Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Periventricular White Matter Hyperintensity in Males is Associated with Post-Stroke Depression Onset at 3 Months
Authors Tu XQ, Lai ZH, Zhang Y, Ding KQ , Ma FY, Yang GY, He JR, Zeng LL
Received 18 March 2021
Accepted for publication 11 May 2021
Published 8 June 2021 Volume 2021:17 Pages 1839—1857
DOI https://doi.org/10.2147/NDT.S311207
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Xuan-qiang Tu,1,* Ze-hua Lai,1,* Yu Zhang,2 Kai-qi Ding,1 Fei-yue Ma,3 Guo-Yuan Yang,1 Ji-rong He,3 Li-li Zeng1
1Department of Neurology and Institute of Neurology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Neurology and Institute of Neurology, Ruijin Hospital North, School of Medicine, Shanghai Jiao Tong University, Shanghai, People’s Republic of China; 3Department of Neurology and Institute of Neurology, Ruijin Hospital Luwan Branch, Shanghai Jiaotong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li-li Zeng No. 197 Ruijin Second Road, Huangpu District, Shanghai, 200025, People’s Republic of China
Tel +86-13816290607
Email [email protected]
Ji-rong He No. 149 Chongqing South Road, Huangpu District, Shanghai, People’s Republic of China
Tel +86-13162762045
Email [email protected]
Objective: This study aimed to explore the correlation between white matter hyperintensity (WMH) and post-stroke depression (PSD) at 3 months, and to further investigate sex differences in the pathogenesis of PSD.
Methods: A total of 238 consecutive patients with acute cerebral infarction were recruited. PSD was assessed at 2 weeks and at 3 months after stroke onset. All stroke cases were divided into four subgroups according to the diagnosis of depression at two time nodes: continuous depression; depression remission; late-onset PSD; and continuous non-depression. The Fazekas and Scheltens visual rating scales were adopted to assess WMH.
Results: Logistic regression revealed that the presence of periventricular white matter hyperintensity (PVWMH) at baseline in male patients was an independent risk factor for PSD at 3 months. Further subgroup analysis revealed that PVWMH was associated with late-onset PSD in males, but not with continuous depression 3 months after stroke. Male acute stroke patients with PVWMH at baseline were more likely to develop PSD at 3 months, especially late-onset PSD.
Conclusion: Our data suggest that sex differences may influence the pathogenesis of PSD.
Keywords: sex difference, acute cerebral infarction, leukoencephalopathy, affective disorder, post-stroke depression
Introduction
Stroke is one of the most common diseases in nervous system, which will do great harm to human beings. The recognition and intervention of stroke and its complications is very important.1,2 Post-stroke depression (PSD) is one complication of stroke, and is associated with serious hazards because it can significantly increase the risk for recurrence of stroke3,4 and mortality.5–7 Depression occurring within 2 weeks of acute stroke is known as early onset PSD, while depression that emerges 3 months after stroke―which was absent in the early stages of stroke―is recognized as late-onset PSD.8,9 Depression occurring 3 months after stroke is more concealed than in patients who experience early onset PSD and, because such patients are easily missed, effective intervention is precluded. Therefore, it is extremely important to devote sufficient attention to depression occurring 3 months after stroke so appropriate measures can be taken to control modifiable risk factors and perform early treatment in time to reduce the downstream harms of stroke. Our previous studies found that the severity of neurological deficits and diabetes mellitus were linked to PSD.10,11 Furthermore, it was reported that age, personality, education level, and stroke location were also correlated with PSD.12–14 In recent years, the correlation between WMH and PSD has attracted widespread attention.
White matter hyperintensity (WMH) is one type of cerebral small vessel disease that is characterized by spot-like or patchy changes in the white matter around the ventricle or in the subcortex. It exhibits high signal changes on T2-weighted magnetic resonance images and fluid attenuation reversal recovery sequences. From the anatomical location of the lesions, it can usually be divided into periventricular WMH (PVWMH) and deep WMH (DWMH). The pathophysiological mechanisms of WMH are related to damage of the blood-brain barrier, neuroinflammation, immunity, and impaired automatic regulation of cerebral blood flow.13 Clinical manifestations include cognitive impairment, balance disorders, gait abnormalities and urinary incontinence.15,16 Although several recent reports have described the correlation between WMH and PSD, the conclusions drawn in these studies have been inconsistent. A study from Hong Kong (China) reported that severe DWMH was linked to PSD,17 whereas earlier research from The Netherlands concluded that WMH was not associated with PSD.18 These inconsistent conclusions may be related to multiple factors including various PSD evaluation time nodes and assessment tools, inconsistent severity of stroke, different sample sizes and methods of group selection, and hospitals of varying specialization, such as general, specialized and community hospitals, or research institutes. Our previous study found that frontal PVWMH was an independent risk factor for early onset PSD.19 The relationship between WMH and late-onset PSD remains unclear. The present investigation further studied the association between WMH and PSD occurring ≥ 3 months after stroke.
Recent studies have revealed that sex can influence the incidence of PSD; however, conclusions from these investigations have not been consistent. In a systematic review of 56 studies published from 1982 to 2006, Poynter (2009)20 found that 35 studies reported a higher prevalence of PSD in women than in men. A study from Brazil found that the prevalence of PSD in female stroke patients was significantly higher than in males.21 Similar conclusions were drawn from a cohort study performed in China investigating PSD.22 In research from Great Britain, however, the incidence of PSD was higher in men.23 There have also been some studies suggesting that there are no sex differences in the occurrence of PSD.24 Similarly, a series of factors, such as different PSD assessment criteria, type of patient care institution, different sample sizes, and varying severity of stroke, may lead to different conclusions. Research has demonstrated that sex differences in ischemic stroke may be primarily manifested in sex hormone levels, apoptotic signaling cascades in neurons and glia, resident microglial activation, neuro-glial response to ionic imbalance, autophagy, and/or mitochondrial toxicity.25–27 Similar cerebral ischemic damage in both sexes could have different outcomes.
The purpose of the present study, therefore, was to explore the correlation among sex, WMH, and PSD occurring 3 months after stroke to further increase the current understanding of risk factors for PSD in efforts to improve interventions for patients with PSD.
Methods
Subjects
A total of 320 consecutive patients diagnosed with acute cerebral infarction in the Department of Neurology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine (Shanghai, China) were hospitalized. 238 of them were recruited as research subjects. All stroke patients underwent brain magnetic resonance imaging (MRI). Inclusion criteria were as follows: (1) diagnosis of ischemic stroke was made in accordance with guidelines for the diagnosis and treatment of acute ischemic stroke in China in 2010, and was confirmed by brain MRI scans; (2) course of disease ≤ 1 week; and age ≥ 18 years; (3) willingness to sign a written consent to participate in the study. Patients who experienced unconsciousness, aphasia or severe cognitive impairment, could not cooperate with the examination, those with psychosis or other psychiatric conditions, such as anxiety and depression, individuals with other severe systemic diseases including infection, cardiac and pulmonary failure, or hepatic and renal dysfunction, and those in whom MRI failed for various reasons, were excluded. The study protocol was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine (2011–12). All participants signed a written consent form. At the onset of the disease, 82 patients were excluded from the study, 67 of them were not willing to participate in the study, 3 of them could not be examined by nuclear magnetic resonance, and 12 patients were excluded due to their condition (such as aphasia, combined with severe organ failure, etc.) could not cooperate with the relevant assessment. During the follow-up of 3 months, 22 of 238 cases were lost, including 1 case of death, 2 cases of stroke recurrence, 1 case with severe other organ dysfunction and 18 cases of loss of follow-up (Figure 1).
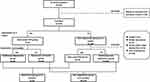 |
Figure 1 The recruitment of patients with acute ischemic stroke in this study. |
Clinical Data Collection
Demographic information and other baseline clinical data were collected on admission, and included age, sex, education level, presence of hypertension, diabetes, hyperlipidemia and/or heart disease, location of stroke, size of acute infarction, TOAST (trial of ORG 10172 in acute stroke treatment) classification, and National Institutes of Health Stroke Scale (NIHSS) score. Multiple blood laboratory examinations, including white blood cell (WBC) count, fasting blood glucose, glycosylated hemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), apolipoprotein A (ApoA) and apolipoprotein B (ApoB), were performed on the fasted vein blood samples collected on the day after admission. At the time of discharge, the TOAST classification was reconfirmed.
Diagnostic criteria for risk factors of stroke included the following:
Hypertension
Systolic pressure > 140 mmHg and/or diastolic pressure > 90 mmHg, or a definite history of hypertension for which medication was used to maintain blood pressure within normal limits.
Diabetes Mellitus
Symptoms of diabetes, venous plasma glucose concentration ≥ 11.1 mmol/L at any time; fasting venous plasma glucose concentration ≥ 7.0 mmol/L; venous plasma glucose concentration ≥ 11.1 mmol/L 2 h after the execution of oral glucose tolerance test). Of the above three criteria, ≥ 1 was required to meet the standard. Moreover, in repeat examination on the following day, of ≥ 1 of the above three items were required to be fulfilled, or there was a confirmed history of diabetes and medication was used to maintain blood glucose levels within normal limits.
Hyperlipidemia
TG > 1.7 mmol/L (0.56–1.7 mmol/L) and/or TC > 5.7 mmol/L (2.33–5.7 mmol/L) and/or LDL-C > 4.3 mmol/L (1.3–4.3 mmol/L), or there was a confirmed history of hyperlipidemia, and medication was used to maintain blood lipid levels within normal limits.
Heart Disease
There was a confirmed history of heart disease, including coronary heart disease, valvular disease and arrhythmia or, in the absence of a definite history, objective evidence of clinical abnormalities confirmed on electrocardiography or echocardiography.
The TOAST classification for ischemic stroke included the following five etiological types:
Large Artery Atherosclerosis
Intracranial and extracranial aortic stenosis ≥ 50%, infarct diameter ≥ 15 mm;
Cardiac Embolism
Cerebral embolism resulting from a variety of heart diseases that could produce cardiogenic emboli;
Small Artery Occlusion
Ischemic stroke caused by stenosis or occlusion of intracranial arteriole, infarct diameter < 15 mm;
Stroke of Other Determined Etiology
Ischemic stroke caused by other rare diseases; and
Stroke of Undetermined Etiology
Multiple examinations failed to reveal the cause of the disease.
Assessment of PSD
The diagnosis of PSD was made after a thorough mental status examination, with all psychiatric interviews performed by a well-trained neurologist. The diagnosis of depression in the stroke patients was based on criteria from the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV). Apart from the DSM-IV, the Hamilton Depression Scale 17-item (HAM-D) was also used to rate the severity of depressive symptoms and as a primary screening test. The assessment procedures for depression were performed at two different time nodes: 2–3 weeks (hereafter referred to as “2 weeks”); and 3 months after stroke. All stroke patients were divided into four subgroups according to the diagnosis of depression at the two time nodes: continuous depression (depression at 2 weeks and 3 months); depression remission (depression at 2 weeks, with no depression at 3 months); late-onset PSD (depression at 3 months, with no depression at 2 weeks); and continuous non-depressed (no depression at 2 weeks or 3 months). The continuous depression group and the late-onset PSD group comprised the depression group at 3 months, while the depression remission group and the continuous non-depressed group comprised the non-depressed group at 3 months (Figure 1).
Assessment of WMH
All stroke patients underwent brain MRI (1.5 Tesla) within 3 days of admission. A qualified neurologist assessed the severity of WMH using the Fazekas and Scheltens visual rating scales and brain magnetic resonance (MR) images.
According to the method described by Fazekas (1987),28 the assessment of WMH was divided into two categories: PVWMH and DWMH. The PVWMH scoring criteria were as follows: no WMH (0 points); caps or pencil-thin lining (1 point); smooth halo (2 points); and irregular PVWMH extending into the deep white matter (3 points). The DWMH scoring criteria were as follows: no WMH (0 points); punctate foci (1 point); beginning confluence of foci (2 points); and large confluent areas (3 points). The total score was the sum of the PVWMH and DWMH scores.
According to the method described by Scheltens (1993),29 the assessment of WMH was divided into four categories: PVWMH, DWMH, basal ganglia WMH, and infratentorial WMH:
PVWMH Scoring Criteria (0–6)
Occipital caps (0–2); frontal caps (0–2); bands (0–2). No WMH (0 points); lesion < 6 mm (1 point); lesion 6–10 mm (2 points); and lesions > 10 mm were recorded as DWMH. The three items were summed to obtain the total PVWMH score.
DWMH Scoring Criteria (0–24)
Frontal (0–6); parietal (0–6); occipital (0–6); temporal (0–6). No WMH (0 points); lesion < 4 mm, n ≤ 5 (1 point); lesion < 4 mm, n > 5 (2 points); lesion 4–10 mm, n ≤ 5 (3 points); lesion 4–10 mm, n > 5 (4 points); lesion > 10 mm, n ≥ 1 (5 points); and large confluent lesions (6 points). These four items were summed to obtain the total DWMH score.
Basal Ganglia WMH Scoring Criteria (0–30)
Caudate nucleus (0–6); putamen (0–6); globus pallidus (0–6); thalamus (0–6); internal/external capsule (0–6). Assessment methods ibid.
Infratentorial WMH Scoring Criteria (0–24)
Cerebellum (0–6); mesencephalon (0–6); pons (0–6); medulla (0–6). Assessment methods ibid.
The total score was the sum of the PVWMH, DWMH, basal ganglia WMH, and infratentorial WMH scores.
The number of lacunes was counted and ventricle-to-brain ratio was calculated using brain MRI30 and the following equation:
Ventricle-to-brain ratio = [(width of anterior horns of lateral ventricle/corresponding brain width at the same level) + (biventricular width at the level of the body of caudate nucleus/corresponding brain width at the same level) + (width of occipital horns of lateral ventricle/corresponding brain width at the same level)]/3.
Statistical Methods
Data were analyzed using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA). Data normality was verified using the Kolmogorov–Smirnov test. Measurement data with normal distribution are expressed as mean ± standard deviation, and were analyzed using two independent samples t test. Measurement data with non-normal distribution are expressed as median and interquartile range, and were analyzed using the Mann–Whitney U rank-sum test. Count data are expressed as constituent ratio (%) or ratio (%). Correlation between WMH and PSD was assessed using multiple logistic regression analysis, with the level of statistical significance set at 0.05.
Results
General Data from All Stroke Cases
In total, 238 patients (162 male, 76 female; mean [± SD] age 66 ± 11 years [range 32–91 years]) were recruited. Forty-two patients experienced depression 2 weeks after stroke, while the other 196 did not. Of the 238 cases, 216 underwent mental status re-evaluation at 3 months after stroke: one died, two experienced stroke recurrence; one had other serious systemic diseases; and 18 were lost to follow-up. Of these 216 patients, 150 were male and 66 were female. According to the assessment results, 43 (19.9%) patients were depressed 3 months after stroke, and the other 173 (80.1%) were not. Of the 216 patients, 25 (11.6%) experienced persistent depression after stroke, 18 (8.3%) had late-onset PSD, 14 (6.5%) had early onset PSD, which was relieved at 3 months, and 159 (73.6%) did not experience any depression after stroke. The depression group and the non-depression group at 3 months did not differ in age, sex, education level, hypertension, hyperlipidemia, heart disease, location of stroke, lateralization, size of acute infarction or TOAST classification (p > 0.05) (Table 1). There were, however, significant differences in diabetes mellitus and NIHSS scores (NIHSS > 3) between the two groups (p = 0.028 and p = 0.000, respectively) (Table 1).
 |
Table 1 Baseline Feature Comparisons Between the Depression Group and the Non Depression Group at 3 Months After Stroke Onset |
Assessment Results of WMH in All Stroke Cases
Between the depression and the non-depression groups at 3 months, there was no significant difference in Fazekas PVWMH score, the presence of PVWMH according to Fazekas, Fazekas DWMH score, the existence of DWMH according to Fazekas, Fazekas total score, Scheltens PVWMH total score, the presence of PVWMH according to Scheltens, occipital caps score, bands score, Scheltens DWMH total score, the presence of DWMH according to Scheltens, frontal score, parietal score, occipital score, temporal score, Scheltens basal ganglia WMH score, Scheltens infratentorial WMH score, Scheltens total score, and the number of lacunes and ventricle-to-brain ratio, in the 216 patients who had been followed up effectively (p > 0.05) (Table 2). Similar negative results were obtained for comparisons between the continuous depression group versus the non-depression group at 3 months, and the continuous depression group versus the depression remission group (p > 0.05) (Table 2).
 |
Table 2 Comparisons of WMH, Number of Lacunes and Ventricle-to-Brain Ratio Between the Depression Group and the Non Depression Group at 3 Months After Stroke Onset |
There was a significant difference in Fazekas DWMH score between the late-onset PSD group and the non-depression group at 3 months (p = 0.032) (Table 2). However, the results could not be confirmed by Scheltens assessment (p > 0.05) (Table 2).
There was a significant difference in Fazekas DWMH score between the late-onset PSD group and the continuous non-depression group (p = 0.047) (Table 2). Similarly, the results could also not be confirmed by Scheltens assessment (p > 0.05) (Table 2).
General Data from Male Stroke Cases
In the male subgroup of 150 cases, 28 (18.7%) patients were depressed at 3 months after stroke, and the other 122 (81.3%) were not. Among these, 15 (10.0%) patients experienced persistent depression after stroke, 13 (8.7%) had late-onset PSD, 10 (6.7%) had early-onset PSD, which was relieved at 3 months, and 112 (74.6%) did not experience any depression after stroke. The depression group and the non-depression group at 3 months in males did not differ in age, education level, hypertension, hyperlipidemia, heart disease, location of stroke, lateralization, size of acute infarction and TOAST classification (p > 0.05) (Table 3), while there were significant differences in diabetes mellitus and NIHSS scores (NIHSS > 3) between the two groups (p = 0.000 and p = 0.003, respectively) (Table 3).
 |
Table 3 Baseline Feature Comparisons Between the Depression Group and the Non Depression Group at 3 Months After Stroke Onset in Male |
Assessment Results of WMH in Males
Between the depression group and the non-depression group at 3 months in males, the proportion of the presence of PVWMH in the depression group at 3 months was significantly higher than that in the non-depression group at 3 months (both p = 0.005 according to Fazekas and Scheltens) (Table 4). There was no significant difference in Fazekas PVWMH score, Fazekas DWMH score, the existence of DWMH according to Fazekas, Fazekas total score, Scheltens PVWMH total score, occipital caps score, bands score, Scheltens DWMH total score, the presence of DWMH according to Scheltens, frontal score, parietal score, occipital score, temporal score, Scheltens basal ganglia WMH score, Scheltens infratentorial WMH score, Scheltens total score, number of lacunes, and ventricle-to-brain ratio (p > 0.05) (Table 4).
 |
Table 4 Comparisons of WMH, Number of Lacunes and Ventricle-to-Brain Ratio Between the Depression Group and the Non Depression Group at 3 Months After Stroke Onset in Male |
There was no significant difference in any of the comparison items between the continuous depression group and the non-depression group at 3 months in males (p > 0.05) (Table 4).
Between the late-onset PSD group and the non-depression group at 3 months in males, the proportion of the presence of PVWMH in the late-onset PSD group was significantly higher than that in the non-depression group at 3 months (both p=0.013 according to Fazekas and Scheltens) (Table 4), while there were no significant differences in the remainder of the comparison items (p > 0.05) (Table 4).
Between the continuous depression group and the depression remission group in males, there were no significant differences in any of the comparison items (p > 0.05) (Table 4).
Between the late-onset PSD group and the continuous non-depression group in males, the proportion of the presence of PVWMH in the late-onset PSD group was significantly higher than that in the continuous non-depression group (both p = 0.012 according to Fazekas and Scheltens) (Table 4). There were no significant differences in the remainder of the comparison items (p > 0.05) (Table 4).
The Presence of PVWMH at Baseline Was Associated with PSD at 3 Months in Males
The presence of PVWMH at baseline in males was further used as an independent variable for the logistic regression model. Logistic regression analysis revealed that PVWMH was closely correlated with PSD at 3 months in males (OR 6.580 [95% CI 1.488–29.094; p = 0.013) (Table 5). In order to control the confounding factors, we took age, education, lesion location, NIHSS score and diabetes mellitus into the logistic regression as well. Logistic regression analysis revealed that PVWMH was closely correlated with PSD at 3 months in males (OR 30.603 [95% CI 3.315–282.535; p = 0.003) (Table 6).
 |
Table 5 Logistic Regression Analysis on Related Factors of PSD at 3 Months in Male |
 |
Table 6 Adjusted Logistic Regression Analysis on Related Factors of PSD at 3 Months in Male |
General Data from Female Stroke Patients
In the female subgroup of 66 cases, 15 (22.7%) patients were depressed at 3 months after stroke, and the other 51 (77.3%) were not. Among these, 10 (15.2%) patients experienced persistent depression after stroke, 5 (7.5%) had late-onset PSD, 4 (6.1%) had early-onset PSD, which was relieved at 3 months, and 47 (71.2%) did not experience any depression after stroke. The depression group and the non-depression group at 3 months in females did not differ in age, education, hypertension, diabetes mellitus, hyperlipidemia, heart disease, location of stroke, lateralization, size of acute infarction and TOAST classification (p > 0.05) (Table 7), while there was a significant difference in NIHSS scores (NIHSS > 3) between the two groups (p = 0.010) (Table 7).
 |
Table 7 Baseline Feature Comparisons Between the Depression Group and the Non Depression Group at 3 Months After Stroke Onset in Female |
Assessment Results of WMH in Females
Between the depression group and the non-depression group at 3 months in females, there were no significant difference in Fazekas PVWMH score, the presence of PVWMH according to Fazekas, Fazekas DWMH score, the presence of DWMH according to Fazekas, Fazekas total score, Scheltens PVWMH total score, the presence of PVWMH according to Scheltens, occipital caps score, bands score, Scheltens DWMH total score, the existence of DWMH according to Scheltens, frontal score, parietal score, occipital score, temporal score, Scheltens basal ganglia WMH score, Scheltens infratentorial WMH score, Scheltens total score, number of lacunes, and ventricle-to-brain ratio (p > 0.05) (Table 8). Similar negative results were obtained between the comparisons of the continuous depression group versus the non-depression group at 3 months, the late-onset PSD group versus the non-depression group at 3 months, the continuous depression group versus the depression remission group, and the late-onset PSD group versus the continuous non depression group in females (p > 0.05) (Table 8).
 |
Table 8 Comparisons of WMH, Number of Lacunes and Ventricle-to-Brain Ratio Between the Depression Group and the Non Depression Group at 3 Months After Stroke Onset in Female |
Discussion
We used two visual rating scales to assess WMH; when two methods reached the same conclusion, we defined the result to be meaningful. Between the depression and the non-depression groups at 3 months in males, the proportion of the presence of PVWMH in the depression group at 3 months was significantly higher than that in the non-depression group at 3 months according to assessment using the two scales. Logistic regression revealed that the presence of PVWMH at baseline in male patients was closely related to depression at 3 months after stroke. PVWMH was an independent risk factor for PSD at 3 months in males (OR 6.367 [95% CI 364–29.718]; p = 0.019). No such correlation was found in the female PSD subgroup. Further subgroup analysis revealed that PVWMH was associated with late-onset PSD in men (p = 0.012), but not with the continuous depression group 3 months after stroke. These findings suggested that male stroke patients with the presence of PVWMH at baseline were more likely to experience PSD at 3 months, especially late-onset PSD. A study from Korea by Kim et al also investigated the association between WMH and PSD at 3 months and concluded that DWMH was associated with delayed mood disorders, including depression and anxiety, in patients with acute ischemic stroke without sex differences31. Kim et al adopted the Hospital Anxiety and Depression Scale as the assessment tool. The use of different rating scales, and the absence of sex-specific subgroup analysis may explain the different conclusions. In another study, Tang et al also reported a link between WMH and PSD at 3 months, but found no sex differences in the link.17 This may also be associated with failing to analyze subgroups according to sex.
Previous research has demonstrated that the limbic circuit present around the ventricles plays a regulatory role in mood and cognition32 The prefrontal cortex communicates with the limbic system through limbic circuits. The prefrontal cortex has extensive neural connections and complex structural patterns, and plays an important role in emotional information processing.33 Many components of the limbic system, including the amygdala, hippocampus gyrus, thalamus and cingulate cortex, are involved in mood processing and regulation, and could affect the brain network in patients experiencing PSD.34 Studies have shown that damage to the prefrontal-limbic circuits could lead to affective disorders and PSD.34,35 Therefore, damage to the PVWMH to the limbic circuits could be a potential contributor to the mechanism of PSD in stroke patients.
In the present study, we compared male and female subgroups and found no significant difference in the incidence of PSD between the sexes. However, the positive results obtained only in males in this study demonstrated significant sex differences in the likelihood of PSD outcomes in stroke patients with the presence of PVWMH at baseline. Statistical research has shown that the cerebral white matter microstructure itself varies between the sexes.36 In males, studies have reported that testosterone secreted by the developing testes weakened the release of luteinizing hormone (LH), resulting in fewer gamma-aminobutyric acid (GABA) and glutamate cells in the brain in men than in women37 As inhibitory neurotransmitters in the brain, GABA and glutamate have significant impact on the regulation of emotions. The anteroventral periventricular nucleus (AVPV) is located around the ventricles of the brain, and is a structure controlling sex-specific LH release. Periventricular white matter injury may lead to AVPV dysfunction, disturbance of the regulation of GABA and glutamate neurotransmitters and, eventually, result in affective disorders such as depression. However, only male stroke patients were susceptible to PSD when experiencing similar PVWMH impairment in both sexes, which may be related to the original difference in the number of GABA and glutamate cells, and the basic variation in the cerebral white matter microstructure itself. The difference in the risk for developing PSD in stroke patients with the presence of PVWMH between the sexes was a key finding illustrating sex differences in the pathogenesis of PSD.
Late-onset PSD is easily overlooked because of its delayed emergence. For male stroke patients with the presence of PVWMH at baseline, close attention should be devoted to assessment of their mental state, and a long-term follow-up system should be established in addition to routine stroke treatment and rehabilitation. Thus, late-onset PSD could be detected and intervention and treatment could be provided in a timely manner, thereby reducing the harm of PSD and improving the prognosis of stroke.
As the limitations, in the present paper, the sample size is small, and we only investigated acute post-stroke depression and 3-month post-stroke depression. Whether the onset of longer-term post-stroke depression is related to gender still needs to be studied. Secondly, the state of PVWMH in normal people and its changes over time also need to be studied in future experimental research to better clarify the risk factors and mechanisms of PSD.
In conclusion, male acute stroke patients with the presence of PVWMH at baseline were more likely to develop PSD at 3 months, especially late-onset PSD. Results of the present study suggest that sex difference may influence the pathogenesis of PSD. The mental state of male stroke patients should be paid attention to in order to detect PSD early and take timely intervention measures to reduce the harm of PSD and improve the prognosis of stroke.
Data Sharing Statement
To protect patient privacy, the data unpublished will not share in public. Any question of original data can email corresponding author.
Ethics Approval and Informed Consent
The study protocol was approved by the Institutional Ethics Committee of Shanghai Ruijin Hospital. All patients provided informed consent to participate, and this study was conducted in accordance with the Declaration of Helsinki.
Consent for Publication
The authors consent that the details of any images, videos, recordings, etc can be published.
Acknowledgments
The authors are sincerely grateful to all study participants. We would like to thank Editage (www.editage.cn) for English language editing.
Author Contributions
All authors meet the authorship guidelines, Li-li Zeng involved in all aspects of the study, supervised tissue assay analysis. Ji-rong He, drafted the enclosed manuscript, performed the evaluation of MRI image and the analysis of data. Xuan-qiang Tu and Ze-hua Lai performed the data analysis and the revision of the article. Yu Zhang performed the collection of blood samples and clinical evaluation in stroke patients. Kai-qi Ding, Fei-yue Ma and Guo-Yuan Yang, participated in the design of the present study and revision of the article. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Hospital specialist development plan of health system in Huangpu District of Shanghai under Grant [number 2019pyzk01-1].
Disclosure
The authors declare no competing interests in this work.
References
1. Leng T, Xiong ZG. Treatment for ischemic stroke: from thrombolysis to thrombectomy and remaining challenges. Brain Circ. 2019;5(1):8–11. doi:10.4103/bc.bc_36_18
2. Tu WJ, Zhao SJ, Xu DJ, Chen H. Serum 25-hydroxyvitamin D predicts the short-term outcomes of Chinese patients with acute ischaemic stroke. Clin Sci. 2014;126(5):339–346. doi:10.1042/CS20130284
3. Larson SL, Owens PL, Ford D, Eaton W. Depressive disorder, dysthymia, and risk of stroke: thirteen-year follow-up from the Baltimore epidemiologic catchment area study. Stroke. 2001;32(9):1979–1983. doi:10.1161/hs0901.094623
4. May M, McCarron P, Stansfeld S, et al. Does psychological distress predict the risk of ischemic stroke and transient ischemic attack? The Caerphilly Study. Stroke. 2002;33(1):7–12. doi:10.1161/hs0102.100529
5. House A, Knapp P, Bamford J, Vail A. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke. 2001;32(3):696–701. doi:10.1161/01.STR.32.3.696
6. Morris PL, Robinson RG, Andrzejewski P, Samuels J, Price TR. Association of depression with 10-year poststroke mortality. Am J Psychiatry. 1993;150(1):124–129.
7. Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am J Psychiatry. 2004;161(6):1090–1095. doi:10.1176/appi.ajp.161.6.1090
8. Shi Y, Xiang Y, Yang Y, et al. Depression after minor stroke: prevalence and predictors. J Psychosom Res. 2015;79(2):143–147. doi:10.1016/j.jpsychores.2015.03.012
9. Sun N, Li QJ, Lv DM, Man J, Liu XS, Sun ML. A survey on 465 patients with post-stroke depression in China. Arch Psychiatr Nurs. 2014;28(6):368–371. doi:10.1016/j.apnu.2014.08.007
10. Zhang Y, Cheng L, Chen Y, Yang GY, Liu J, Zeng L. Clinical predictor and circulating microRNA profile expression in patients with early onset post-stroke depression. J Affect Disord. 2016;193:51–58. doi:10.1016/j.jad.2015.12.061
11. Zhang Y, He JR, Liang HB, et al. Diabetes mellitus is associated with late-onset post-stroke depression. J Affect Disord. 2017;221:222–226. doi:10.1016/j.jad.2017.06.045
12. Hama S, Yamashita H, Shigenobu M, et al. Post-stroke affective or apathetic depression and lesion location: left frontal lobe and bilateral basal ganglia. Eur Arch Psychiatry Clin Neurosci. 2007;257(3):149–152. doi:10.1007/s00406-006-0698-7
13. Teoh V, Sims J, Milgrom J. Psychosocial predictors of quality of life in a sample of community-dwelling stroke survivors: a longitudinal study. Top Stroke Rehabil. 2009;16(2):157–166. doi:10.1310/tsr1602-157
14. Unalan D, Ozsoy S, Soyuer F, Ozturk A. Poststroke depressive symptoms and their relationship with quality of life, functional status, and severity of stroke. Neurosciences. 2008;13(4):395–401.
15. Lin J, Wang D, Lan L, Fan Y. Multiple Factors Involved in the Pathogenesis of White Matter Lesions. Biomed Res Int. 2017;2017:9372050. doi:10.1155/2017/9372050
16. Zheng JJ, Delbaere K, Close JC, Sachdev PS, Lord SR. Impact of white matter lesions on physical functioning and fall risk in older people: a systematic review. Stroke. 2011;42(7):2086–2090. doi:10.1161/STROKEAHA.110.610360
17. Tang WK, Chen YK, Lu JY, et al. White matter hyperintensities in post-stroke depression: a case control study. J Neurol Neurosurg Psychiatry. 2010;81(12):1312–1315. doi:10.1136/jnnp.2009.203141
18. Nys GM, van Zandvoort MJ, van der Worp HB, de Haan EH, de Kort PL, Kappelle LJ. Early depressive symptoms after stroke: neuropsychological correlates and lesion characteristics. J Neurol Sci. 2005;228(1):27–33. doi:10.1016/j.jns.2004.09.031
19. He JR, Zhang Y, Lu WJ, et al. Age-Related Frontal Periventricular White Matter Hyperintensities and miR-92a-3p Are Associated with Early-Onset Post-Stroke Depression. Front Aging Neurosci. 2017;9:328. doi:10.3389/fnagi.2017.00328
20. Poynter B, Shuman M, Diaz-Granados N, Kapral M, Grace SL, Stewart DE. Sex differences in the prevalence of post-stroke depression: a systematic review. Psychosomatics. 2009;50(6):563–569. doi:10.1016/S0033-3182(09)70857-6
21. Carod-Artal FJ, Trizotto DS, Coral LF, Moreira CM. Determinants of quality of life in Brazilian stroke survivors. J Neurol Sci. 2009;284(1–2):63–68. doi:10.1016/j.jns.2009.04.008
22. Wang Z, Zhu M, Su Z, et al. Post-stroke depression: different characteristics based on follow-up stage and gender-a cohort perspective study from Mainland China. Neurol Res. 2017;39(11):996–1005. doi:10.1080/01616412.2017.1364514
23. Morrison V, Pollard B, Johnston M, MacWalter R. Anxiety and depression 3 years following stroke: demographic, clinical, and psychological predictors. J Psychosom Res. 2005;59(4):209–213. doi:10.1016/j.jpsychores.2005.02.019
24. Berg A, Palomaki H, Lehtihalmes M, Lonnqvist J, Kaste M. Poststroke depression: an 18-month follow-up. Stroke. 2003;34(1):138–143. doi:10.1161/01.STR.0000048149.84268.07
25. Ahnstedt H, McCullough LD, Cipolla MJ. The Importance of Considering Sex Differences in Translational Stroke Research. Transl Stroke Res. 2016;7(4):261–273. doi:10.1007/s12975-016-0450-1
26. Demarest TG, McCarthy MM. Sex differences in mitochondrial (dys)function: implications for neuroprotection. J Bioenerg Biomembr. 2015;47(1–2):173–188. doi:10.1007/s10863-014-9583-7
27. Hendrix SL, Wassertheil-Smoller S, Johnson KC, et al. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113(20):2425–2434. doi:10.1161/CIRCULATIONAHA.105.594077
28. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356. doi:10.2214/ajr.149.2.351
29. Scheltens P, Barkhof F, Leys D, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114(1):7–12. doi:10.1016/0022-510X(93)90041-V
30. de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47(2):145–151. doi:10.1002/1531-8249(200002)47:2<145::AID-ANA3>3.0.CO;2-P
31. Kim JT, Park MS, Yoon GJ, et al. White matter hyperintensity as a factor associated with delayed mood disorders in patients with acute ischemic stroke. Eur Neurol. 2011;66(6):343–349. doi:10.1159/000332585
32. Barber R, Gholkar A, Scheltens P, Ballard C, McKeith IG, O’Brien JT. MRI volumetric correlates of white matter lesions in dementia with Lewy bodies and Alzheimer’s disease. Int J Geriatr Psychiatry. 2000;15(10):911–916. doi:10.1002/1099-1166(200010)15:10<911::AID-GPS217>3.0.CO;2-T
33. DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR. Testing predictions from personality neuroscience. Brain structure and the big five. Psychol Sci. 2010;21(6):820–828. doi:10.1177/0956797610370159
34. Shi Y, Zeng Y, Wu L, et al. A Study of the Brain Abnormalities of Post-Stroke Depression in Frontal Lobe Lesion. Sci Rep. 2017;7(1):13203. doi:10.1038/s41598-017-13681-w
35. Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28(2):206–215. doi:10.1038/sj.npp.1300045
36. Kanaan RA, Chaddock C, Allin M, et al. Gender influence on white matter microstructure: a tract-based spatial statistics analysis. PLoS One. 2014;9(3):e91109. doi:10.1371/journal.pone.0091109
37. Del Pino Sans J, Clements KJ, Suvorov A, Krishnan S, Adams HL, Petersen SL. Developmental exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin may alter LH release patterns by abolishing sex differences in GABA/glutamate cell number and modifying the transcriptome of the male anteroventral periventricular nucleus. Neuroscience. 2016;329:239–253. doi:10.1016/j.neuroscience.2016.04.051
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
