Back to Journals » International Journal of General Medicine » Volume 17
Perioperative Risk Factors for Postoperative Pulmonary Complications After Minimally Invasive Esophagectomy
Authors Li X, Yu L , Fu M, Yang J, Tan H
Received 28 November 2023
Accepted for publication 5 February 2024
Published 15 February 2024 Volume 2024:17 Pages 567—577
DOI https://doi.org/10.2147/IJGM.S449530
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Xiaoxi Li, Ling Yu, Miao Fu, Jiaonan Yang, Hongyu Tan
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Anesthesiology, Peking University Cancer Hospital & Institute, Beijing, China
Correspondence: Hongyu Tan, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Anesthesiology, Peking University Cancer Hospital & Institute, #52 Fucheng Street, Haidian District, Beijing, 100142, China, Tel/Fax +8610-88196107, Email [email protected]
Background: Postoperative pulmonary complications (PPCs) are the most prevalent complication after esophagectomy and are associated with a worse prognosis. This study aimed to investigate the perioperative risk factors for PPCs after minimally invasive esophagectomy (MIE).
Methods: Seven hundred and sixty-seven consecutive patients who underwent McKeown MIE via thoracoscopy and laparoscopy were retrospectively studied. Patient characteristics, perioperative data, and postoperative complications were analyzed.
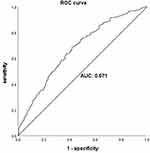
Results: The incidence of PPCs after MIE was 25.2% (193/767). Univariate analysis identified age (odds ratio [OR] 1.022, P = 0.044), male sex (OR 2.955, P < 0.001), pulmonary comorbidities (OR 1.746, P = 0.032), chronic obstructive pulmonary disease (COPD) (OR 2.821, P = 0.003), former smoking status (OR 1.880, P = 0.001), postoperative albumin concentration (OR 0.941, P = 0.007), postoperative creatinine concentration (OR 1.011, P = 0.019), and perioperative transfusion (OR 2.250, P = 0.001) as risk factors for PPCs. In multivariate analysis, the independent risk factors for PPCs were male sex (OR 3.135, P < 0.001), body mass index (BMI) (OR 1.088, P = 0.002), COPD (OR 2.480, P = 0.012), neoadjuvant chemoradiotherapy (OR 2.057, P = 0.035), postoperative albumin concentration (OR 0.929, P = 0.002), and perioperative transfusion (OR 1.939, P = 0.013). The area under the receiver operating characteristic curve for the predictive model generated by multivariate logistic regression analysis was 0.671 (95% confidence interval 0.628– 0.713).
Conclusions: Male sex, BMI, COPD, neoadjuvant chemoradiotherapy, postoperative albumin concentration, and perioperative transfusion were independent predictors of PPCs after MIE.
Keywords: esophageal cancer, minimally invasive esophagectomy, postoperative pulmonary complications, perioperative risk factors, predictive model
Introduction
Esophagectomy is associated with high risks of mortality and morbidity, particularly postoperative pulmonary complications (PPCs),1 which are strongly associated with increased durations of ventilation, intensive care, and hospitalization, and are reported to adversely affect overall survival.2–5 Recently, minimally invasive esophagectomy (MIE) has become a standard procedure for the surgical treatment of esophageal cancer. MIE is reportedly associated with significantly better short-term outcomes than open esophagectomy.6,7 Studies have also found a lower incidence of PPCs in patients undergoing MIE compared with open surgery.8–10 In a multi-center randomized controlled trial of patients undergoing esophagectomy, the incidence of a major pulmonary complication was 18% in the hybrid-procedure group and 30% in the open-procedure group.9 Additionally, MIE reportedly decreases the risk of respiratory failure after esophagectomy.11 However, PPCs remain a major concern after MIE, and the risk factors related to PPCs after MIE have not been fully investigated. Therefore, it would be beneficial for both patients and healthcare providers to identify the perioperative risk factors that influence the development of PPCs, especially regarding the best protective strategies during perioperative management of MIE to enhance clinical decision-making. The aim of this study was to assess the perioperative risk factors that influence the occurrence of PPCs after total MIE.
Materials and Methods
This study was approved by the Ethics Committee of Peking University Cancer Hospital, Beijing, China. The need for patient consent was waived by the ethics committee due to the retrospective study design. The data analyzed in the study were anonymized and the study was conducted in accordance with the Declaration of Helsinki. The study was registered in the Chinese Clinical Trial Registry (No. ChiCTR2300071822). Consecutive patients who were diagnosed with esophageal cancer and underwent McKeown MIE via thoracoscopy and laparoscopy between January 2016 and February 2023 were retrospectively studied. Patients with cervical esophageal cancer, esophageal cancer recurrence, an American Society of Anesthesiologists grade of greater than class III, a diagnosis of COVID-19 within 1 month prior to surgery or during hospitalization, unresectable tumors found during surgery, conversion of thoracoscopy or laparoscopy to an open procedure, unplanned resection of other organs, or a lack of complete case records were excluded from the study.
All patients were admitted to the same general ward and were perioperatively managed in accordance with the standard protocol for MIE. Patients routinely underwent chest physiotherapy and were nutritionally supported if required before surgery. All patients underwent general anesthesia or general anesthesia combined with regional anesthesia (intercostal nerve block/paravertebral nerve block/combined paravertebral nerve block and transversal plane block) managed by the same team of anesthesiologists specialized in thoracic anesthesia. Epidural anesthesia was not routinely used for total MIE in our institution and therefore was not included in the analysis. After establishing standard monitoring, anesthesia was induced intravenously with sufentanil/oxycodone, propofol/etomidate, and cisatracurium/rocuronium. Patients were intubated with either a single-lumen endotracheal tube, bronchial blocker, or double-lumen endobronchial tube based on the decision of the anesthesiologists and surgeons. In patients intubated with a single-lumen endotracheal tube, the thoracic procedure was completed with the facilitation of artificial pneumothorax. In patients intubated with a bronchial blocker or a double-lumen endobronchial tube, the thoracic procedure was completed under one-lung ventilation. The tidal volume was set at 4–6 mL/kg during the thoracic phase, and was set at 6 mL/kg during the remaining surgery. The respiratory rate was set at 12 to 20 breaths/minute and adjusted according to the end-tidal carbon dioxide and arterial blood gas analysis measurements. General anesthesia was maintained with sevoflurane, propofol, remifentanil, and cisatracurium/rocuronium. Neuromuscular blockade was reversed by neostigmine at the end of surgery. Neuromuscular monitoring was not routinely performed during surgery, but was monitored before extubation. Postoperative analgesia was managed with patient-controlled intravenous opioids. All surgeries were performed by one of three experienced thoracic surgeons from the same general ward, and consisted of MIE with either two-field or three-field lymphadenectomy. The thoracic procedures were performed under thoracoscopy in the left lateral decubitus position. The abdominal procedures were performed under laparoscopy in the supine position. Gastroesophageal anastomosis was performed in the neck. After surgery, patients were transferred to the post-anesthesia care unit and extubated after making a full recovery, and then transferred to the general ward; patients who required continued mechanical ventilation after surgery and patients with poor cardiopulmonary reserve were transferred to the intensive care unit (ICU). Postoperative care was managed by doctors and nurses on the same general ward or in the ICU. Perioperative transfusion was defined as the infusion of red blood cells and/or fresh frozen plasma intraoperatively or within 7 days postoperatively.
Patient demographic characteristics, tumor-specific characteristics, preoperative evaluations, surgical and anesthesia-related data, and postoperative complications during hospitalization were retrieved from the electronic medical records. Preoperative pulmonary comorbidities were defined as a history of chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis, pulmonary bulla, pulmonary tuberculosis, or pulmonary infection within 1 month before surgery. The primary outcome was the occurrence of PPCs by postoperative day 7, as most primary PPCs occur within the first postoperative week.12 PPCs were assessed using classification criteria adapted from the Clavien-Dindo classification regarding the respiratory system: grade I was defined as atelectasis requiring physiotherapy, grade II was defined as pneumonia treated with antibiotics on the ward, grade III was defined as the need for suction during bronchoscopy, grade IV was defined as respiratory failure requiring endotracheal or non-invasive ventilation/respiratory failure with failure of another organ, and grade V was defined as death of the patient. Patients with a grade of II or above were considered to have developed PPCs.13 The secondary outcomes were the occurrences of non-pulmonary complications including anastomotic leakage, cardiac complications (arrhythmia, myocardial infarction, and heart failure), wound infection, chylothorax, and recurrent laryngeal nerve injury during hospitalization. All complications were identified from the patients’ electronic medical records. The incidences of unplanned postoperative intubations, tracheostomies, prolonged ICU stay (> 2 days), or re-admission into the ICU during hospitalization were recorded. The length of hospital stay was also documented.
Sample Size
According to previous studies, the estimated incidence of PPCs after MIE is reported to be around 20%.14 For 10 or fewer independent predictors and a target number of events of more than 10 per variable analyzed in logistic regression, the required sample size was a minimum of 500 patients.15
Statistical Analysis
SPSS version 26.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Categorical variables were analyzed with the Pearson chi-squared test or Fisher’s exact test and reported as number (percentage). Continuous variables were analyzed with the independent-samples t-test (normally distributed data) or Mann–Whitney U-test (non-normally distributed data) and reported as mean ± SD or median (interquartile range). Univariate analyses using the forced entry method were performed to identify potential risk factors associated with PPCs. Covariates included in the multivariate logistic regression analysis were selected based on the results of the univariate analysis (factors with a p < 0.1) and previously reported risk factors in the literature. The receiver operating characteristic (ROC) curve was used to describe the discriminative abilities of the predictive model. The area under the curve was used as the quantitative index to describe the ROC curve. P < 0.05 was considered statistically significant.
Results
During the study period, a total of 838 patients were scheduled for MIE via thoracoscopy and laparoscopy; of these, 767 patients met the inclusion and exclusion criteria and were enrolled in the study. The overall incidence of PPCs was 25.2% (193/767). The demographic and perioperative characteristics are shown in Table 1.
 |
Table 1 Demographic and Perioperative Characteristics |
Compared with patients without PPCs (non-PPCs group), patients who developed PPCs (PPCs group) had significantly higher incidences of unplanned re-intubation (P < 0.001), tracheostomy (P < 0.001), re-admission to the ICU (P < 0.001), and prolonged ICU stay (P < 0.001). The PPCs group also had a higher incidence of non-pulmonary complications (P < 0.001), namely anastomotic leakage (P = 0.015), recurrent laryngeal nerve injury (P = 0.005), and cardiac complications (P < 0.001) (Table 2). The length of hospital stay was significantly longer in the PPCs group than the non-PPCs group (14.0 vs 13.0 days, P = 0.005).
 |
Table 2 Postoperative Data and Non-Pulmonary Complications |
The univariate logistic regression analysis results are shown in Table 3. The following variables were entered as covariates into the multivariable logistic regression analysis: age, sex, body mass index (BMI), pulmonary comorbidities, COPD, former smoking status, neoadjuvant chemoradiotherapy, fluid intake, blood loss, surgery duration, postoperative albumin concentration, postoperative creatinine concentration, and perioperative transfusion. The independent risk factors for PPCs were male sex (odds ratio [OR] 3.135, P < 0.001), high BMI (OR 1.088, P = 0.002), COPD (OR 2.480, P = 0.012), neoadjuvant chemoradiotherapy (OR 2.057, P = 0.035), low postoperative albumin concentration (OR 0.929, P = 0.002), and perioperative transfusion (OR 1.939, P = 0.013) (Table 4). The predictive model generated by multivariate logistic regression analysis included the factors of sex, BMI, COPD, neoadjuvant chemoradiotherapy, postoperative albumin concentration, and perioperative transfusion, and its predictive value was examined using the ROC curve. The area under the curve for the generated model was 0.671 (95% confidence interval 0.628–0.713) (Figure 1).
 |
Table 3 Risk Factors Associated with PPCs: Univariate Logistic Regression Analysis |
 |
Table 4 Independent Risk Factors Associated with PPCs: Multivariate Logistic Regression Analysis |
Discussion
In the present study, the overall incidence of PPCs was 25.2%, which was within the range reported in previous studies (18–32%).9,10,14 The PPCs group had significantly higher incidences of unplanned re-intubation and tracheostomy postoperatively than the non-PPCs group. The PPCs group also had a prolonged duration of ICU stay, higher incidence of re-admission to the ICU, and prolonged hospital stay, which may not only affect patient outcomes, but could also cause a high economic burden. Therefore, it is of great value to identify the possible predictive factors of PPCs after MIE to provide adequate perioperative monitoring and management, and appropriate prophylactic or treatment modalities. Previous studies have focused on conventional open esophagectomy, while few studies have investigated the risk factors for PPCs after MIE. In the present study, we focused on the predictive risk factors for PPCs after MIE via thoracoscopy and laparoscopy.
In general, preoperative respiratory comorbidities are reported as strong predictors of PPCs.3 In particular, a recent study found that COPD is an independent risk factor for PPCs after MIE.14 Similarly, COPD was found to be an independent risk factor for PPCs after MIE in the present study. However, because of the retrospective design, we only categorized the patients as having preoperative COPD or not, while the impact of different grades of COPD was not analyzed. A high BMI is a well-recognized patient-related risk factor associated with PPCs after esophagectomy.16 Obesity causes substantial changes to the mechanics of the lung and chest wall. The pulmonary function of patients with obesity is characterized by reduced respiratory system compliance, expiratory reserve volume, and functional residual capacity.17 Furthermore, a previous study reported that obesity is associated with increased respiratory complications after esophagectomy.16 In the present study, a high BMI was also revealed to be an independent risk factor for PPCs after MIE. Sex was the strongest patient-related predictor identified in the present study, with a threefold increased risk of PPCs in males. This result was consistent with a study that reported female sex as an overall protective factor against PPCs after abdominal surgery.18 Previous investigations of patients undergoing major abdominal surgery have also reported an association between male sex and postoperative pneumonia.19 One study found that male patients are five times more likely to develop postoperative pneumonia than female patients.20 It was demonstrated that cell-mediated immune responses after trauma are depressed in males.21 This might lead to the increased incidence of PPCs in males after surgical trauma.
A low postoperative serum albumin concentration was recognized as another independent risk factor for PPCs in the present study. This result was in accordance with an earlier study.22 Malnutrition is reported to be a significant predictor of postoperative pneumonia after general and digestive surgery, and perioperative nutritional support may prevent postoperative pneumonia in patients with malnutrition.23 A possible explanation for the association of the postoperative albumin concentration with the development of PPCs may be that hypoalbuminemia contributes to decreased plasma osmotic pressure, which may induce pulmonary interstitial edema. The independent patient-related risk factors for PPCs after MIE identified in the present study (such as sex and COPD) are non-adjustable, and the potentially modifiable factor BMI is unlikely to be altered in a short period of time. Therefore, the only improvable patient-related factor was a low postoperative albumin concentration. Physicians should be vigilant when treating patients with multiple patient-related risk factors identified in the current study, as they might have a higher risk of developing PPCs after MIE. Efforts to prevent PPCs should be actively pursued; in particular, malnutrition should be corrected to maintain an adequate albumin concentration.
Studies have shown a relationship between neoadjuvant therapy and a worse prognosis after esophagectomy. A meta-analysis involving 2,311 patients with esophageal cancer found an apparent increase in treatment-related mortality in patients who received neoadjuvant treatment, mainly in patients who received neoadjuvant chemoradiotherapy.24 A recent retrospective study using propensity-score matching found a higher incidence of pneumonia and pleural effusion in patients treated with neoadjuvant combined immunotherapy and chemotherapy.25 Additionally, salvage esophagectomy after definitive chemoradiotherapy is an independent factor associated with the occurrence of PPCs.26 However, other studies have reported contradictory results.11,27 Therefore, it remains unclear whether neoadjuvant treatment has a negative impact on PPCs. In the present study, multivariate analysis revealed that PPCs after MIE were associated with pretreatment neoadjuvant chemoradiotherapy, but not neoadjuvant chemotherapy. Further investigation is needed to better understand the influence of neoadjuvant chemoradiotherapy on PPCs after MIE. However, current studies have also reported the benefits of neoadjuvant pretreatment, such as a higher complete resection rate, an improved 3-year survival rate, and reduced local-regional cancer recurrences, which might outweigh the possible disadvantages of increased short-term complications.28,29 In addition, although one study found a higher incidence of PPCs in patients with pre-surgical neoadjuvant treatment, there were no differences regarding postoperative hospital stay, hospital cost, and 30-day mortality.25 Therefore, we speculate that although neoadjuvant chemoradiotherapy is associated with the development of PPCs, it may not be detrimental to the patients’ overall outcomes.
In the present study, we investigated the impact of perioperative transfusion of red blood cells and/or fresh frozen plasma, as lung injury can occur following the transfusion of any type of blood product. The pre- and postoperative hemoglobin concentrations and the intraoperative blood loss volume were comparable between patients who developed PPCs and those who did not. However, patients with PPCs had a higher incidence of perioperative transfusion. Multivariate analysis revealed that perioperative transfusion was an independent risk factor for PPCs, and was associated with a nearly twofold increased risk of PPCs after MIE. One study reported that transfusion is an independent procedural risk factor for PPCs.30 Furthermore, a previous meta-analysis of 3,659 patients investigating transfusion and postoperative lung injury found evidence of a strong association between perioperative transfusion of blood products and increased risk of postoperative ARDS.31 Similarly, other investigations have also recognized a relationship between perioperative transfusion and postoperative pulmonary infection.22 This finding might be related to the fact that allogeneic blood transfusion induces inflammation, immunosuppression, and predisposes patients to postoperative infection.32,33 A recent retrospective study found that blood cell transfusion has an OR of 2.02 for the development of postoperative complications in patients undergoing MIE.34 In the present study, the PPCs group also had higher incidences of non-pulmonary complications than the non-PPCs group. Therefore, it is unclear whether the risk of development of PPCs was caused by transfusion or was related to a higher incidence of non-pulmonary complications. However, we consider it more likely that the association between perioperative transfusion and PPCs was related to other aspects, such as the severity of illness or complexity of treatments (for non-pulmonary complications), which resulted in an increased requirement for transfusion. Nevertheless, based on our results and previous findings, clinicians should carefully consider the risks versus the benefits regarding the use of blood products, and should avoid unnecessary transfusions.
Our results revealed that patients with PPCs also had significantly higher incidences of non-pulmonary complications such as anastomotic leakage, recurrent laryngeal nerve injury, and cardiac complications compared with patients without PPCs. The pathophysiology of recurrent laryngeal nerve injury and PPCs are linked. Patients with recurrent laryngeal nerve injury might have resultant vocal cord dysfunction, manifested clinically by hoarseness, ineffective cough, dysphagia, and aspiration. There is a reported correlation between recurrent laryngeal nerve palsy and pneumonia after MIE,35 and recurrent laryngeal nerve paralysis is significantly associated with PPCs and the requirements for tracheostomy and mechanical ventilation.36,37 Similarly, atrial fibrillation is also frequently associated with PPCs.38 However, anastomotic leakage is reportedly a predictive factor for secondary pulmonary complications.12 Leaks are more likely to result in patients with severe illness, which also makes those patients more inclined to develop PPCs. However, the causal relationship between these non-pulmonary complications and PPCs was not evaluated in our study. Despite this, unlike the non-adjustable risk factors, physicians could strive to prevent potential procedural risk factors, such as recurrent laryngeal nerve injury, through improvements in surgical techniques and early interventions to minimize their impact on the respiratory system.
Limitations
This study has several limitations. First, this was a retrospective study and the results may have been affected by potential bias, such as variability in standard practice among clinicians. Information related to perioperative care may also be biased by the experiences and preferences of different clinicians, and by the time-related changes in medical strategies, which is unavoidable given the nature of retrospective analyses. Second, intraoperative ventilator parameters such as tidal volume and positive end-expiratory pressure were not recorded because of the retrospective design. These data may affect PPCs. Third, only the short-term results during hospitalization were analyzed. It is possible that postoperative non-pulmonary complications may have occurred after hospital discharge. Therefore, the incidence of postoperative non-pulmonary complications may be underestimated. Finally, this study was conducted in a single institution and our findings may not be generalizable. Multi-center, randomized controlled trials are warranted.
Conclusions
Male sex, high BMI, COPD, neoadjuvant chemoradiotherapy, low postoperative albumin concentration, and perioperative transfusion were independent predictors of PPCs after MIE. Physicians should carefully monitor patients with these risk factors for the development of PPCs in the clinical setting. Malnutrition after surgery should be corrected in a timely manner to maintain an adequate albumin concentration. Furthermore, the risks and benefits should be weighed carefully with regard to the use of perioperative blood products.
Abbreviations
PPCs, postoperative pulmonary complications; MIE, minimally invasive esophagectomy; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; ROC, receiver operating characteristic; BMI, body mass index; OR, odds ratio.
Data Sharing Statement
The study datasets are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The study was approved by the Institutional Review Board at Peking University Cancer Hospital (No. 2023YJZ36). The requirement for written informed consent was waived by the Institutional Review Board because of the retrospective study design. The data analyzed in the study were anonymized and the study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We thank Kelly Zammit, BVSc, from Liwen Bianji (Edanz) (http://www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking complications associated with esophagectomy. Ann Surg. 2019;269(2):291–298. doi:10.1097/SLA.0000000000002611
2. Booka E, Takeuchi H, Nishi T, et al. The impact of postoperative complications on survivals after esophagectomy for esophageal cancer. Medicine. 2015;94(33):e1369. doi:10.1097/MD.0000000000001369
3. Tanaka K, Yamasaki M, Kobayashi T, et al. Postoperative pneumonia in the acute phase is an important prognostic factor in patients with esophageal cancer. Surgery. 2021;170(2):469–477. doi:10.1016/j.surg.2021.03.051
4. Schlottmann F, Patti MG. Prevention of postoperative pulmonary complications after esophageal cancer surgery. J Thorac Dis. 2019;11(Suppl 9):S1143–s1144. doi:10.21037/jtd.2019.04.57
5. Deana C, Vetrugno L, Bignami E, Bassi F. Peri-operative approach to esophagectomy: a narrative review from the anesthesiological standpoint. J Thorac Dis. 2021;13(10):6037–6051. doi:10.21037/jtd-21-940
6. Dyas A R, Stuart C M, Bronsert M R, Schulick R D, McCarter M D and Meguid R A. (2023). Minimally invasive surgery is associated with decreased postoperative complications after esophagectomy. The Journal of Thoracic and Cardiovascular Surgery, 166(1), 268–278. 10.1016/j.jtcvs.2022.11.026
7. Brown AM, Pucci MJ, Berger AC, et al. A standardized comparison of peri-operative complications after minimally invasive esophagectomy: Ivor Lewis versus McKeown. Surg Endosc. 2018;32(1):204–211. doi:10.1007/s00464-017-5660-4
8. Souche R, Nayeri M, Chati R, et al. Thoracoscopy in prone position with two-lung ventilation compared to conventional thoracotomy during Ivor Lewis procedure: a multicenter case-control study. Surg Endosc. 2020;34(1):142–152. doi:10.1007/s00464-019-06742-w
9. Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med. 2019;380(2):152–162. doi:10.1056/NEJMoa1805101
10. van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg. 2019;269(4):621–630. doi:10.1097/SLA.0000000000003031
11. Zingg U, Smithers BM, Gotley DC, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann Surg Oncol. 2011;18(5):1460–1468. doi:10.1245/s10434-010-1474-5
12. Fang W, Kato H, Tachimori Y, Igaki H, Sato H, Daiko H. Analysis of pulmonary complications after three-field lymph node dissection for esophageal cancer. Ann Thorac Surg. 2003;76(3):903–908. doi:10.1016/S0003-4975(03)00549-6
13. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
14. Ohi M, Toiyama Y, Omura Y, et al. Risk factors and measures of pulmonary complications after thoracoscopic esophagectomy for esophageal cancer. Surg Today. 2019;49(2):176–186. doi:10.1007/s00595-018-1721-0
15. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi:10.1016/S0895-4356(96)00236-3
16. Healy LA, Ryan AM, Gopinath B, Rowley S, Byrne PJ, Reynolds JV. Impact of obesity on outcomes in the management of localized adenocarcinoma of the esophagus and esophagogastric junction. J Thorac Cardiovasc Surg. 2007;134(5):1284–1291. doi:10.1016/j.jtcvs.2007.06.037
17. Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755–767. doi:10.1080/17476348.2018.1506331
18. Yang CK, Teng A, Lee DY, Rose K. Pulmonary complications after major abdominal surgery: national surgical quality improvement program analysis. J Surg Res. 2015;198(2):441–449. doi:10.1016/j.jss.2015.03.028
19. Kawasaki K, Yamamoto M, Suka Y, et al. Development and validation of a nomogram predicting postoperative pneumonia after major abdominal surgery. Surg Today. 2019;49(9):769–777. doi:10.1007/s00595-019-01796-8
20. Mohri Y, Tonouchi H, Miki C, Kobayashi M, Kusunoki M. Incidence and risk factors for hospital-acquired pneumonia after surgery for gastric cancer: results of prospective surveillance. World J Surg. 2008;32(6):1045–1050. doi:10.1007/s00268-008-9534-8
21. Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14(2):81–90. doi:10.1097/00024382-200014020-00001
22. Li S, Su J, Sui Q, Wang G. A nomogram for predicting postoperative pulmonary infection in esophageal cancer patients. BMC Pulm Med. 2021;21(1):283. doi:10.1186/s12890-021-01656-7
23. Baba H, Tokai R, Hirano K, et al. Risk factors for postoperative pneumonia after general and digestive surgery: a retrospective single-center study. Surg Today. 2020;50(5):460–468. doi:10.1007/s00595-019-01911-9
24. Kaklamanos IG, Walker GR, Ferry K, Franceschi D, Livingstone AS. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta-analysis of randomized clinical trials. Ann Surg Oncol. 2003;10(7):754–761. doi:10.1245/ASO.2003.03.078
25. Hong ZN, Zhang Z, Chen Z, et al. Safety and feasibility of esophagectomy following combined neoadjuvant immunotherapy and chemotherapy for locally advanced esophageal cancer: a propensity score matching. Esophagus. 2022;19(2):224–232. doi:10.1007/s10388-021-00899-x
26. Yoshida N, Watanabe M, Baba Y, et al. Risk factors for pulmonary complications after esophagectomy for esophageal cancer. Surg Today. 2014;44(3):526–532. doi:10.1007/s00595-013-0577-6
27. Sihag S, Ku GY, Tan KS, et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg. 2021;161(3):836–843.e1. doi:10.1016/j.jtcvs.2020.11.106
28. Shen D, Chen Q, Wu J, Li J, Tao K, Jiang Y. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol. 2021;12(1):1–10. doi:10.21037/jgo-20-599
29. Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185(6):538–543. doi:10.1016/S0002-9610(03)00066-7
30. Kaufmann KB, Baar W, Glatz T, et al. Epidural analgesia and avoidance of blood transfusion are associated with reduced mortality in patients with postoperative pulmonary complications following thoracotomic esophagectomy: a retrospective cohort study of 335 patients. BMC Anesthesiol. 2019;19(1):162. doi:10.1186/s12871-019-0832-5
31. Serpa Neto A, Juffermans NP, Hemmes SNT, et al. Interaction between peri-operative blood transfusion, tidal volume, airway pressure and postoperative ARDS: an individual patient data meta-analysis. Ann Transl Med. 2018;6(2):23. doi:10.21037/atm.2018.01.16
32. Xu X, Zhang Y, Gan J, Ye X, Yu X, Huang Y. Association between perioperative allogeneic red blood cell transfusion and infection after clean-contaminated surgery: a retrospective cohort study. Br J Anaesth. 2021;127(3):405–414. doi:10.1016/j.bja.2021.05.031
33. Suzuki G, Ichibayashi R, Masuyama Y, et al. Association of red blood cell and platelet transfusions with persistent inflammation, immunosuppression, and catabolism syndrome in critically ill patients. Sci Rep. 2022;12(1):629. doi:10.1038/s41598-021-04327-z
34. Deana C, Vetrugno L, Stefani F, et al. Postoperative complications after minimally invasive esophagectomy in the prone position: any anesthesia-related factor? Tumori. 2021;107(6):525–535. doi:10.1177/0300891620979358
35. Oshikiri T, Takiguchi G, Hasegawa H, et al. Postoperative recurrent laryngeal nerve palsy is associated with pneumonia in minimally invasive esophagectomy for esophageal cancer. Surg Endosc. 2021;35(2):837–844. doi:10.1007/s00464-020-07455-1
36. Yang Y, Li B, Xu X, et al. Short-term and long-term effects of recurrent laryngeal nerve injury after robotic esophagectomy. Eur J Surg Oncol. 2023;49(10):107009. doi:10.1016/j.ejso.2023.107009
37. Koyanagi K, Igaki H, Iwabu J, Ochiai H, Tachimori Y. Recurrent laryngeal nerve paralysis after esophagectomy: respiratory complications and role of nerve reconstruction. Tohoku J Exp Med. 2015;237(1):1–8. doi:10.1620/tjem.237.1
38. Stawicki SP, Prosciak MP, Gerlach AT, et al. Atrial fibrillation after esophagectomy: an indicator of postoperative morbidity. Gen Thorac Cardiovasc Surg. 2011;59(6):399–405. doi:10.1007/s11748-010-0713-9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.