PUFU-PEVA stents a gradual and sustained release of 5FU was observed over a period of 161 d, reaching a maximum release of ~ 86% (~ 479 µg/cm2). In contrast, the Ba-PUFU-PEVA stents provided biphasic release kinetics with an initial burst effect (~ 22%) during the first day followed by a relatively sustained release up to 30 d, reaching a maximum release of ~ 94% (~ 215 µg/cm2). As a point of reference, release studies were also conducted on single-layer Si-PUFU stents (data not shown), which displayed a rapid release of 5FU (100% in 4 d) due to its high aqueous solubility (octanol/water partition coefficient, Pow = −0.8360,61) and highlighted the importance of the additional PEVA topcoat in modulating the release kinetics. Similar results have also been reported by others, with Shin et al noting the rapid release of gemcitabine from single layer ChronoFlex AL (PU) films in the absence of PEVA,62 and various others demonstrating diffusion-controlled drug release from PEVA-based polymer matrices.18,19,63,64
The disparity of 5FU release periods between the two drug-loaded stents was attributed to structural (including geometry) differences and the effect of this on the deposited polymer coatings. The Si-covered stents possessed different surface topographies and thickness (for both the PU-5FU basecoat and PEVA topcoat) as compared to that of the bare stents.32 Furthermore, in the case of Si-covered stents the primary Si membrane backing layer results in abluminal and luminal PU-5FU basecoat layers, whereas only a single PU-5FU basecoat layer is formed over the mesh interstices of the bare stents.
Invariably, the successful performance of DESs depends on the delivery of an effective drug dose with controlled drug release kinetics.3,16 The in vitro release studies showed that both the Si-PUFU-PEVA and Ba-PUFU-PEVA stents can provide a sustained release of 5FU across two different time scales, which may be useful in different scenarios. According to the European Society of Gastrointestinal Endoscopy (ESGE) guidelines, the placement of SEMS is recommended when the patient’s expected survival time is > 4 months,9 and therefore, the sustained release provide by the Si-PUFU-PEVA stent (> 5 months) might make it a promising candidate for the treatment of various obstructing GI cancers. In contrast, the relatively fast and short-term 5FU release from the Ba-PUFU-PEVA stents may be particularly effective for the treatment of palliative GI cancer patients, many of which would be likely to continue with the stent on their GI tract until death.
Furthermore, chemotherapeutics consistently released from DESs over a sustained period of time are more likely to result in optimal diffusion and uptake by the surrounding cancer cells over several cell division cycles and may lead to better control of GI tumor cell replication and subsequent cancer spread.1,65 Hence, GI DESs could potentially inhibit the growth of cancer cells around the stent more efficiently than conventional systemic administration of chemotherapeutics and non-DESs.6,9,10,13,18–20,23,66–68
Mathematical Modeling of Drug Release Profiles
To study the mechanism of 5FU release from the bilayer coated DESs, various mathematical models (SI, Table S10) were applied to the drug release (at least 80%) data obtained from in vitro release studies in PBS.8,34,35 The goodness of fit of the experimental release profiles was evaluated using three common statistical criteria in combination; the adjusted R2, the RMSE, and the AIC.8,34,35 For the Si-PUFU-PEVA and Ba-PUFU-PEVA stents the experimental release data were best fitted by the Weibull model and Peppas-Sahlin model, respectively. The β value of the Weibull model is the shape parameter which describes the release curve as either sigmoidal (S-shaped) with upward bend followed by a turning point (when β > 1) or exponential (when β = 1), or parabolic with a steeper initial slope and rest consistent with the exponential (when β < 1).35,38,69 The shape parameter (β value) of the Weibull model was calculated to be < 0.75 (SI, Table S10), indicating a more pronounced parabolic shape to the release profile35,38 that is consistent with a predominantly Fickian diffusion-controlled mechanism for the Si-PUFU-PEVA stents.69 The Peppas-Sahlin model describes drug release as a combination of the Fickian diffusion and polymer phase transition from a semi-rigid to a flexible structure that corresponds to relaxation of the polymer chains.34,35 However, determination of the Peppas-Sahlin model rate constants for the Ba-PUFU-PEVA stents (k1 = 24.038 and k2 = −1.694) implied that Fickian diffusion was the predominant factor because of a larger k1 value.
Determination of Residual Solvents from Manufacturing of DESs
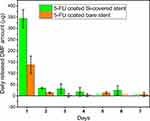
During the stent coating process, several organic solvents were used to prepare dip-coating solutions: THF (boiling point (BP) = 66 °C), DMF (BP = 153 °C), and DCM (BP = 40 °C).70 While the majority of the solvents are evaporated during the stent drying process following dip-coating, it is conceivable that residual traces remain entrapped within the polymeric matrices, especially for less volatile solvents. Given the inherent toxicities of these solvents, the amount of THF and DMF in the Si-PUFU-PEVA and Ba-PUFU-PEVA stents was quantified, disregarding DCM due to its low BP. The polymeric coatings on stent sections were dissolved and the solutions analyzed via GC, which determined that no residual THF was present in either the Si-PUFU-PEVA or Ba-PUFU-PEVA stents (SI, Figure S8). However, residual DMF was detected at levels of 1201 ± 115 and 1319 ± 194 ppm (SI, Figure S9) respectively, both of which are above the FDA recommended concentration limit of 880 ppm (SI, Table S11).71
The relatively high amounts of residual DMF in the coated stents may be correlated with the drying time and process (oven drying), and further optimization of the process is likely to reduce residual levels. Nevertheless, the inherent design of DESs is that they slowly release their payload over time, and it was hypothesized that a gradual release of DMF would also be observed. Therefore, the release of DMF from the stents over time was measured to better understand the true DMF exposure levels and assess the compliance of the stents (Figure 3). Day 1–7 samples were collected from the 5FU release study in PBS (pH 7.4, 37 °C) and analyzed for DMF via HPLC (SI, Figures S10–12). Compared to the total amount of DMF extracted from the stents, the daily release of DMF was significantly lower (Figure 3), and well below the FDA permitted daily exposure (PDE) limit of 8.8 mg (SI, Table S11). For Si-PUFU-PEVA and Ba-PUFU-PEVA stents, the highest amount of DMF was detected after the first day (343.4 and 137.7 µg, respectively), which then decreased significantly on subsequent days. These results demonstrated that the amount of residual DMF that could leach from the stents in a single day after placement is unlikely to cause DMF-related localized or systemic toxicities. This claim was further confirmed by in vitro cytotoxicity tests with drug-free (blank control) PU coated stents, which showed the (relative) viability of HCT 116 human colon cancer cells was almost similar (~ 97%) to untreated (media) control.32
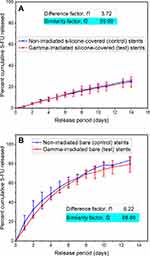
Effect of Sterilization on Stents
Sterilization of medical devices and DESs with ionizing radiation (for example, gamma irradiation) can potentially lead to drug or polymer degradation that may result in unanticipated effects on the critical quality attributes of DESs, such as loss of coating integrity, and drug and polymer carrier instability. However, according to FDA recommendations, in vitro drug release testing is a powerful tool for assessing any changes in DES critical performance parameters,8 and therefore, the 5FU release profiles were compared for the stents before and after gamma irradiation (25 kGy72) (Figure 4). Overall, no significant differences were noted in the release profiles for irradiated and non-irradiated stents, which was further confirmed by calculation of the difference (f1) and similarity (f2) factors. Generally two dissolution profiles are considered similar when f1 value range between 0 and 15 and f2 value range between 50 and 100, as suggested by the US FDA.36–38 For the irradiated and non-irradiated Si-PUFU-PEVA and Ba-PUFU-PEVA stents the f1 and f2 values were within acceptable limits (f1 = 3.72 and f2 = 95.99 for Si-PUFU-PEVA; f1 = 6.22 and f2 = 68.88 for Ba-PUFU-PEVA) indicating that gamma irradiation sterilization had no significant effect on the in vitro 5FU release.
Stability Studies
To investigate the stability of 5FU in the stents and the integrity of the polymer coating under the influence of temperature/humidity variations over time, stability studies were conducted with the Si-PUFU-PEVA and Ba-PUFU-PEVA stents under the storage conditions prescribed in the International Conference on Harmonisation (ICH) Q1A(R2) guidelines.73,74 The stents were individually packed in aluminum foil bags (SI, Figure S5) and stored at 25 °C/60% RH or 40 °C/75% RH, and evaluated after 1 and 3 months for appearance, weight variation, drug content, degradation products/impurities, and in vitro drug release (SI, Table S12).
The appearance of the stents (abluminal and luminal surfaces) was visualised via brightfield microscopy (40× magnification) before and after storage, which revealed no significant differences and implied that the stents are physically stable under the conditions tested over a period of three months; this was further supported by the absence of any weight changes. To determine the stability of 5FU, the drug content in the Si-PUFU-PEVA or Ba-PUFU-PEVA stents was determined via HPLC over the 3 months storage and compared to the initial amount, which revealed no significant change (SI, Table S12).
However, to assess the effect of accelerated storage conditions on the 5FU release from the Si-PUFU-PEVA and Ba-PUFU-PEVA stents, the release profiles of stents stored at 40 °C ± 2 °C/75% RH ± 5% RH for 3 months were compared to stents stored at 4 °C (Figure 5). For both the Si-PUFU-PEVA and Ba-PUFU-PEVA stents stored under accelerated conditions the f1 and f2 factors were within acceptable limits versus stents stored at 4 °C (SI, Table S12), and univariate analysis of variance (ANOVA) indicated that the 5FU release profiles were not significantly different statistically. Together, these results imply that the stents are stable under accelerated storage conditions over 3 months without alteration in their in vitro drug release profiles.
Conclusions
Two anticancer drug-eluting stent (DES) platforms were fabricated successfully via dip-coating of either silicone membrane-covered or bare self-expanding GI nitinol stents sequentially with a 5FU (6.5% w/w)-incorporated polyurethane (PU) basecoat and a blank (drug-free) poly(ethylene-co-vinyl acetate) (PEVA) topcoat. Optimization of the stent coating formulations and dip-coating process parameters allowed the highly hydrophilic 5FU to be successfully encapsulated in a hydrophobic PU polymer matrix, and top-coating with PEVA allowed the burst release of 5FU to be controlled effectively. Determination of drug loading content in the stents confirmed that the 5FU was uniformly distributed throughout the PU matrix without significant variation in its content along the length of an individual coated stent. With the chemical stability of 5FU in the in vitro release medium confirmed for at least 100 d, drug release studies demonstrated controlled and sustained 5FU release profiles across two different time scales (161 and 30 d), which may be useful in different clinical scenarios. No residual THF was detected in either of the coated 5FU-loaded stents. While residual DMF was detected, the amount that leached from the stents in a single day was determined to be significantly lower than the FDA recommended daily exposure limit of 8.8 mg, and is unlikely to cause any DMF-related local/systemic toxicities. In addition, the 5FU-loaded stents exhibited no significant change in the in vitro drug release profiles after sterilization with gamma radiation, or after incubation for 3 months under accelerated storage conditions.
The series of tests performed in this study are typically considered quality assessment tests recommended to be performed during development and commercial production of DESs. However, other aspects of DESs, such as mechanical characteristics of the polymer coating which may influence the delivery and deployment of DESs in vivo, are required to be explored further, before taking this preclinical experimental study of GI DESs into confirmatory animal and human studies. Nevertheless, the results from the critical quality attributes tested herein comply with the current FDA regulatory requirements for DESs, suggesting the newly developed 5FU-eluting DESs could be potential candidates for effective chemotherapeutic treatment of gastrointestinal cancers and associated obstructions.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Arafat M, Fouladian P, Blencowe A, Albrecht H, Song Y, Garg S. Drug-eluting non-vascular stents for localised drug targeting in obstructive gastrointestinal cancers. J Controlled Release. 2019;308:209–231. doi:10.1016/j.jconrel.2019.07.001
2. European Medicines Agency. EMEA/CHMP/EWP/110540/2007. Guideline on the clinical and non clinical evaluation during the consultation procedure on medicinal substances contained in drug-eluting (medicinal substance-eluting) coronary stents. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003275.pdf.
3. Guo S-R, Chen W-L, Rong H-J LF. Stents as a platform for drug delivery AU - Lei, Lei. Expert Opin Drug Deliv. 2011;8(6):813–831. doi:10.1517/17425247.2011.572068
4. Baron TH Enteral stents for the management of malignant colorectal obstruction. Available from: https://www.uptodate.com/contents/enteral-stents-for-the-management-of-malignant-colorectal-obstruction?source=search_result&search=enteral%20stent&selectedTitle=2~22.
5. Boam AB. Regulatory issues facing the development of drug-eluting stents: a US FDA perspective. Expert Rev Med Devices. 2006;3(3):297–300. doi:10.1586/17434440.3.3.297
6. Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials. 2006;27(11):2450–2467. doi:10.1016/j.biomaterials.2005.11.031
7. Schmidt W, Lanzer P. Instrumentation. In: Lanzer P, editor. Catheter-Based Cardiovascular Interventions: A Knowledge-Based Approach. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013:445–472.
8. Food and Drug Administration Center for Devices and Radiological Health (CDRH). Guidance for Industry: Coronary Drug-Eluting Stents-Nonclinical and Clinical Studies (Draft). Rockville, MD, USA: Food and Drug Administration (FDA); 2008:89.
9. Kim S-Y, Kim M, Kim M-K, et al. Paclitaxel-eluting nanofiber-covered self-expanding nonvascular stent for palliative chemotherapy of gastrointestinal cancer and its related stenosis. Biomed Microdevices. 2014;16(6):897–904. doi:10.1007/s10544-014-9894-9
10. Kwak TW, Lee HL, Song YH, et al. Vorinostat-eluting poly(DL-lactide-co-glycolide) nanofiber-coated stent for inhibition of cholangiocarcinoma cells. Int J Nanomedicine. 2017;12:7669–7680. doi:10.2147/IJN.S141920
11. Lee DK, Kim HS, Kim K-S, et al. The effect on porcine bile duct of a metallic stent covered with a paclitaxel-incorporated membrane. Gastrointest Endosc. 2005;61(2):296–301. doi:10.1016/S0016-5107(04)02570-2
12. Lee JW, Yang S-G NK. Gemcitabine-releasing polymeric films for covered self-expandable metallic stent in treatment of gastrointestinal cancer. Int J Pharm. 2012;427(2):276–283. doi:10.1016/j.ijpharm.2012.02.016
13. Moon S, Yang S-G NK. An acetylated polysaccharide-PTFE membrane-covered stent for the delivery of gemcitabine for treatment of gastrointestinal cancer and related stenosis. Biomaterials. 2011;32(14):3603–3610. doi:10.1016/j.biomaterials.2011.01.070
14. Clark JW, Grothey A Systemic chemotherapy for metastatic colorectal cancer. Available from: https://www.uptodate.com/contents/systemic-chemotherapy-for-metastatic-colorectal-cancer-completed-clinical-trials/print?source=see_link.
15. Pinto AC, Moreira J, Simoes SR. Combination chemotherapy in cancer: principles, evaluation and drug delivery strategies. In: Ozdemir O, editor. Current Cancer Treatment - Novel Beyond Conventional Approaches. InTech; 2011:693–714.
16. Thipparaboina R, Khan W, Domb AJ. Eluting combination drugs from stents. Int J Pharm. 2013;454(1):4–10. doi:10.1016/j.ijpharm.2013.07.005
17. National Cancer Institute. Drugs Approved for colon and rectal cancer. https://www.cancer.gov/about-cancer/treatment/drugs/colorectal. Available from:
18. Guo Q, Guo S, Wang Z. A type of esophageal stent coating composed of one 5-fluorouracil-containing EVA layer and one drug-free protective layer: in vitro release, permeation and mechanical properties. J Controlled Release. 2007;118(3):318–324. doi:10.1016/j.jconrel.2006.12.030
19. Guo S-R, Wang Z-M, Zhang Y-Q, et al. In Vivo Evaluation of 5-Fluorouracil-Containing Self-Expandable Nitinol Stent in Rabbits: efficiency in Long-Term Local Drug Delivery. J Pharm Sci. 2010;99(7):3009–3018. doi:10.1002/jps.22066
20. Li G, Chen Y, Hu J, et al. A 5-fluorouracil-loaded polydioxanone weft-knitted stent for the treatment of colorectal cancer. Biomaterials. 2013;34(37):9451–9461. doi:10.1016/j.biomaterials.2013.08.055
21. Olukman M, Sanli O, Solak EK. Release of Anticancer Drug 5-Fluorouracil from Different Ionically Crosslinked Alginate Beads. J Biomater Nanobiotechnol. 2012;3(4):12. doi:10.4236/jbnb.2012.34048
22. Fouladian P, Kohlhagen J, Arafat M, et al. Three-dimensional printed 5-fluorouracil eluting polyurethane stents for the treatment of oesophageal cancers. Biomater Sci. 2020;8(23):6625–6636. doi:10.1039/D0BM01355B
23. Liu J, Wang Z, Wu K, et al. Paclitaxel or 5-fluorouracil/esophageal stent combinations as a novel approach for the treatment of esophageal cancer. Biomaterials. 2015;53(SupplementC):592–599. doi:10.1016/j.biomaterials.2015.03.009
24. Wang Z, Liu J, Wu K, et al. Nitinol stents loaded with a high dose of antitumor 5-fluorouracil or paclitaxel: esophageal tissue responses in a porcine model. Gastrointest Endosc. 2015;82(1):153–160.e151. doi:10.1016/j.gie.2015.02.034
25. Armstrong EJ, Waltenberger J, Rogers JH. Percutaneous coronary intervention in patients with diabetes: current concepts and future directions. J Diabetes Sci Technol. 2014;8(3):581–589. doi:10.1177/1932296813517058
26. Htay T, Liu MW. Drug-Eluting Stent: a Review and Update. Vasc Health Risk Manag. 2005;1(4):263–276. doi:10.2147/vhrm.2005.1.4.263
27. Kwon H, Park S. Local delivery of antiproliferative agents via stents. Polymers. 2014;6(3):755. doi:10.3390/polym6030755
28. Mani G, Feldman MD, Patel D, Agrawal CM. Coronary stents: a materials perspective. Biomaterials. 2007;28(9):1689–1710.
29. Rizas Konstantinos D, Mehilli J. Stent Polymers. Circ Cardiovasc Interv. 2016;9(6):e002943. doi:10.1161/CIRCINTERVENTIONS.115.002943
30. Hsu LSF, Marrs TC. Determination of 5-Fluorouracil in human plasma by high-pressure ion-exchange chromatography. Ann Clin Biochem. 1980;17(5):272–276. doi:10.1177/000456328001700510
31. Chappa RA, Hergenrother RW, Wadman SA, Wormuth KR Coating systems for the controlled delivery of hydrophilic bioactive agents. Google Patents; 2013.
32. Arafat M, Fouladian P, Wignall A, et al. Development and in vitro evaluation of 5-fluorouracil-eluting stents for the treatment of colorectal cancer and cancer-related obstruction. Pharmaceutics. 2021;13(1):17. doi:10.3390/pharmaceutics13010017
33. United States Pharmacopeial Convention. Fluorouracil. United States Pharmacopeial Convention; 2019.
34. Freire MCLC, Alexandrino F, Marcelino HR, et al. Understanding Drug Release Data through Thermodynamic Analysis. Materials. 2017;10(6):651. doi:10.3390/ma10060651
35. Zhang Y, Huo M, Zhou J, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12(3):263–271. doi:10.1208/s12248-010-9185-1
36. Zuo J, Gao Y, Bou-Chacra N, Löbenberg R. Evaluation of the DDSolver Software Applications. Biomed Res Int. 2014;2014:204925. doi:10.1155/2014/204925
37. Shaikh M, Roy Choudhury N, Knott R, Kanwar JR, Garg S. Effect of polymer microstructure on the docetaxel release and stability of polyurethane formulation. Eur J Pharm Biopharmaceutics. 2016;101:82–89. doi:10.1016/j.ejpb.2016.01.015
38. Yuksel N, Kanık AE, Baykara T. Comparison of in vitro dissolution profiles by ANOVA-based, model-dependent and -independent methods. Int J Pharm. 2000;209(1):57–67. doi:10.1016/S0378-5173(00)00554-8
39. Chung MJ, Kim H, Kim KS, Park S, Chung JB, Park SW. Safety evaluation of self-expanding metallic biliary stents eluting gemcitabine in a porcine model. J Gastroenterol Hepatol. 2012;27(2):261–267. doi:10.1111/j.1440-1746.2011.06866.x
40. Jang SI, Kim JH, Kim M, et al. Porcine feasibility and safety study of a new paclitaxel-eluting biliary stent with a Pluronic-containing membrane. Endoscopy. 2012;44(09):825–831. doi:10.1055/s-0032-1309881
41. Lee SS, Shin JH, Han JM, et al. Histologic influence of paclitaxel-eluting covered metallic stents in a canine biliary model. Gastrointest Endosc. 2009;69(6):1140–1147. doi:10.1016/j.gie.2008.08.005
42. Seo EH, Na K. Polyurethane membrane with porous surface for controlled drug release in drug eluting stent. Biomater Res. 2014;18(1):15.
43. Lei L, Liu X, Guo S, Tang M, Cheng L, Tian L. 5-Fluorouracil-loaded multilayered films for drug controlled releasing stent application: drug release, microstructure, and ex vivo permeation behaviors. J Controlled Release. 2010;146(1):45–53. doi:10.1016/j.jconrel.2010.05.017
44. Thakkar A, Raval A, Mandal R, et al. Development and evaluation of drug eluting stent having biphasic release from a single layer of biodegradable polymer. J Med Device. 2013;7(1). doi:10.1115/1.4023414
45. Whitbourne RJ, Chamberlain AM, Hullihen DG, Rosebrough SF, Calistri-Yeh M Medicated stent having multi-layer polymer coating. Google Patents; 2012.
46. Amasya G, Gumustas M, Badilli U, Ozkan SA, Tarimci N. Development of a HILIC method for the determination of 5-fluorouracil from nano drug delivery systems and rat skin extracts. J Pharm Biomed Anal. 2018;154:285–293. doi:10.1016/j.jpba.2018.03.021
47. Mattos A, Khalil NM, Mainardes RM. Development and validation of an HPLC method for the determination of fluorouracil in polymeric nanoparticles. Br J Pharm Sci. 2013;49:117–126. doi:10.1590/S1984-82502013000100013
48. United States Pharmacopeial Convention. (1225) Validation of Compendial Procedures. United States Pharmacopeial Convention; 2019.
49. Zafar H, Mau M, Arshad S, Altaf H, Ma M, Rehman M. Simultaneous Quantification of 5-Fluorouracil and Leucovorin in Pharmaceutical Dosage Form and Human Spiked Plasma by Using RP- HPLC Method. J Chromatography Separation Tech. 2014;05.
50. Sanganal S, Kulkarni GB, Karegoudar TB. Development and validation of high performance liquid chromatographic analysis of residual N,N-Dimethylformamide in Spent Medium after Biodegradation by Paracoccus denitrificans SD1. ISRN Chromatography. 2013;2013:401629. doi:10.1155/2013/401629
51. Chan CM, Frimberger AE, Moore AS. A literature review of reports of the stability and storage of common injectable chemotherapy agents used in veterinary patients. Vet Comp Oncol. 2017;15(4):1124–1135. doi:10.1111/jedm.12046
52. Galanti L, Lebitasy MP, Hecq JD, Cadrobbi J, Vanbeckbergen D, Jamart J. Long-term stability of 5-Fluorouracil in 0.9% sodium chloride after freezing, microwave thawing, and refrigeration. Can J Hosp Pharm. 2009;62(1):34–38. doi:10.4212/cjhp.v62i1.115
53. Milano G, Etienne M-C, Cassuto-Viguier E, et al. Long-term stability of 5-fluorouracil and folinic acid admixtures. Eur J Cancer. 1993;29(1):129–132. doi:10.1016/0959-8049(93)90590-C
54. Williams DA, Lokich J. A review of the stability and compatibility of antineoplastic drugs for multiple-drug infusions. Cancer Chemother Pharmacol. 1992;31(3):171–181. doi:10.1007/BF00685544
55. Ibrahim RA, Suhail FSA, Al-Hakeim HK. Stability of Anticancer Drug 5-Fluorouracil in Aqueous Solution: an Assessment of Kinetic Behavior. Nano Biomed Eng. 2018;10(3):224–234.
56. Charalambides D, Segal I. Colonic pH: a comparison between patients with colostomies due to trauma and colorectal cancer. Am J Gastroenterol. 1992;87(1):74–78.
57. Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29(8):1035–1041.
58. McDougall CJ, Wong R, Scudera P, Lesser M, DeCosse JJ. Colonic mucosal pH in humans. Dig Dis Sci. 1993;38(3):542–545. doi:10.1007/BF01316512
59. Pye G, Evans DF, Ledingham S, Hardcastle JD. Gastrointestinal intraluminal pH in normal subjects and those with colorectal adenoma or carcinoma. Gut. 1990;31(12):1355–1357. doi:10.1136/gut.31.12.1355
60. Chinembiri TN, Gerber M, Du Plessis L, Du Preez J, Du Plessis J. Topical Delivery of 5-Fluorouracil from Pheroid™ formulations and the in vitro efficacy against human melanoma. AAPS PharmSciTech. 2015;16(6):1390–1399. doi:10.1208/s12249-015-0328-7
61. Vermaas M. Formulation of 5-Fluorouracil for Transdermal Delivery. Potchefstroom, North-West: School of Pharmacy, North-West University; 2010.
62. Shin MS, Hong JY, Park S. Gemcitabine release behavior of polyurethane matrixes designed for local anti-cancer drug delivery via stent. J Drug Deliv Sci Technol. 2012;22(4):301–306. doi:10.1016/S1773-2247(12)50050-X
63. Bege N, Steinmüller SO, Kalinowski M, et al. Drug eluting stents based on Poly(ethylene carbonate): optimization of the stent coating process. Eur J Pharm Biopharmaceutics. 2012;80(3):562–570. doi:10.1016/j.ejpb.2011.12.006
64. Mc Conville C, Major I, Friend DR, Clark MR, Woolfson AD, Malcolm RK. Development of polylactide and polyethylene vinyl acetate blends for the manufacture of vaginal rings. J Biomed Mater Res B Appl Biomater. 2012;100B(4):891–895. doi:10.1002/jbm.b.31919
65. Wolinsky JB, Colson YL, Grinstaff MW. Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Control Release. 2012;159(1):14–26. doi:10.1016/j.jconrel.2011.11.031
66. Jang SI, Lee DK. Stents with specialized functions: drug-eluting stents and stents with antireflux devices. Gastrointestinal Intervent. 2015;4(1):50–54. doi:10.1016/j.gii.2015.03.004
67. Kim DH, Jeong Y-I, Chung C-W, et al. Preclinical evaluation of sorafenib-eluting stent for suppression of human cholangiocarcinoma cells. Int J Nanomedicine. 2013;8:1697–1711. doi:10.2147/IJN.S43508
68. Zhao L, Gao Y, Gu G, et al. Rational design of drug-eluting stents via electrospray and in vivo evaluation of preventing oesophageal stricture. RSC Adv. 2014;4(32):16885–16892. doi:10.1039/C4RA01300J
69. KobryD J, Sowa S, Gasztych M, Musia BW. Influence of hydrophilic polymers on the factor in weibull equation applied to the release kinetics of a biologically active complex of aesculus hippocastanum. Int J Polym Sci. 2017;2017:1–8. doi:10.1155/2017/3486384
70. Smallwood IM. Methylene chloride. In: Smallwood IM, editor. Handbook of Organic Solvent Properties. Oxford: Butterworth-Heinemann; 1996:137–139.
71. United States Pharmacopeial Convention. Residual Solvents; 2019. Available from: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisions/gc-467-residual-solvents-ira-20190927.pdf.
72. Harrell CR, Djonov V, Fellabaum C, Volarevic V. Risks of using sterilization by gamma radiation: the other side of the coin. Int J Med Sci. 2018;15(3):274–279. doi:10.7150/ijms.22644
73. ICH Harmonised Tripartite Guideline. Stability testing of new drug substances and products Q1A(R2). Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-products-step-5_en.pdf.
74. Kamberi M, Rapoza R. Stability testing of drug eluting stents. J Drug Deliv Sci Technol. 2016;35:58–68. doi:10.1016/j.jddst.2016.05.006
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.