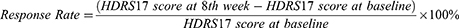Back to Journals » Journal of Inflammation Research » Volume 16
Peripheral Blood Inflammatory Cytokine Factors Expressions are Associated with Response to Acupuncture Therapy in Postpartum Depression Patients
Authors Xu YQ, Gou Y, Yuan JJ, Zhu YX, Ma XM, Chen C, Huang XX, Yang ZX, Zhou YM
Received 28 August 2023
Accepted for publication 31 October 2023
Published 13 November 2023 Volume 2023:16 Pages 5189—5203
DOI https://doi.org/10.2147/JIR.S436907
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Yu-Qin Xu,1 YanHua Gou,1 Jin-Jun Yuan,1 Yan-Xian Zhu,1 Xiao-Ming Ma,1 Chen Chen,2 Xing-Xian Huang,1 Zhuo-Xin Yang,1 Yu-Mei Zhou1
1The Fourth Clinical Medical College of Guangzhou University of Chinese Medicine, Shenzhen Traditional Chinese Medicine Hospital, Shenzhen, Guangdong Province, People’s Republic of China; 2Shenzhen Maternity and Child Healthcare Hospital, Shenzhen, Guangdong Province, People’s Republic of China
Correspondence: Zhuo-Xin Yang; Yu-Mei Zhou, Email [email protected]; [email protected]
Background: Increasing evidences demonstrate that immune dysregulation can result in depression, and it is reported that persistent inflammatory response is related to the unresponsiveness of antidepressant treatment.
Purpose: This study aimed to explore the reason why some responded but some not responded to acupuncture in treating postpartum depression (PPD), and whether it related to the levels of inflammatory cytokines.
Patients and Methods: Women diagnosed with PPD were recruited in to accept 8-week acupuncture. All subjects were assessed the 17-item Hamilton Depression Rating Scale (HDRS17) at baseline, week 1, week 2, week 4 and week 8 during the treatment. A panel of 9 cytokines was measured at baseline and 8 weeks.
Results: Of the 121 participants, 96 completed the 8-week assessment and 46 completed the blood sample collection. HDRS17 scores of 96 subjects showed significant statistical reduction since the first week (P = 0.002) and reached to 5.31 (P < 0.000) at the end of therapy. And we divided the 46 subjects into responders and non-responders according to the response rate of HDRS17 scores. Responders and non-responders did not differ significantly between-group in changes in the 9 cytokines. In responders, IL-6, IL-10 and IFN-γ levels were statistically lower (P = 0.006; P = 0.033; P = 0.024), while TGF-β 1 was statistically higher after 8 weeks treatment (P < 0.000). In non-responders, the levels of IL-5, TNF-α and TGF-β 1 were statistically higher (P = 0.018; P < 0.000; P < 0.000), while IFN-γ was statistically lower (P = 0.005).
Conclusion: Acupuncture could alleviate depressive symptoms of patients with PPD and might through adjusting peripheral inflammatory response by up-regulating anti-inflammatory cytokines and down-regulating pro-inflammatory cytokines.
Keywords: acupuncture, postpartum depression, inflammatory cytokines
Introduction
Postpartum depression (PPD) is one of the most common complications in perinatal period,1 classified as a subtype of major depressive disorder (MDD) with postpartum onset in American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV).2
The prevalence of PPD ranged from 5 to 25% around the world,1,3,4 approximately 10 to 20% in China.5–8 PPD affects offspring’s cognitive development such as language and intelligence,9 and the impact might be worse if mother’s depressive episode went severe and prolonged.10–12
The specific pathogenic mechanism of PPD is still unknown. The main underlying mechanisms contain neuroendocrine, genetic factors, neurotransmitters, neuroinflammatory, and environmental milieu.13 Substantial evidence demonstrating a connection between immune dysregulation and depression.14,15 Longitudinal studies provided supportive evidence for inflammation preceding depression or depressive symptoms over a wide range of timescales.16–18 Some studies have investigated how immune therapies can treat primary immunological illnesses that comorbid with depressive symptoms in individuals or how they can influence immune processes that disrupt synaptic function in animal models.19,20 Furthermore, robust preclinical data suggest that peripheral and central immunological processes may impair neuronal function and result in anhedonia, social withdrawal, and other depressive-like behaviors in animals.21–23 Based on that, a number of studies have drawn attention to correlations between depression symptoms and elevated peripheral inflammatory marker levels,21,24,25 and the existing antidepressants were found that it could lower peripheral inflammation in people and animal models.26,27 Hence, anti-inflammatory agents have been tested as adjunctive antidepressant therapy approaches.20
Inflammatory cytokine or proinflammatory cytokine is a type of signaling molecule, secreted from immune cells like helper T cells (Th) and macrophages which contribute to inflammation process. Inflammatory cytokines are classified as proinflammatory or anti-inflammatory. Proinflammatory cytokines consist of IL-2 and tumor necrosis factor-α (TNF-α),28 mainly mediated by T-helper 1 (Th1) cells. Anti-inflammatory cytokines include IL-4, IL-5 and IL-13, mainly mediated by T-helper 2 (Th2) cells.29 In addition, IL-6 and IL-10 act as both a pro-inflammatory cytokine and an anti-inflammatory myokine, TGF-β1 produced by T-helper 3 (Th3) cytokine is capable of inhibiting both Th1 and Th2 development and regulating the balance of Th1 and Th2 cytokines.30–32 Proinflammatory biomarkers could activate inflammatory response manifesting as fever, drowsiness, fatigue, decreased food intake, decreased interests, and depressed mood.33 Mainly because inflammatory cytokines in peripheral tissues act directly on the central nervous system (CNS) via the blood–brain barrier to cause such behavioral symptoms.21 Wide-spread immune dysregulation triggers off a tendency to reduce inflammation and suppress effector immune cells through the induction of IL-10 and TGF-β while promoting the development of anti-inflammatory immune cell populations.
According to a meta-analysis, acupuncture showed comparable efficacy in the severity of depression and remission of depression and manifested greater safety compared with medication.34 Approximately one-third of all patients with depression fail to respond to conventional antidepressant therapies, and this may concern with interactions between inflammatory pathways and neurocircuits in the brain.14 Acupuncture has been reported to play an immunomodulatory role in inflammatory diseases, just like allergic asthma,35 knee osteoarthritis,36 and inflammatory bowel disease.37 Compelling evidence showed that acupuncture could drive sympathetic pathways in somatotopy- and intensity-dependent manners to produce anti-inflammatory effects.38 Since the cohesive relationship between immune dysregulation and depression as mentioned above, the alteration of inflammatory cytokine undergoing to acupuncture therapy may somehow contribute to reveal the mechanism of acupuncture in treatment of depression.
In recent years, increasing trials reported the positive efficacy and safety of acupuncture for PPD population, and also demonstrated that acupuncture could be a promising and alternative therapy for PPD.39,40 However, few studies have focused on the underlying mechanisms of responders and non-responders in PPD patients undergoing acupuncture intervention. It is found that persistent inflammatory response was also associated with the unresponsiveness of antidepressant treatment.41 This finding inspired us to conduct the present study to explore why there were some responded but some not responded to acupuncture in treating PPD, and whether it relates to the levels of inflammatory cytokines? The chosen immunological biomarkers in this current study covered pro-inflammatory and anti-inflammatory to investigate the potential immunoregulation mechanism of acupuncture in treating PPD.
Materials and Methods
Study Design and Setting
We registered this prospective clinical study (registration number: ChiCTR2100041687) on Chinese Clinical Trial Registry. This study was conducted in Shenzhen Traditional Chinese Medicine Hospital and Shenzhen Maternity & Child Healthcare Hospital (Shenzhen, China) from March 2021 to February 2022.
This trial was approved by the institutional review board of the Shenzhen Traditional Chinese Medicine Hospital Ethics Committee (NO. K2020-027-01), and written informed consent was obtained from all participants. All authors certified to the data’s accuracy and completeness and the trial’s adherence to the protocol.
Procedure
All patients were recruited from Shenzhen Traditional Chinese Medicine Hospital and Shenzhen Maternity and Child Healthcare Hospital. We used the Chinese version of the Edinburgh Postnatal Depression Scale (EPDS) in the clinic for primary screening.42 Those who scored over 7 and were diagnosed with PPD were introduced by their doctors to our researchers. After preliminary introduction of this program through the mobile phone, the researchers invited the patients for a face-to-face interview. All interviews were launched in a quiet and dedicated room.
First, the researcher explained the program in detail to the interviewee in a one-to-one setting and determined if the interviewee met all the inclusion criteria and did not meet the exclusion criteria. If all conditions were met, the researcher used the 17-item Hamilton Depression Rating Scale (HDRS17) to assess depressive symptoms and asked the interviewee whether she was willing to participate.43
All volunteers signed written informed consent. The course of acupuncture treatment was 8 weeks, with 16 sections in total. All subjects were assessed at baseline, week 1, week 2, week 4 and week 8 during the treatment. The blood samples were collected before and after the 8 weeks acupuncture treatment. Participants were not compensated for study participation (Figure 1).
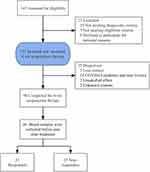 |
Figure 1 Trial flow diagram. |
Participants
We recruited the participants who met the following criterion: (1) aged 20 to 49 years old; (2) diagnosed as PPD by psychiatrist (diagnostic criteria according to Fourth Edition of the Diagnostic and Statistical Manual of Mental Disorders, DSM-IV); (3) the scores of HDRS17 ranging from 8 to 24; and (4) volunteer to join this research and sign the informed consent form. Participants were not compensated for study participation.
Participants with any one of the following items were excluded: (1) with serious mental disorders, such as schizophrenia (diagnosed by DSM-IV); (2) dysnoesia or having difficulty in understanding the content of the questionnaire due to brain diseases or other reasons, or incapable of effective interview; (3) pregnancy; (4) the score of “suicide” item in HDRS17 over 2; or (5) attempted suicide within 1 year.
We distributed the participants by the response rate to compare cytokine levels of the responders and non-responders after 8 weeks acupuncture treatment. The participants whose response rate, based on the change of HDRS17 scores between the baseline and 8-week treatment, ≥50% were defined as Responders, and those response rates <50% were defined as non-responders.44 The formula for calculating the response rate is as follows:
Intervention
All participants accepted acupuncture therapy. Therapy was performed by at least 5-year-experienced licensed acupuncturists in clinic. All were acupunctured at Baihui (GV20), Yintang (EX-HN3), Zhongwan (CV12), Qihai (CV6), Guanyuan (CV4), bilateral Neiguan (PC6), Shenmen (HT7), Hegu (LI4), Sanyinjiao (SP6), and Taichong (LR3) (Figure 2). After skin disinfection, disposable sterilized acupuncture needles (Product type: HuanQiu, Suzhou, China; 0.3 mm × 40 mm/0.3 mm × 75 mm; C-160630) were inserted into the skin with specified angle and depth. For Baihui (GV20) and Yintang (EX-HN3), the needles were inserted 0.5~0.8 cun (10mm~15mm) into skin followed in an angle of 15°. For Zhongwan (CV12), Neiguan (PC6), Shenmen (HT7), Hegu (LI4), Sanyinjiao (SP6), and Taichong (LR3), needles were inserted vertically 0.8~1.0 cun (15mm~20mm) into skin. For Qihai (CV6) and Guanyuan (CV4), needles were inserted 1.0~1.2 cun (20mm~25mm) into skin with an angle approximately 30° to 45°.
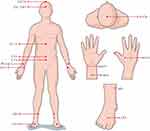 |
Figure 2 Locations of the selected acupoints. |
Once every 10 minutes, the acupuncturist rotated, lifted, and thrust equally on all of the needles to achieve de qi (pain, numbness, distention, and heaviness feelings). Each therapy lasted 30 minutes. For 8 weeks straight, a total of 16 sessions, participants accepted twice weekly therapy sessions.
Study Measures
HDRS17
Change of HDRS17 from baseline to end of 8-week treatment was used to evaluate the clinical efficacy of acupuncture on PPD. HDRS17 is a clinician-rated scale, which was designed to measure individuals’ frequency and severity of depressive symptoms. HDRS17 score ranges from 0 to 52, which higher score indicates more severe depressive clinical symptoms (mild, 8–17; moderate, 18–24; severe, ≥25).1 Each item is divided into four grades: never (0 point), occasionally (1 point), often (2 points), and always (3 points). Minimal important difference (MID) of HDRS17 was reported likely between 3 and 5 points. All subjects were evaluated with HDRS17 before and after the 8 weeks of treatment by our trained researchers.
Inflammatory Cytokine Factors
All participants were collected blood sample before and after 8 weeks of treatment. Blood samples were obtained using EDTA coated tubes. After collecting the blood samples were immediately centrifuged at 3500g for 5 minutes at 4°C, and serum was removed and stored at −80°C. Cytokine levels were measured by Bioscience ELISA kits (R&D systems, United States), including Human IL-2 Quantikine ELISA Kit, Human IL-4 Quantikine ELISA Kit, Human IL-5 Quantikine ELISA Kit, Human IL-6 Quantikine ELISA Kit, Human IL-10 Quantikine ELISA Kit, Human IL-13 Quantikine ELISA Kit, Human TNF-alpha Quantikine ELISA Kit, Human TGF-beta 1 Quantikine ELISA Kit and Human IFN-gamma Quantikine ELISA Kit. The inflammatory factors include IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, TNF-α, TGF-β and IFN-γ. Briefly, samples were performed after a twofold dilution with diluent-S (1×) (100μL serum +100μL diluent-S). Briefly, different concentrations of standard substances, samples or quality control substances were added into the corresponding well in each microwell, and incubated for 2 hours at room temperature. Repeat washing the plate 4 times. Then, 200μL enzyme-labeled antibody was added to each microwell and incubated at room temperature for another 2 hours, after that repeat washing the plate 4 times. Next, 200μL of TMB substrate solution was added to each microwell and incubated at room temperature for 30 minutes. After that 50μL termination solution was added into each microwell to make the solution mixed evenly. Last, fluorescence was quantified by measuring the absorbance value at 450nm using a microplate reader, and 540nm or 570nm was set as the correction wavelength (Varioskan LUX, Thermo, United States).
The corrected absorbance values (OD450-OD540/OD570), multiplex readings were averaged for each standard and sample, and then the mean zero standard OD value was subtracted. Four-parameter logic curve fitting was performed using computer software to create standard curves. Alternatively, a curve can be generated by plotting the logarithm of the standard concentration against the logarithm of the corresponding OD value, and the best fitting line can be determined by regression analysis. This process produces an adequate but less accurate fit to the data. If the sample has been diluted, the concentration should be multiplied by the dilution.
Adverse Events
If an adverse event (AE) was observed during any treatment, it was assessed and recorded in the case report form (CRF). Some adverse events related to acupuncture, such as sharp pain or hematomas around the inserted region, bleeding, fainting, or nausea, occurred during therapy. Other uncomfortable conditions less relevant to acupuncture, such as cough and headache, were also recorded.
Concomitant Medications
Throughout the research, it was suggested that subjects not receive any extra therapies, including medication or alternative therapies such as moxibustion, herbal medicine, massage, etc. In necessary conditions, conventional treatments, such as antidepressants or psychological therapy, were conducted by the psychiatrist. All concomitant medications were recorded in CRFs for further analysis. The name of the disease, the drug’s name, the dosage, the date, and the precise time that the medications were used were also reported to the researchers.
Follow-Up
During the follow-up period, if subjects’ HDRS17 score was over 24, the suicide item was over 2, or there was a tendency toward infanticide, psychiatrists would intervene and make appropriate suggestions for the patient, even including termination of the trial.
Statistical Methods
All inflammatory factors’ concentration levels were log-transformed. For continuous and normally distributed data, we performed the Student t test for between-group differences and paired t test for within-group differences. If data distributed non-normally, we used proper variable transformation to reduce skewness coefficient, for example logarithmic transformation, then performed Student t test or paired t test. Or we used the Mann–Whitney U-test for between-group differences and the Wilcoxon test for within-group differences if data distributed non-normally. The normally distributed data were expressed as mean ± standard deviation (SD). Non-normally distributed data is expressed as median or inter-quartile range (IQR). For categorical variables, the χ2 test were used for examining the between-group differences, and percentages and frequencies were presented to describe the effect size. All clinical data analyses were performed using the SPSS V.22.0 software. A two-tailed test will be conducted, and a P value <0.05 was considered statistically significant.
Results
Participants Baseline Characteristics
A total of 143 outpatients were screened for eligibility; 121 qualified. A total of 96 persons had completed the interventions and among them 46 completed the blood sample collections before and after treatment (Figure 1). We divided them into the responders (n = 21) and non-responders (n = 25) according to the remission rate based on the reduction of HDRS17. As CRFs recorded, none of the participants had history of rheumatoid arthritis, allergic rhinitis, mastitis or active uncontrolled inflammatory bowel disease (IBD). Comes to medication, no one had used of antidepressants, antibiotics or anti-inflammatory medications, accepted psychotherapy or other related therapy during the trial. Table 1 shows the baseline characteristics of the study population. Except the education level, the two groups were no significant difference with other baseline characteristics (including age, BMI, manner of last delivery, family history of MDD, number of delivery, number of survival newborn, postpartum duration, EPDS scores and HDRS17 scores).
 |
Table 1 Participant Baseline Characteristics |
HDRS17
HDRS17 scores of acupuncture group showed significant statistical reduction since the first week (95% CI: 0.40 to 1.68, P = 0.002). At the end of treatment, the reduction reached to 5.31 (95% CI: 4.33 to 6.30, P < 0.000) (Table 2).
 |
Table 2 HDRS17 Scores of Acupuncture Group at Each Assessment Time Point |
From baseline to 8 weeks, responders had mean reductions of 8.14 (95% CI: 6.55 to 9.74, P < 0.000). Slighter improvements were observed in the non-responders: mean reduction of 2.56 (95% CI: 1.33 to 3.79, P < 0.000) in 8 weeks. The difference of two groups was statistically significant of 5.58 (95% CI: 3.66 to 7.51, P < 0.000) (Table 3).
 |
Table 3 HDRS17 Scores of Response Group and Non-Response Group Before and After Treatment |
Inflammatory Cytokine Factors
Since the large skewness of the data, we uniformly used logarithmic functions to transform. After transformation, the skewness of data decreased, which closed to or turned into normal distribution. As Table 4 shows, IL-6, IL-10 and IFN-γ levels of responders were statistically lower (95% CI: 0.48 to 0.55, P = 0.006; 95% CI: 0.93 to 1.24, P = 0.033; 95% CI: 0.14 to 0.21, P = 0.024), while TGF-β1 was statistically higher after 8 weeks treatment (95% CI: 1.51 to 2.07, P < 0.000) (Figure 3). In non-responders, the levels of IL-5, TNF-α and TGF-β1 were statistically higher (95% CI: 0.94 to 0.98, P = 0.018; 95% CI: 0.58 to 1.22, P < 0.000; 95% CI: 1.62 to 2.02, P < 0.000), while IFN-γ was statistically lower (95% CI: 0.19 to 0.29, P = 0.005) (Figure 4). There was no between-group difference was found (Table 4).
 |
Table 4 Log-Transformed Inflammatory Cytokine Scores Comparison Between Responders and Non-Responders |
 |
Figure 3 Significant alteration of log-transformed inflammatory cytokine scores in responders. |
 |
Figure 4 Significant alteration of log-transformed inflammatory cytokine scores in non-responders. |
Discussion
This study evaluated the clinical effect and investigated the relationship between peripheral cytokine levels and responsiveness to acupuncture treatment in PPD. We found that acupuncture treatment resulted in a statistically significant reduction in the HDRS17 score at the end of 8 weeks treatment. What’s more, our results suggested that PPD patients who showed better response to acupuncture treatment manifested higher TGF-β1 and lowered IL-6, IL-10 and IFN-γ. While non-responders showed increased IL-5, TNF-α, TGF-β1 and decreased IFN-γ. There was no significantly difference found of these selected cytokines between responders and non-responders.
In this study, the HDRS17 score difference began to show statistical significance at week 1. At the end of the 8-wk intervention, a 5.31-point decrease in HDRS17 scores was observed in the acupuncture group. According to both anchor-based and distribution-based approaches, the minimum clinical difference (MID) of HDRS17 in the therapeutic effect of depression is estimated to range from 3 to 5 points.45 Hence, at the end of 8 weeks of acupuncture therapy, the subjects with PPD showed clinically significant improvement.
In terms of cytokine levels, IL-5, produced by Th2 cells, has long been associated with the cause of several allergic diseases, like allergic rhinitis and asthma.46 According to Ho et al, the anti-inflammatory cytokine IL-5 could be considered as a good predictor of depression, and higher level of IL-5 correlated to more serious depressive symptoms.47 Besides, a previous meta-analysis found the anti-depressant decreased IL-5 in responders.28 In our study, the increase of IL-5 peripheral level in non-responders is consistent with previous results.
IL-6 could activate a trans-signaling pathway, which may have anti- and proinflammatory effects.48 IL-6 contribute to the pathophysiology of a subset of patients with MDD via excessive release of corticotropin-releasing hormone (CRH) and by the promotion of glucocorticoid receptor resistance, which may ultimately impair the negative feedback regulation of the hypothalamic–pituitary–adrenal (HPA) axis.49 What’s more, IL-6 is mediated through the repression of brain-derived neurotrophic factor (BDNF) expression in the brain.50 Upregulating IL-6 level can inhibit BDNF levels and then induce decreased connectivity in the brain, which could be marked as depression.51 A meta-analysis found the IL-6 level increased in MDD group compared with health control.52 Previous studies have shown that acupuncture can activate the cholinergic anti-inflammatory pathway by down-regulating IL-6 level in the hippocampus, prefrontal cortex, and serum, thereby regulating peripheral inflammatory response.53–55 Wu et al found a significant decrease in IL-6 level in PPD patients undergoing duloxetine combined with cognitive behavioral therapy, which is consistent with our finding.56 Our results suggested that, undergoing acupuncture therapy, the IL-6 level of responders was more sensitive to non-responders.
IL-10 is an important immunomodulatory molecule, it can prevent the development of immunopathological lesions caused by the aggravation of protective immune responses to acute and chronic infections.57 IL-10 secretion by Th1 cells represents a powerful autoregulatory feedback loop that prevents excessive inflammation and potential tissue destruction during proinflammatory Th1-driven immune responses to infection.58 The fluctuation in IL-10 levels in depression is still unclear. Some have hypothesized that a feed-forward loop has the potential to inhibit inflammatory processes through its immunomodulatory/anti-inflammatory effects, and the activation of this feed-forward loop may be associated with IL-10 release induced by IL-6.59 In some stages, there was an inflammatory response involving an increase in IL-10 level, but high concentrations of IL-10 are detrimental and directly eliminate the inflammatory response. In a large cohort of patients in Mali, 133 patients with complicated malaria had higher circulating levels of IL-10 and inflammatory cytokines than those without complications.60 In a population-based study, IL-10 was found significantly increased in patients with MDD (with anti-depressant) and bipolar disorder (BD) compared to healthy control.59 Another meta-analysis also found the IL-10 level increased in MDD patients among 17 studies.52 A Chinese clinical study also found significant increase of IL-10 level in PPD patients with anti-depressant combined with psychotherapy. In summary, the alteration of IL-10 in MDD or PPD showed increased tendency. However, in our study, after 8-week acupuncture intervention, IL-10 level decreased in both responders and non-responders, and the reduction in responders showed statistically significant. It may relate to the bidirectional regulation of IL-10 function, increased IL-10 level could cause inflammatory response, while high concentration of IL-10 could inhibit inflammatory response. Secondly, it may relate to the above-mentioned mechanism of IL-6-driven induction of IL-10 release. To our observation, the level of IL-6 in PPD patients also showed a downward trend after 8-week acupuncture treatment, which might result in IL-10 concentration reduced. More studies are needed to confirm this hypothesis.
Overactivation of the pro-inflammatory cytokines in the CNS of the TNF-α is likely to cause damage, such as excitotoxicity and dysregulation in neurotransmission.61,62 TNF-α was found to be elevated in patients with MDD compared to healthy controls in previous studies.52,63 It was hypothesized that under conditions of chronic inflammation, increased levels of TNF-α would enhance SERT-mediated 5-HT uptake and significantly impact the available extracellular 5-HT.64 As reported, acupuncture could induce the down-regulation of TNF-α by inhibiting Th1 cell responses,65,66 which is closely related to the function of TGF-β1. Two clinical studies found that TNF-α level of PPD patients was negatively correlated with depressive symptoms.56,67 Significantly, we found TNF-α in non-responders at a higher level than baseline, and the inadequate inhibition of TNF-α by Th1 cell may be relevant to the depressive symptoms.48
TGF-β1 plays a crucial role in maintaining the homeostasis of the immune system and is clinically used in the diagnosis and treatment of various inflammatory diseases.68 It was proposed that some designated Th3 cells exert their action primarily by secreting TGF-β1.69 The action of TGF-β1 was shown to suppress production of Th1 cytokines to manifest the anti-inflammatory effect.70 A nested cohort study found that the level of TGF-β1 in colostrum of PPD patients was significantly lower than healthy controls, which means that low level of TGF-β1 is associated with postpartum depressive symptoms.71 In this study, both responders and non-responders showed a significant increase in TGF-β1 level, indicating that acupuncture could activate the up-regulation of anti-inflammatory cytokines in peripheral.
IFN-γ, produced by Th1 cells and natural killer cells, plays an important role in immune regulation.72 Interestingly, high level of IFN-γ could negatively influence cognitive behaviors. Zhang et al found that IFN-γ could cross through the permeable blood–brain barrier and induces microglia activation which triggers a series of inflammatory response. Such inflammatory response affects adult hippocampus neurogenesis and results in depression-like behaviors and cognitive impairments.73 At gene polymorphisms aspect, functional polymorphisms in regulatory gene promoter regions are expected to predetermine the phenotype manifestation of a specific cytokine profile and might thus be employed as disease-associated indicators.74 Based on that, it is found that IFN-γ + 874 A/T s is associated with suicide behavior in MDD.73,75 Acupuncture stimulation down-regulated serum IFN-γ levels in collagen-induced arthritic rats, thereby reducing arthritis incidence and avoiding joint histological deterioration.76 We found that IFN-γ level in both responders and non-responders decreased significantly, indicating that acupuncture may activate mechanisms that inhibits IFN-γ hence to alleviate depressive symptoms.
We found that the alterations of TGF-β1 and IFN-γ were consistent between the responders and non-responders. Based on the above evidence, acupuncture may play an immunomodulatory role by up-regulating TGF-β1 and inhibiting IFN-γ, and Th3 and Th1 cells might also be involved. The failure of acupuncture in non-responders was related to the rising levels of pro-inflammatory factors (such as IL-5 and TNF-α). Further pre-clinical studies are warranted to confirm the reasons for the failure to down-regulate the pro-inflammatory factors after acupuncture treatment.
Through the observation of the alterations of inflammatory factors in different cytokines in the responders and the non-responders, it is suggested that the response of acupuncture was closely related to the inhibition and activation of pro- and anti-inflammatory cytokines. Previous studies have shown that the occurrence of peripheral inflammatory response is closely related to the balance of pro-inflammatory and anti-inflammatory cytokines.77,78 Impaired balance between pro-inflammatory and anti-inflammatory cytokines leads to production of a high concentration of the neurodegenerative metabolite, quinolinic acid, in the brain79 in turn affects cognition and mood.
The findings of this study suggested that acupuncture could play a holistic role in regulating inflammatory cytokines, while the function of cytokines is considered as chemical messengers for regulating the innate and adaptive immune systems,14 and the upstream and downstream mechanism of acupuncture regulates inflammation is still unknown. Currently, the mechanism pathways of acupuncture by means of inflammatory reaction leading to improvement in depressive symptoms can be divided into three types: (1) inhibition of the release of inflammatory cytokines by activating the vagal nerve;80 (2) regulation of the brain-gut axis through intestinal microbiota;81(3) regulation of HPA axis.82 Moreover, for PPD patients who did not respond after 8 weeks of acupuncture treatment, whether they can achieve significant clinical efficacy by increasing frequency of acupuncture, prolonging treatment courses, or combining with other therapies (such as psychotherapy) needs to be explored in future research.
Limitation
The sample size of this study was small, thus the findings need to be replicated in a larger and more diverse sample in the future. Due to lack of inflammatory cytokine indicators from blank control group, it cannot be determined whether these mentioning inflammatory factors are treatment-specific. This present study could not answer whether inflammatory cytokines have specificity with PPD in contrast to MDD. More clinical studies are needed in the future.
Conclusion
Acupuncture could alleviate depressive symptoms of patients with PPD and might through adjusting peripheral inflammatory response by up-regulating anti-inflammatory cytokines and down-regulating pro-inflammatory cytokines.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author (YM Zhou) on reasonable request.
Ethics Approval and Consent to Participate
The protocol was carried out according to GCP (Good Clinical Practice) guidelines and in accordance with the principles of the Declaration of Helsinki. This study was approved by the institutional review board of the Shenzhen Traditional Chinese Medicine Hospital Ethics Committee (No. K2020-027-01). All participants signed written informed consent.
Acknowledgments
We thank the Shenzhen Maternity and Child Healthcare Hospital and the Shenzhen Mental Health Center/Shenzhen Kangning Hospital for their expert advice.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This trial was supported by the National Natural Science Foundation of China (No. 82004470), the Shenzhen Traditional Chinese Medicine Hospital “3030 Program” Chinese Medicine Clinical Research Project (No. G3030202119), the Medical Research Foundation of Guangdong Province (No. B2023099), and the National Veteran Chinese Medicine Expert Inheritance Studio Construction Project (No. [2022]75).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Stewart DE, Vigod S. Postpartum Depression. N Eng J Med. 2016;375(22):2177–2186. doi:10.1056/NEJMcp1607649
2. Do LJSU. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Encyclopedia Child Behav Dev. 2011:84.
3. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal Depression: prevalence, Screening Accuracy, and Screening Outcomes. AHRQ Evidence Rep Summaries. 2005;119(119):1–8.
4. Munk-Olsen T, Laursen TM, Pedersen CB, et al. New Parents and Mental DisordersA Population-Based Register Study. JAMA. 2006;296(21):2582–2589. doi:10.1001/jama.296.21.2582
5. Chen C, et al. Postpartum depression and influencing factors in Shenzhen. Modern Preventive Med. 2021;48(2):235–240.
6. Tao J-Q, Gong J-R, J-Y L. Meta Analysis of the Prevalence Rate of Post Depressive Depression in China. China J Health Psychol. 2018;26(02):171–174.
7. Xu S-J, Abudurexiti M, Nuermaimaiti A, et al. Analysis of the incidence and related influencing factors of postpartum depression in parturients. Practical Preventive Med. 2016;23(2):3.
8. Y-Q. X, Cui Y, Li J, et al. Cross-sectional study of the incidence of perinatal depression and the risk factors in Beijing area. China J Health Psychol. 2021;1–7.
9. Stein A, Pearson RM, Goodman SH, et al. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):1800–1819. doi:10.1016/S0140-6736(14)61277-0
10. Kaplan PS, Danko CM, Diaz A, et al. An associative learning deficit in 1-year-old infants of depressed mothers: role of depression duration. Infant Behav Dev. 2011;34(1):35–44. doi:10.1016/j.infbeh.2010.07.014
11. Quevedo LA, Silva RA, Godoy R, et al. The impact of maternal post-partum depression on the language development of children at 12 months. Child Care Health Dev. 2012;38(3):420–424. doi:10.1111/j.1365-2214.2011.01251.x
12. Sutter-Dallay AL, Murray L, Dequae-Merchadou L, et al. A prospective longitudinal study of the impact of early postnatal vs. chronic maternal depressive symptoms on child development. Eur Psychiatry. 2011;26(8):484–489. doi:10.1016/j.eurpsy.2010.05.004
13. Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. doi:10.1016/S0140-6736(18)31948-2
14. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi:10.1038/nri.2015.5
15. Bullmore E. The art of medicine: inflamed depression. Lancet. 2018;392(10154):1189–1190. doi:10.1016/S0140-6736(18)32356-0
16. Khandaker GM, Pearson RM, Zammit S, et al. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71(10):1121–1128. doi:10.1001/jamapsychiatry.2014.1332
17. Bell JA, Kivimäki M, Bullmore ET, et al. Repeated exposure to systemic inflammation and risk of new depressive symptoms among older adults. Transl Psychiatry. 2017;7(8):e1208. doi:10.1038/tp.2017.155
18. Lamers F, Milaneschi Y, Smit JH, et al. Longitudinal Association Between Depression and Inflammatory Markers: results From the Netherlands Study of Depression and Anxiety. Biol Psychiatry. 2019;85(10):829–837. doi:10.1016/j.biopsych.2018.12.020
19. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12):1381–1391. doi:10.1001/jamapsychiatry.2014.1611
20. Drevets WC, Wittenberg GM, Bullmore ET, et al. Immune targets for therapeutic development in depression: towards precision medicine. Nat Rev Drug Discov. 2022;21(3):224–244. doi:10.1038/s41573-021-00368-1
21. Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi:10.1038/nrn2297
22. Laumet G, Edralin JD, Chiang AC-A, et al. Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology. 2018;43(13):2597–2605. doi:10.1038/s41386-018-0154-1
23. Hodes GE, Pfau ML, Leboeuf M, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111(45):16136–16141. doi:10.1073/pnas.1415191111
24. Franceschi C. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J Gerontol. 2014;69(Suppl_1):S4–S9.
25. Miller AH. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Rev Immunol. 2016;16(1):22–34.
26. Eyre HA, Air T, Pradhan A, et al. A meta-analysis of chemokines in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;68:1–8. doi:10.1016/j.pnpbp.2016.02.006
27. Sun Y, Drevets W, Turecki G, et al. The relationship between plasma serotonin and kynurenine pathway metabolite levels and the treatment response to escitalopram and desvenlafaxine. Brain Behav Immunity. 2020;87:404–412. doi:10.1016/j.bbi.2020.01.011
28. Liu JJ, Wei YB, Strawbridge R, et al. Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Mol Psychiatry. 2020;25(2):339–350. doi:10.1038/s41380-019-0474-5
29. O’Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10(12):542–550. doi:10.1016/s0962-8924(00)01856-0
30. Swain SL, Huston G, Tonkonogy S, et al. Transforming growth factor-beta and IL-4 cause helper T cell precursors to develop into distinct effector helper cells that differ in lymphokine secretion pattern and cell surface phenotype. J Immunol. 1991;147(9):2991–3000.
31. Schmitt E, Hoehn P, Huels C, et al. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin‐12 and interferon‐γ and is inhibited by transforming growth factor‐β. Eur J Immunol. 1994;24(4):793–798. doi:10.1002/eji.1830240403
32. O’Garra AJI. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Nature Rev Immunol. 1998;8(3):275–283.
33. Anisman H, Merali Z. Cytokines, stress and depressive illness: brain-immune interactions. Ann Med. 2003;35(1):2–11. doi:10.1080/07853890310004075
34. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):Cd004046. doi:10.1002/14651858.CD004046.pub4
35. Joos S, Schott C, Zou H, et al. Immunomodulatory effects of acupuncture in the treatment of allergic asthma: a randomized controlled study. J Altern Complement Med. 2000;6(6):519–525. doi:10.1089/acm.2000.6.519
36. Ruan A, Wang Q, Ma Y, et al. Efficacy and Mechanism of Electroacupuncture Treatment of Rabbits With Different Degrees of Knee Osteoarthritis: a Study Based on Synovial Innate Immune Response. Front Physiol. 2021;12:642178. doi:10.3389/fphys.2021.642178
37. Song G, Fiocchi C, Achkar JP. Acupuncture in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25(7):1129–1139. doi:10.1093/ibd/izy371
38. Liu S, Wang Z-F, Su Y-S, et al. Somatotopic Organization and Intensity Dependence in Driving Distinct NPY-Expressing Sympathetic Pathways by Electroacupuncture. Neuron. 2020;108(3):436–450.e7. doi:10.1016/j.neuron.2020.07.015
39. Li W, Yin P, Lao L, et al. Effectiveness of Acupuncture Used for the Management of Postpartum Depression: a Systematic Review and Meta-Analysis. Biomed Res Int. 2019;2019:6597503. doi:10.1155/2019/6597503
40. Li S, Zhong W, Peng W, et al. Effectiveness of acupuncture in postpartum depression: a systematic review and meta-analysis. Acupunct Med. 2018;36(5):295–301. doi:10.1136/acupmed-2017-011530
41. Carvalho L, Torre JP, Papadopoulos AS, et al. Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J Affective Disorders. 2013;148(1):136–140. doi:10.1016/j.jad.2012.10.036
42. Lee DT, Yip SK, Chiu HFK, et al. Detecting postnatal depression in Chinese women. Validation of the Chinese version of the Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1998;172:433–437. doi:10.1192/bjp.172.5.433
43. Hamilton M. A Rating Scale For Depression. J Neurol Neurosurgery Psychiatry. 1960;23(1):56–62. doi:10.1136/jnnp.23.1.56
44. Brunoni AR, Moffa AH, Sampaio-Junior B, et al. Trial of Electrical Direct-Current Therapy versus Escitalopram for Depression. N Engl J Med. 2017;376(26):2523–2533. doi:10.1056/NEJMoa1612999
45. Hengartner MP, Plöderl M. Estimates of the minimal important difference to evaluate the clinical significance of antidepressants in the acute treatment of moderate-to-severe depression. BMJ Evid Based Med. 2022;27(2):69–73. doi:10.1136/bmjebm-2020-111600
46. Shen HH, Ochkur SI, McGarry MP, et al. A Causative Relationship Exists Between Eosinophils and the Development of Allergic Pulmonary Pathologies in the Mouse1. J Immunol. 2003;170(6):3296–3305. doi:10.4049/jimmunol.170.6.3296
47. Ho HY, Chin-Hung Chen V, Tzang B-S, et al. Circulating cytokines as predictors of depression in patients with breast cancer. J Psychiatr Res. 2021;136:306–311. doi:10.1016/j.jpsychires.2021.02.037
48. Maes M, Anderson G, Kubera M, et al. Targeting classical IL-6 signalling or IL-6 trans-signalling in depression? Exp Opinion Therapeutic Targets. 2014;18(5):495–512. doi:10.1517/14728222.2014.888417
49. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi:10.1016/j.biopsych.2008.11.029
50. Sharma RP, Tun N. Depolarization induces downregulation of DNMT1 and DNMT3 in primary cortical cultures. Nature Rev Immunol. 2008;3(2):74–80.
51. Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Bio Psychiatry. 2007;62(5):429–437.
52. Köhler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135(5):373–387. doi:10.1111/acps.12698
53. Son Y-S, Park H-J, Kwon O-B, et al. Antipyretic effects of acupuncture on the lipopolysaccharide-induced fever and expression of interleukin-6 and interleukin-1β mRNAs in the hypothalamus of rats. Neurosci Lett. 2002;319(1):45–48. doi:10.1016/s0304-3940(01)02538-1
54. Lu J, Shao R-H, Hu L, et al. Potential antiinflammatory effects of acupuncture in a chronic stress model of depression in rats. Neurosci Lett. 2016;618:31–38. doi:10.1016/j.neulet.2016.02.040
55. Yue N, Li B, Yang L, et al. Electro-Acupuncture Alleviates Chronic Unpredictable Stress-Induced Depressive- and Anxiety-Like Behavior and Hippocampal Neuroinflammation in Rat Model of Depression. Front Mol Neurosci. 2018;11:149. doi:10.3389/fnmol.2018.00149
56. Wu PP, Lin FB. To investigate the effect and dysfunction of duloxetine combined with cognitive behavioral therapy in patients with postpartum depression Effects of Cognition. Mater Child Health Care China. 2021;36(20):4642–4645.
57. Wei H, et al. Interleukin-10 Family Cytokines Immunobiology and Structure. Adv Exp Med Biol. 2019;1172:79–96.
58. Ouyang W, Rutz S, Crellin NK, et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29(1):71–109. doi:10.1146/annurev-immunol-031210-101312
59. Chi CH, Huang -Y-Y, Ye S-Z, et al. Interleukin-10 level is associated with post-stroke depression in acute ischaemic stroke patients. J Affect Disord. 2021;293:254–260. doi:10.1016/j.jad.2021.06.037
60. Lyke KE, Burges R, Cissoko Y, et al. Serum Levels of the Proinflammatory Cytokines Interleukin-1 Beta (IL-1β), IL-6, IL-8, IL-10, Tumor Necrosis Factor Alpha, and IL-12(p70) in Malian Children with Severe Plasmodium falciparum Malaria and Matched Uncomplicated Malaria or Healthy Controls. Infect Immun. 2004;72(10):5630–5637. doi:10.1128/IAI.72.10.5630-5637.2004
61. Simen BB, Duman CH, Simen AA, et al. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59(9):775–785. doi:10.1016/j.biopsych.2005.10.013
62. Perry VH. Stress primes microglia to the presence of systemic inflammation: implications for environmental influences on the brain. Brain Behav Immun. 2007;21(1):45–46. doi:10.1016/j.bbi.2006.08.004
63. Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi:10.1016/j.biopsych.2009.09.033
64. Kopschina Feltes P, Doorduin J, Klein HC, et al. Anti-inflammatory treatment for major depressive disorder: implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. J Psychopharmacol. 2017;31(9):1149–1165. doi:10.1177/0269881117711708
65. Tian L, et al. Downregulation of electroacupuncture at ST36 on TNF-α in rats with ulcerative colitis. World J Gastroenterol. 2003;9(5):1028. doi:10.3748/wjg.v9.i5.1028
66. Wang J, Zhao H, Mao-Ying Q-L, et al. Electroacupuncture downregulates TLR2/4 and pro-inflammatory cytokine expression after surgical trauma stress without adrenal glands involvement. Brain Res Bulletin. 2009;80(1–2):89–94. doi:10.1016/j.brainresbull.2009.04.020
67. Yang ZY, Liu LJ. Effects of sertraline combined with fluoxetine hydrochloride on the expression of neurotransmitters and inflammatory cytokines in patients with postpartum depression. Mater Child Health Care China. 2022;37(16):2909–2913. doi:10.19829/j.zgfybj.issn.1001-4411.2022.16.001
68. Clark DA, Coker R. Transforming growth factor-beta (TGF-beta). Int J Biochem Cell Biol. 1998;30(3):293–298. doi:10.1016/S1357-2725(97)00128-3
69. Carrier Y, Yuan J, Kuchroo VK, et al. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol. 2007;178(1):179–185. doi:10.4049/jimmunol.178.1.179
70. Lee KM, Kim YK. The role of IL-12 and TGF-beta1 in the pathophysiology of major depressive disorder. Int Immunopharmacol. 2006;6(8):1298–1304. doi:10.1016/j.intimp.2006.03.015
71. Xiong Z, Zhou L, Chen J, et al. 产后抑郁与初乳中转化生长因子-β水平的关系:90例巢氏队列研究 [Association between postpartum depression and concentrations of transforming growth factor-beta in human colostrum: a nested cohort study]. Nan Fang Yi Ke Da Xue Xue Bao. 2022;42(9):1426–1430. Chinese. doi:10.12122/j.issn.1673-4254.2022.09.21
72. Schroder K. Interferon‐γ: an overview of signals, mechanisms and functions. J Leukocyte Biol. 2004;75(2):163–189. doi:10.1189/jlb.0603252
73. Zhang J, He H, Qiao Y, et al. Priming of microglia with IFN‐γ impairs adult hippocampal neurogenesis and leads to depression‐like behaviors and cognitive defects. Glia. 2020;68(12):2674–2692. doi:10.1002/glia.23878
74. Mihailova S, Ivanova-Genova E, Lukanov T, et al. A study of TNF-α, TGF-β, IL-10, IL-6, and IFN-γ gene polymorphisms in patients with depression. J Neuroimmunol. 2016;293:123–128. doi:10.1016/j.jneuroim.2016.03.005
75. Misener V, Gomez L, Wigg KG, et al. Cytokine Genes TNF, IL1A, IL1B, IL6, IL1RN and IL10, and childhood-onset mood disorders. Neuropsychobiology. 2008;58(2):71–80. doi:10.1159/000159775
76. Yim Y-K, et al. Electro-acupuncture at acupoint ST36 reduces inflammation and regulates immune activity in collagen-induced arthritic mice. J Affective Disorders. 2007;4(1):51–57.
77. Myint A-M, Leonard BE, Steinbusch HW, et al. Th1, Th2, and Th3 cytokine alterations in major depression. J Affective Disorders. 2005;88(2):167–173.
78. Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune‐related diseases. Ann N York Acad Sci. 2006;1069(1):62–76. doi:10.1196/annals.1351.006
79. Myint AM, Kim YK. Cytokine–serotonin interaction through IDO: a neurodegeneration hypothesis of depression. Med Hypotheses. 2003;61(5–6):519–525. doi:10.1016/s0306-9877(03)00207-x
80. Fang JF, Fang J-Q, Shao X-M, et al. Electroacupuncture treatment partly promotes the recovery time of postoperative ileus by activating the vagus nerve but not regulating local inflammation. Sci Reports. 2017;7(1):39801. doi:10.1038/srep39801
81. Sun Z, Wang X, Feng S, et al. A review of neuroendocrine immune system abnormalities in IBS based on the brain–gut axis and research progress of acupuncture intervention. Front Neurosci. 2023;17:934341. doi:10.3389/fnins.2023.934341
82. Irwin MR. Reciprocal regulation of the neural and innate immune systems. Bio Psychiatry. 2011;11(9):625–632.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.