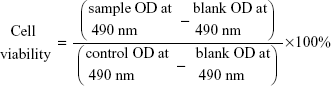Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
pH-Sensitive carboxymethyl chitosan-modified cationic liposomes for sorafenib and siRNA co-delivery
Authors Yao Y, Su Z, Liang Y, Zhang N
Received 15 June 2015
Accepted for publication 14 August 2015
Published 1 October 2015 Volume 2015:10(1) Pages 6185—6198
DOI https://doi.org/10.2147/IJN.S90524
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Lei Yang
Yao Yao, Zhihui Su, Yanchao Liang, Na Zhang
School of Pharmaceutical Sciences, Shandong University, Jinan, Shandong, People’s Republic of China
Abstract: Combination of chemotherapeutic drug and small interfering RNA (siRNA) can affect multiple disease pathways and has been proven effective in suppressing tumor progression. Co-delivery of drug and siRNA within a same nanocarrier is a vital means in this field. The present study aimed at the development of a pH-sensitive liposome to co-deliver drug and siRNA to tumor region. Driven by the electrostatic interaction, the pH-sensitive material, carboxymethyl chitosan (CMCS), was coated onto the surface of the cationic liposome (CL) preloaded with sorafenib (Sf) and siRNA (Si). To evaluate whether the resulting CMCS-modified Sf and siRNA co-delivery cationic liposome (CMCS-SiSf-CL) enhanced antitumor efficiency after systematic administration, in vitro and in vivo experiments were evaluated in HepG2 cells and the H22 cells-bearing Kunming mice model. The experimental results demonstrated that CMCS-SiSf-CL was able to condense siRNA efficiently and protect siRNA from being degraded by serum and RNase. The release rate of Sf from CMCS-modified liposome exhibited pH-sensitive release behavior. Furthermore, in vitro cellular uptake results showed that CMCS-SiSf-CL yielded higher fluorescence intensity at pH 6.5 than at pH 7.4, and that siRNA could be delivered to tumor site by CMCS-SiSf-CL in vivo. The in vivo antitumor efficacy showed that CMCS-Sf-CL inhibits tumor growth effectively when compared with free Sf solution. In current experimental conditions, this liposomal formulation did not show significant toxicity both in vitro and in vivo. Therefore, co-delivering Sf with siRNA by CMCS-SiSf-CL might provide a promising approach for tumor therapy.
Keywords: co-delivery, sorafenib, gene, charge conversion, cancer therapy
Introduction
The occurrence and development of cancer is characterized by multiple gene mutations and is further complicated by multistep communication paths between cancer cells that constitute tumors. Complex mechanisms and signaling pathways to evade programmed cell death, as well as to proliferate with impunity, make cancer treatment present great challenges when designing treatment modalities.1 Therefore, combination therapy with two or more therapeutic strategies has emerged on the basis of affecting different tumorigenesis mechanisms to enhance the therapeutic efficacy and reduce undesired toxicities. Recently, combination of antitumor drug with small interfering RNA (siRNA) has been employed as a promising therapeutic modality.1,2 siRNA is able to inhibit the progression of cell cycle, proliferation, angiogenesis, or other cellular pathways3 through the formation of RNA-induced silencing complex (RISC) in cytoplasm and inducing sequence-specific gene silencing. Correspondingly, siRNA shows great potential in treating specific tumor types4 and several studies have demonstrated the efficiency with drug/siRNA combination therapy on downregulating cancer-related genes to enhance chemotherapeutic effect at the tumor site.5–7
In recent studies, the most prevalent method to achieve the synergistic effort of drug and siRNA is incorporating anticancer drug and siRNA into one nanocarrier that allows effective co-delivery to tumor site. For example, PEGylated liposomes were designed to co-encapsulate anti-bcr-abl siRNA and imatinib mesylate for chronic myeloid leukemia treatment.8 Dual-stimuli-sensitive micelles were used to co-deliver doxorubicin and bcl-2 siRNA.9 Quantum dots functionalized by β-cyclodextrin were designed for co-delivery of doxorubicin and mdr1 siRNA.10 Among these delivery carriers, liposomes represent one of the most attractive candidates and have been approved in clinical practice.11 The biocompatible lipid bilayer with aqueous inner capacity allows carrying various drugs with different physicochemical properties.11 Especially, cationic liposomes (CL) not only protect siRNA against degradation by nuclease accumulation in the tumor via the enhanced permeability and retention (EPR) effects, but also facilitate cellular uptake through electrostatic interactions with the membrane of the target cell in vitro.12
On the other hand, although a number of advances have been made in the field of combinational therapy, recent studies on drug/siRNA co-delivery are frequently focusing on the development of multifunctional synergy strategies that base upon regulating multiple pathways rather than altering single target. Sorafenib (Sf) is a multi-target inhibitor that blocks Raf kinases, the VEGF/PDGF receptors, and MEK tyrosine kinases,13 inhibiting both angiogenesis and cell proliferation. Besides nanotechnology strategies focusing on improving its solubility and antitumor efficacy,9,14,15 many investigations have proved the synergistic effect of Sf with other therapeutic agents.16–18 Thus, it was envisaged that the co-delivery of Sf and siRNA would be feasible when mediated by liposomes.
Moreover, to ensure maximal deposition of siRNA and antitumor drug in the target tissue, nanocarriers should be rationally designed to enhance passive target efficiency. Recent studies are focusing on the exploitation of the “smart liposome” that goes through structural changes when exposed to in vivo microenvironmental stimuli such as pH, enzyme, temperature, and magnetic field. The advantage of the smart strategy is to achieve the selective homing and control release of the payload at the target site.12 Most advanced of these, which is also a hot topic, is the pH-sensitive liposome that is stable in physiological conditions (pH 7.4) but disassembles in the acidic microenvironment (pH<6.8). To obtain the precise pH-sensitive release, the sensory component of liposome should be tuned and selected. Notably, pH-sensitive polyanionic polymers have been conferred to liposomes to respond to the mild pH differences between normal and cancerous tissues, triggering the release of the encapsulated drug. Wang et al anchored the pH-sensitive octylamine grafted poly aspartic acid on liposomes for efficient cytarabine delivery. The pH-sensitive liposomes killed 70% of the tumor cells but did not cause injury to the normal cells.19 Hardiansyah et al immobilized carboxymethyl-hexanoyl chitosan onto 1,2-distearoylglycero-3 phosphatidylethanolamine (DSPE) liposomes. The release rate of doxorubicin from pH-sensitive liposomes was faster at pH 4 than at pH 7.4.20
Carboxymethyl chitosan (CMCS) is an amphoteric polysaccharide that possesses pH-sensitive property, since it bears both acidic (–COOH) and basic groups (–NH2). It is negatively charged in the physiological pH and positively charged in the acidic environment of the tumor. In addition, CMCS is a biocompatible and biodegradable material that is suitable for various applications such as sustained or controlled release drug delivery, pH-responsive drug delivery, gene delivery as permeation enhancer, etc.21 Being a coating material on nanocarriers, its excellent pH-sensitivity and nontoxicity have been proved in our previous studies.22–24
In this work, cationic liposome co-delivering Sf and siRNA (SiSf-CL) was developed. Then pH-sensitive CMCS was coated onto the positively charged complex at physiological pH via electrostatic interaction to construct a CMCS-modified pH-sensitive Sf/siRNA co-delivery cationic liposome (CMCS-SiSf-CL). It was hypothesized that CMCS-SiSf-CL was able to simultaneously load Sf and siRNA, protect siRNA from degradation during systemic circulation, and finally accumulate in the tumor region via the EPR effects. Then CMCS shell was expected to peel off from CMCS-SiSf-CL under the mild environment in the tumor and the exposed SiSf-CL could contact with and enter into negatively charged cell membrane, resulting in enhanced therapeutic effects compared with control, which was treated with free Sf solution.
Thus, the physicochemical properties and characteristics of CMCS-SiSf-CL, including morphology, particle size, zeta potential, siRNA retarding ability, and pH-sensitive release, were determined. In vitro toxicity and cellular uptake were evaluated in HepG2 cells. Tumor accumulation and antitumor efficiency was examined in H22 cells-bearing Kunming mice to assess their potential applications in the development of co-delivery therapeutics.
Materials and methods
Materials
1,2-dioleoyl-3-trimethylammonium-propane (chloride salt) (DOTAP) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Cholesterol was purchased from Sigma-Aldrich Co., (St Louis, MO, USA). CMCS (average MW =50,000 Da; degree of carboxymethyl substitution =60%; degree of deacetylation =85%) was obtained from Jinan Haidebei Biological Engineering Co. (Jinan, People’s Republic of China). Sf was purchased from Biochempartner (Shanghai, People’s Republic of China). Lipofectamine-2000 was purchased from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA); Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were purchased from Hangzhou Sijiqing Biological Engineering Materials Co (Hangzhou, People’s Republic of China). Cy3 or Cy5-labeled nonspecific siRNA (Cy3-siRNA or Cy5-siRNA) obtained from Ribobio Co., Ltd (Guangzhou, People’s Republic of China) was used as a negative control. The Cy3-siRNA was synthesized on the solid support using Cy3-phosphoramidite (sense: 5′-Cy3-UUCUCCGAACGUGUCACGUdTdT-3′, antisense: 5′-AAGAGGCUUGCACAGUGCAdTdT-3′). The Cy5-siRNA was also prepared in the same sequence with Cy5 labeling. MTT was purchased from Sigma-Aldrich Co. All other reagents were of commercial special grade and were used without further purification. Human hepatocellular liver carcinoma cell lines HepG2 and murine hepatic cancer cell line H22 were kindly provided by Institute of Immunopharmacology and Immunotherapy of Shandong University (Jinan, People’s Republic of China). HepG2 and H22 were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% FBS and incubated at 37°C and 5% CO2. Female Kunming mice weighing 18–22 g were supplied by the Medical Animal Test Center of the New Drugs Evaluation Center, Shandong University.
Preparation and characterization of CMCS-SiSf-CL
CL composed of DOTAP, DOPE, cholesterol (2.6:6.7:3.2, mol/mol) were prepared by the thin-film hydration method as previously reported25,26 with some modifications. Briefly, all lipids and Sf were dissolved with absolute ethanol and were evaporated in a pear-shaped flask to form a thin lipid dried film using a rotary evaporator under vacuum at 40°C. The lipid film was subsequently hydrated with RNase-free diethyl pyrocarbonate water and incubated at 40°C for 30 minutes. Then, the obtained dispersion was extruded through 400, 200, and 100 nm polycarbonate membranes for five cycles, respectively, using a LiposoFast™ basic extruder (Avestin, Ottawa, ON, Canada). To incorporate siRNA, Sf-CL were simply mixed with siRNA solution (20 μM) in RNase-free diethyl pyrocarbonate water and incubated at room temperature for 30 minutes. For the CMCS-SiSf-CL preparation, the resulting cationic lipoplex SiSf-CL were added dropwise to CMCS solution of different concentrations and then kept at room temperature for 30 minutes. Blank CL and CMCS-CL were prepared under the conditions similar to those mentioned earlier. The particle size and zeta potential were measured by dynamic light scattering using the Malvern Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). The volume of samples needed for detection was 1 mL. Data were collected and processed with the Zetasizer Nano software. The particle size was expressed with intensity-based distribution. Raw data were collected at 25°C and an angle of 90°C. The morphology was observed using transmission electron microscope (TEM; JEM-120 0EX, JEOL, Tokyo, Japan).
Encapsulation and loading efficiency
To determine the amount of Sf loaded in CL, 100 μL of samples were added to 1.9 mL of phosphate-buffered saline (PBS) (pH 7.4) containing 0.4% (w/v) Tween 80 and shaken with gently vortex to dissolve the free drugs and then centrifuged at 16,000 rpm for 30 minutes. The Sf content in the supernatant after centrifugation was measured by high-pressure liquid chromatography (HPLC) (SPD-10Avp UV–vis detector, Shimadzu, Kyoto, Japan) with InertSustain® C18 chromatographic column (4.6×250 mm2, Shimadzu), where the measured wavelength was 265 nm. The mobile phase was a mixture of acetonitrile:water (63:37, v/v) containing 0.03% triethylamine at the flow rate of 1 mL/min.27 Sf encapsulation efficiency (EE) and drug loading (DL) efficiency was calculated by the following formula:
EE = (Wt–W)/Wt ×100%, | (1) |
where W is the mass of Sf in the supernatant and Wt is the total amount of Sf added in dispersion before centrifugation.
DL = Wd/W0 ×100%, | (2) |
where Wd is the weight of encapsulated Sf and W0 is the total weight of Sf and lipids.
Agarose gel retardation assay
To evaluate siRNA binding ability of SiSf-CL, different weight ratios of CL to siRNA were mixed with appropriate volume of 6× DNA loading buffer and then separated on 2% agarose gel in 1% Tris-acetate-EDTA (TAE) buffer at 95 V for 12 minutes. The siRNA bands were visualized by a Bio-Rad transilluminator. Similarly, to examine the effect of CMCS coating on the inner SiSf-CL, CMCS-SiSf-CL based on different weight ratios of CMCS to siRNA was also tested on agarose gel.
siRNA protection
To evaluate the serum stability or siRNA protection against RNase, free siRNA or equivalent CMCS-SiSf-CL were mixed with equal volume of FBS (50% serum concentration) or 1 μL RNase A (1 mg/mL) at 37°C. The samples collected at different time points were immediately frozen at −80°C. Prior to running electrophoresis, 10% heparin was added for a further 1 hour at room temperature to withdraw siRNA from CMCS-SiSf-CL and analyzed by 2% gel retardation assay as described earlier. Untreated free siRNA was used as a control.
Release of Sf from liposomes
In vitro release of Sf from CMCS-Sf-CL and CMCS-SiSf-CL was monitored by a dialysis method. In brief, 2 mL CMSS-Sf-CL or CMCS-SiSf-CL dispersions (Sf concentration was 17 μg/mL) were added into dialysis bags (molecular weight cut off 8,000–14,000 Da) and sealed. Then the dialysis bags were placed into 13 mL PBS (pH 7.4 or 6.5) containing 1% (w/v) Tween 80 as release medium and maintained in a thermostat oscillator at 37°C and a speed of 100 rpm for 72 hours. At time points, 2 mL release media was withdrawn and replaced with equal volume of fresh release medium. Then the concentration of Sf in each release sample was analyzed by HPLC as described earlier.
In vitro cytotoxicity
Cytotoxicity assays of HepG2 cells were evaluated by MTT assay. HepG2 cells were loaded in 96-well plates at a density of 4,500 cells per well in 150 μL of RPMI 1640 containing 10% FBS and incubated overnight at 37°C. After reaching 60%–80% confluence, the cells were treated with 50 μL of free Sf solution or Sf-loaded liposomes at different concentrations and further incubated for 24 hours. Then 20 μL of MTT solution (5 mg/mL) was added to each well and incubated for 4 hours, followed by replacing the supernatants with 100 μL dimethyl sulfoxide (DMSO). Finally, the plates were placed on a microplate reader (TECAN, Salzburg, Austria) and the absorbance was read at 490 nm wavelength. The cell viability was calculated according to the following formula:
|
|
Cellular uptake study
To analyze the in vitro cellular uptake, HepG2 cells were seeded into the 12-well plates at a density of 1.5×105 per well and incubated overnight at 37°C. Then the culture medium was replaced by fresh RPMI 1640 containing free Cy3-labled siRNA or different liposomal formulation. The final concentration of Cy3-labled siRNA was 20 nM. After incubating for 4 hours at 37°C, the cells were washed with cold PBS and imaged by fluorescence microscope (BX40; Olympus Corporation, Tokyo, Japan). To quantify the cellular uptake, the cells were trypsinized, centrifuged, and resuspended in 200 μL of PBS, followed by detecting the fluorescence intensity using flow cytometry (BD Biosciences, San Jose, CA, USA).
Tumor distribution study of CMCS-SiSf-CL
Female Kunming mice (18–22 g) bearing H22 tumor model was established by inoculating subcutaneously 1×106 H22 cells into the right axillary space. When the tumors grew to approximately 70 mm3, the mice were given intravenous (iv) injections of CMCS-SiSf-CL at a dose of 1.0 mg/kg Cy5-siRNA (20 nM) or free Cy5-siRNA. After 6 hours of injection, the in vivo images were observed with the in vivo real-time fluorescence imaging system (IVIS) Spectrum (Cailper PerkinElemer, Waltham, MA, USA) at appropriate wavelength (Cy5: excitation wavelength of 640 nm, emission wavelength of 680 nm). Then mice were sacrificed and tumors were excised from the mice and embedded in optimum cutting temperature medium. Then the tumors were snap frozen at -20°C and were cut into 5 μm sections using a freezing microtome (Leica CM 1,950; Leica, Wetzlar, Germany). Cell nuclei were stained with Hoechst 33342 for 15 minutes at room temperature. Fluorescence images were acquired with fluorescence microscope (BX40; Olympus).
In vivo tumor growth inhibition study
Kunming mice bearing H22 tumor model was established as described in the section “Tumor distribution study of CMCS-SiSf-CL”. The mice were randomly divided into six groups (five animals per group), and were treated with different formulations of 1) saline, 2) Sf solution (4.5 mg/kg), 3) CL, 4) Sf-CL (4.5 mg/kg), 5) CMCS-Sf-CL (4.5 mg/kg), and 6) CMCS-CL by iv injection once every 3 days (total for six doses) for 19 days. Tumor volume was measured with a vernier caliper every other day by using the formula (length×width2)/2, where length is the longest dimension and width is the widest dimension. In addition, body weight of each mouse was simultaneously recorded. On the 19th day, the animals were sacrificed to isolate tumor tissue and organ tissue. All experiments were carried out in compliance with the Animal Management Rules of the Ministry of Health of the People’s Republic of China (document 55, 2001). The study was reviewed and approved by the Experimental Animal Ethical Committee of Shandong University.
Immunohistochemical analysis and HE staining
As mentioned in the section “Tumor distribution study of CMCS-SiSf-CL”, the excised organ tissue including heart, liver, spleen, lung, and kidney were fixed, embedded in paraffin, and cut into 5 μm thick sections for hematoxylin and eosin (HE) staining. Images were collected using an Olympus light microscope.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). Statistical comparisons used Student’s t-test and analysis of variance. P<0.05 was considered statistically significant, and P<0.01 was considered as highly significant.
Results and discussion
Characterization of CMCS-SiSf-CL
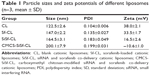
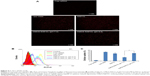
The Sf-loaded liposome was prepared by thin-film hydration technique, the particle size of which was controlled by repeated extrusion. Then siRNA and CMCS were adsorbed on the surface of the Sf-CL through electrostatic interactions layer by layer. The mean sizes, polydispersity index (PDI), and zeta potentials of CL, Sf-CL, SiSf-CL, and CMCS-SiSf-CL are exhibited in Table 1. Transmission electron microscope of the CL showed its uniform spherical appearance, demonstrating the successful formation of the liposome (Figure 1A). Mean particle size was 122.5±2.6 nm with a narrow PDI of 0.1, and the positive charge (38.0±2.1 mV) was due to the presence of DOTAP as cationic lipid on the surface of liposomes. In the case of hydrophobic Sf being encapsulated into the lipid bilayer, the Sf-CL had a slightly increased particle size (147.0±2.2 nm) with no significant change in surface charge (33.5±1.7 mV). The EE of Sf in Sf-CL was 90.36%±0.63%, which indicated that Sf was almost completely entrapped within the CL, and the Sf drug loading efficiency was 5.19%±0.035%, which was suitable for Sf delivery.
Agarose gel retardation assay was used to evaluate the siRNA binding affinity of Sf-CL and determine the appropriate ratio of Sf-CL to siRNA that favors binding. As shown in Figure 2A, as the mass ratio increased, more siRNA was condensed by the CL. When the ratio reached 10:1 or higher, the siRNA was completely trapped in the gel wells with Sf-CL. The results of dynamic light scattering technique (Figure 2C) revealed that the particle size of SiSf-CL complex varied in a range from 140 to 250 nm at different mass ratios, likely due to the compressible lipid shell. The zeta potential of different SiSf-CL complexes varied along with changing the mass ratio from 10:1 to 100:1, increasing from +16 mV to +35 mV. To ensure effective adhesion to the negative cell membrane with a low cationic cytotoxicity, SiSf-CL complex of mass ratio 20:1 with a positive surface charge 16.5±2.55 mV was adopted for further experiments. It was observed that relatively low weight ratio provided the possibility to load more therapeutics with reduced toxicity.28
To evaluate whether the anionic CMCS would affect siRNA adsorption, different amounts of CMCS were added to form CMCS-SiSf-CL complexes. As shown in Figure 2B, no free siRNA was observed on gel, demonstrating that the CMCS did not disassemble the SiSf-CL. The size of CMCS-SiSf-CL ternary complexes were between 170 and 320 nm at the CMCS/siRNA weight ratio from 1.9:1 to 5.7:1 (Figure 2D). When the weight ratio was 3.8:1, the particle size was 200 nm; this condition mediates the EPR effect that promotes nanocomplexes to accumulate in tumors.29 And the corresponding zeta potential was −10 mV, which was suitable for avoiding aggregation in circulation and extending the halftime. At high ratios, particle size increased measurably; thus, the CMCS/siRNA weight ratio of 3.8:1 was chosen as the optimized ratio to prepare CMCS-SiSf-CL. The electron microscopic images revealed a coreshell structure of CMCS-SiSf-CL, confirming the uniform CMCS coating on the liposomal inner core (Figure 1D). Notably, the liposome size measured by dynamic light scattering technique was larger than that obtained from TEM. This was because the size measured by dynamic light scattering technique was the hydrodynamic size, while the results of TEM reflected the dried state of liposomes.30
Sf release behaviors were studied in release medium at 37°C and pH 7.4 and 6.5 (Figure 3). The latter was to mimic acidic microenvironment in the tumor. Through the release period, Sf was sustainably released from CMCS-SiSf-CL and CMCS-Sf-CL at a release rate of 26% and 31%, respectively, in pH 7.4 PBS. The release rate of Sf was over 30% and 40% from CMCS-SfSi-CL and CMCS-Sf-CL, respectively, in pH 6.5 PBS, faster than that in pH 7.4, which indicated that the release rate of Sf was enhanced under the acidic environment. A relatively slower Sf release profile was observed in CMCS-SiSf-CL due to the presence of siRNA on the outer surface, which might hinder the drug release.31
Protection of the siRNA encapsulated in CMCS-SiSf-CL
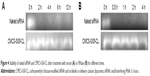
siRNA is inclined to degradation by nuclease in vivo after systematic administration. The carrier designed should be qualified for protecting the entrapped siRNA from such degradation. Therefore, stability study of siRNA was carried out at higher serum or RNase concentration, and integrity of siRNA was analyzed by gel electrophoresis. Figure 4 shows gel electrophoresis analysis for naked siRNA and siRNA-encapsulated CMCS-SiSf-CL. It demonstrated that naked siRNA was seriously degraded after 4 hours of incubation in the presence of serum or RNase. In contrast, siRNA was still intact in the CMCS-SiSf-CL group after treating with RNase for 12 hours or incubating with serum for 4 hours.
Cellular uptake study
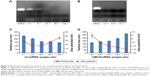
To evaluate the influence of CMCS charge reversal on cellular uptake of CMCS-SiSf-CL, the Cy3-siRNA uptake intensity in HepG2 cells was observed by fluorescence microscope after 4 hours of treatment both in pH 7.4 and 6.5 culture medium, with Lipofectamine-2000/siRNA complex as the positive control and free siRNA as the negative control. Flow cytometry was used for the quantitative analysis. The microscopy images (Figure 5A) showed that Lipofectamine-2000/siRNA complex, SiSf-CL, and CMCS-SiSf-CL could be internalized into HepG2 cells efficiently. According to flow cytometric analysis (Figure 5B and C), the concentration of Cy3-positive HepG2 cells was approximately twofold greater (P<0.05) when the cells were incubated with CMCS-SiSf-CL in pH 6.5 culture medium in comparison to that in pH 7.4 culture medium. These results were mainly due to the charge reversal of CMCS in the acidic environment of the tumor. At pH 6.5, CMCS shell became positively charged and fell off from the surface of cationic SiSf-CL, followed by the exposure of SiSf-CL to cells and the cellular uptake.22 It indicated that pH-sensitive CMCS-SiSf-CL provided an ideal platform for siRNA to enter into tumor cells in the acidic environment of the tumor.
In vitro cytotoxicity
MTT assay was used to investigate the cytotoxicity of free Sf solution, Sf CL, CMCS-Sf-CL, CMCS-SiSf-CL, CL, and CMCS-CL on HepG2 cells. As shown in Figure 6, CMCS-CL showed >80% cell viability at the concentration (equal to Sf) from 1.5 to 25 μM, and approximately 73% cell viability at 37.5 μM in HepG2 cells for 24 hours. CL showed 10%–20% reduction compared with CMCS-CL, which was attributed to the cationic nature of the DOTAP liposome.32 In the same cells, all the formulations – free Sf solution, Sf-CL, CMCS-Sf-CL, CMCS-SiSf-CL – exhibited a dose-dependent cytotoxicity. Free Sf solution, CMCS-Sf-CL, and CMCS-SiSf-CL showed nearly similar cytotoxicity in all the concentrations. The half maximal inhibitory concentration (IC50) value of free Sf solution, Sf-CL, CMCS-Sf-CL, and CMCS-SiSf-CL was calculated to be 12.16, 7.80, 11.82, and 11.44 μM, respectively (Table 2). These results suggested that CMCS-CL was a biocompatible carrier and did not weaken the inhibitory effect of the encapsulated Sf in vitro.
Tumor accumulation study
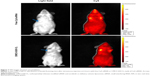
To characterize the accumulation of CMCS-SiSf-CL in tumor tissue, CMCS-SiSf-CL (CMCS/siRNA =3.8:1, weight ratio) was administered to Kunming mice bearing H22 cells via the tail vein and the fuorescence signal was acquired by the IVIS Spectrum imaging system at 6 hours, and free Cy5-siRNA was also injected as control. As shown in Figure 7, free Cy5-siRNA displayed weak fluorescence intensity at tumor site after 6 hours injection, which indicated the instability and rapid clearance of naked siRNA in vivo. In contrast, CMCS-SiSf-CL containing the same amount of Cy5-siRNA showed clear fluorescence solely at the tumor site, demonstrating the in vivo stability and successful delivery to tumor region of siRNA via CMCS-SiSf-CL.
To further prove the cellular delivery ability at tumor site, tumor sections were excised and observed by fluorescence microscope. Figure 8 shows that the fluorescence intensity is higher in the CMCS-SiSf-CL treated tumor section than free Cy5-siRNA. The data suggested that the CMCS-SiSf-CL efficiently delivered siRNA to the tumor tissues, which was consistent with the siRNA stability and cellular uptake in vitro. Both the live image and tumor section image illustrated that CMCS-SiSf-CL could passively target the tumor through the EPR effect and deliver siRNA to tumor cells via the exposed positively charged liposomal complex.
In vivo antitumor efficacy
To evaluate the in vivo tumor growth inhibition, H22 tumor model was generated in female Kunming mice. Various formulations, including free Sf solution, CL, CMCS-CL, Sf-CL, and CMCS-Sf-CL were injected intravenously via the tail vein once every 3 days. As shown in Figure 9A and C, CL and CMCS-CL did not inhibit the tumor growth during the administration period and achieved maximum tumor volume. The group injected with free Sf solution showed smaller tumors at the end of treatment. Tumors of CMCS-Sf-CL group were even significantly smaller (P<0.01) than those from free Sf solution and saline treated tumors, whereas Sf-CL group did not exhibit statistical differences compared with the group treated with free Sf solution. The tumor weight was lighter in the Sf-CL treated group (P<0.05) and CMCS-Sf-CL (P<0.01) treated group than of the control. These data suggested that Sf encapsulated in CMCS-Sf-CL could achieve enhanced tumor inhibition and provide improved therapeutic effect mainly owing to the CMCS shell.
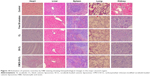
Considering that CL may cause immune responses and systemic toxicity after iv injection, the body weight changes in mice were recorded once every 2 days, intending to evaluate in vivo systemic toxicity of Sf and the delivery carrier. As shown in Figure 9B, free Sf solution induced a slight body weight reduction, while other formulations did not exhibit significant loss of weight throughout the period of experiments in animals. HE staining images (Figure 10) indicated that not all the formulations caused significantly toxic pathological changes in the heart, liver, spleen, lung, and kidney, revealing that CMCS-CL had low toxicity and was a promising candidate delivery carrier in present experimental conditions.
Conclusion
In this study, pH-sensitive CL were developed for co-delivery of Sf and siRNA to the tumor tissue. The results showed that CMCS-SiSf-CL could protect siRNA against serum and RNase. CMCS-SiSf-CL exhibited increased Sf release and significantly enhanced cellular uptake at pH 6.5 compared to that at pH 7.4, which demonstrated the pH-sensitivity of CMCS shell. In addition, CMCS-SiSf-CL showed more siRNA tumor accumulation compared to naked siRNA owing to protection of siRNA by CMCS-SiSf-CL and passively target via EPR effect. Besides, CMCS-CL showed lower toxicity than CL, and CMCS-Sf-CL had significant tumor growth inhibition based on the enhancement of Sf accumulation in tumor cells via EPR effect. In future, the Sf and siRNA co-delivery liposomes could be further modified with target ligands in order to construct pH-sensitive and active targeting carrier.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81573368 and 81503008), The Science and Technology Development Project of Shandong Province (2014GGE27121), and the Independent Innovation Foundation of Shandong University, IIFSDU (2015GN019).
Disclosure
The authors report no conflicts of interest in this work.
References
Li J, Wang Y, Zhu Y, et al. Recent advances in delivery of drug-nucleic acid combinations for cancer treatment. J Control Release. 2013;172(2):589–600. | ||
Tsouris V, Joo MK, Kim SH, et al. Nano carriers that enable co-delivery of chemotherapy and RNAi agents for treatment of drug-resistant cancers. Biotechnol Adv. 2014;32(5):1037–1050. | ||
Resnier P, Montier T, Mathieu V, et al. A review of the current status of siRNA nanomedicines in the treatment of cancer. Biomaterials. 2013;34(27):6429–6443. | ||
Oh YK, Park TG. siRNA delivery systems for cancer treatment. Adv Drug Delivery Rev. 2009;61(10):850–862. | ||
Costa PM, Cardoso AL, Custodia C, et al. MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: a new multimodal gene therapy approach for glioblastoma. J Control Release. 2015;207:31–39. | ||
Kato T, Natsume A, Toda H, et al. Efficient delivery of liposome-mediated MGMT-siRNA reinforces the cytotoxity of temozolomide in GBM-initiating cells. Gene Ther. 2010;17(11):1363–1371. | ||
Wang M, Lu X, Dong X, et al. pERK1/2 silencing sensitizes pancreatic cancer BXPC-3 cell to gemcitabine-induced apoptosis via regulating Bax and Bcl-2 expression. World J Surg Oncol. 2015;13(1):66. | ||
Mendonça LS, Moreira JN, de Lima MCP, et al. Co-encapsulation of anti-BCR-ABL siRNA and imatinib mesylate in transferrin receptor-targeted sterically stabilized liposomes for chronic myeloid leukemia treatment. Biotechnol Bioeng. 2010;107(5):884–893. | ||
Chen J, Sheu AY, Li WG, et al. Poly(lactide-co-glycolide) microspheres for MRI-monitored transcatheter delivery of sorafenib to liver tumors. J Control Release. 2014;184:10–17. | ||
Li J-M, Wang Y-Y, Zhao M-X, et al. Multifunctional QD-based co-delivery of siRNA and doxorubicin to HeLa cells for reversal of multidrug resistance and real-time tracking. Biomaterials. 2012;33(9):2780–2790. | ||
Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. | ||
Movahedi F, Hu RG, Becker DL, et al. Stimuli-responsive liposomes for the delivery of nucleic acid therapeutics. Nanomedicine. 2015;11(6):1575–1584. | ||
Chen Z, Han L, Xu M, et al. Rationally designed multitarget anticancer agents. Curr Med Chem. 2013;20(13):1694–1714. | ||
Liu J, Boonkaew B, Arora J, et al. Comparison of sorafenib-loaded poly (lactic/glycolic) acid and DPPC liposome nanoparticles in the in vitro treatment of renal cell carcinoma. J Pharm Sci. 2015;104(3):1187–1196. | ||
Zhang H, Zhang FM, Yan SJ. Preparation, in vitro release, and pharmacokinetics in rabbits of lyophilized injection of sorafenib solid lipid nanoparticles. Int J Nanomedicine. 2012;7:2901–2910. | ||
Cao HQ, Wang YX, He XY, et al. Codelivery of sorafenib and curcumin by directed self-assembled nanoparticles enhances therapeutic effect on hepatocellular carcinoma. Mol Pharm. 2015;12(3):922–931. | ||
Shen J, Sun H, Meng Q, et al. Simultaneous inhibition of tumor growth and angiogenesis for resistant hepatocellular carcinoma by co-delivery of sorafenib and survivin small hairpin RNA. Mol Pharm. 2014;11(10):3342–3351. | ||
Wang JL, Xi Y, Liu YL, et al. Combination of targeted PDT and anti-VEGF therapy for rat CNV by RGD-modified liposomal photocyanine and sorafenib. Invest Ophthalmol Vis Sci. 2013;54(13):7983–7989. | ||
Wang L, Geng D, Su H. Safe and efficient pH sensitive tumor targeting modified liposomes with minimal cytotoxicity. Colloids Surf B Biointerfaces. 2014;123:395–402. | ||
Hardiansyah A, Huang LY, Yang MC, et al. Novel pH-sensitive drug carriers of carboxymethyl-hexanoyl chitosan (Chitosonic (R) Acid) modified liposomes. Rsc Advances. 2015;5(30):23134–23143. | ||
Mourya V, Inamdar NN, Tiwari A. Carboxymethyl chitosan and its applications. Adv Mat Lett. 2010;1(1):11–33. | ||
Liu FX, Li M, Liu CX, et al. pH-Sensitive self-assembled carboxymethyl chitosan-modified DNA/polyethylenimine complexes for efficient gene delivery. J Biomed Nanotechnol. 2014;10(11):3397–3406. | ||
Liu T, Wang M, Wang T, et al. Co-delivery of doxorubicin and siRNA by a simplified platform with oligodeoxynucleotides as a drug carrier. Colloids Surf B Biointerfaces. 2015;126:531–540. | ||
Wang MF, Liu TX, Han LQ, et al. Functionalized O-carboxymethyl-chitosan/polyethylenimine based novel dual pH-responsive nanocarriers for controlled co-delivery of DOX and genes. Polymer Chemistry. 2015;6(17):3324–3335. | ||
Feng Q, Yu MZ, Wang JC, et al. Synergistic inhibition of breast cancer by co-delivery of VEGF siRNA and paclitaxel via vapreotide-modified core-shell nanoparticles. Biomaterials. 2014;35(18):5028–5038. | ||
Chen W, Yuan Y, Cheng D, et al. Co-delivery of doxorubicin and siRNA with reduction and pH dually sensitive nanocarrier for synergistic cancer therapy. Small. 2014;10(13):2678–2687. | ||
Liu Y, Feng L, Liu T, et al. Multifunctional pH-sensitive polymeric nanoparticles for theranostics evaluated experimentally in cancer. Nanoscale. 2014;6(6):3231–3242. | ||
Deng ZJ, Morton SW, Ben-Akiva E, et al. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. Acs Nano. 2013;7(11):9571–9584. | ||
Dawidczyk CM, Kim C, Park JH, et al. State-of-the-art in design rules for drug delivery platforms: lessons learned from FDA-approved nanomedicines. J Control Release. 2014;187:133–144. | ||
Wang F, Chen L, Zhang R, et al. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J Control Release. 2014;196:222–233. | ||
Qu M-H, Zeng R-F, Fang S, et al. Liposome-based co-delivery of siRNA and docetaxel for the synergistic treatment of lung cancer. Int J Pharm. 2014;474(1):112–122. | ||
Fan Y, Sahdev P, Ochyl LJ, et al. Cationic liposome-hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J Control Release. 2015;208:121–129. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.