Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Perioperative and Postoperative Complications of Supraclavicular, Ultrasound-Guided, Totally Implantable Venous Access Port via the Brachiocephalic Vein in Adult Patients: A Retrospective Multicentre Study
Authors Yu Z , Sun X , Bai X, Ding W, Wang W , Xu L, Qin W, Wen L, Jin Y
Received 21 November 2020
Accepted for publication 20 January 2021
Published 4 February 2021 Volume 2021:17 Pages 137—144
DOI https://doi.org/10.2147/TCRM.S292230
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Zepeng Yu,1,* Xingwei Sun,1,* Xuming Bai,1 Wei Ding,2 WeiDong Wang,2 Liang Xu,3 Wenming Qin,4 Ling Wen,5 Yong Jin1
1Department of Intervention, The Second Affiliated Hospital of Soochow University, Suzhou, 215004, Jiangsu, People’s Republic of China; 2Department of Intervention, Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi, 214023, Jiangsu Province, People’s Republic of China; 3Department of General Surgery, Changshu Hospital Affiliated to Nanjing University of Chinese Medicine, Changshu, 215500, Jiangsu, People’s Republic of China; 4Department of Anesthesiology, Bazhong Central Hospital, Bazhong, Sichuan, 636000, People’s Republic of China; 5Department of Radiology, The First Affiliated Hospital of Soochow University, Suzhou, 215004, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yong Jin
Department of Intervention, The Second Affiliated Hospital of Soochow University, Suzhou, 215004, Jiangsu, People’s Republic of China
Tel +86 13776097707
Email [email protected]
Ling Wen
Department of Radiology, The First Affiliated Hospital of Soochow University, Suzhou, 215004, Jiangsu, People’s Republic of China
Email [email protected]
Purpose: The totally implantable venous access port (TIVAP) provides patients with safe, effective and long-term convenient venous access for the administration of medications such as chemotherapy drugs. The implantation and long-term use of TIVAP are related to thrombosis, infection and other complications. In this study, the medical records of multicentre patients were collected, and the perioperative and postoperative complications were retrospectively analysed to objectively evaluate the safety of the implantation of supraclavicular, ultrasound-guided TIVAP via the brachiocephalic vein (BCV).
Patients and Methods: We retrospectively analysed the clinical data of 433 adult patients who had undergone ultrasound-guided TIVAP implantation via the BCV at four hospitals in China from March 2018 to May 2019. The success rates of the first puncture, operation time, and perioperative and postoperative complications were analysed.
Results: All the TIVAPs were implanted successfully (100%). The average TIVAP carrying time was 318.15 ± 44.22 days (range: 38– 502 days) for a total of 197,694 catheter days. The success rate of the first puncture was 94.92% (411/433), and the average operation time was 29.66 ± 7.45 min (range: 18– 60 min). The perioperative complications included arterial puncture in 4 patients and pneumothorax in 1 patient. The incidence of postoperative complications was 5.08% (22/433), including poor incision healing (n = 2), catheter-related infection (n = 3), port infection (n = 6), thrombosis (n = 2) and fibrin sheath formation (n = 8). Another patient had infusion disturbance 2 days after the operation, and chest X-ray showed bending at the connection between the catheter and port. No other serious complications occurred, such as catheter rupture and drug leakage. The total incidence of complications was 6.24% (27/433).
Conclusion: This study showed excellent tolerance of supraclavicular, ultrasound-guided BCV puncture to implant TIVAP and a low incidence of complications. As a safe and effective method of TIVAP implantation, it can provide a new choice for clinicians.
Keywords: complications, totally implantable venous access ports, ultrasound-guided, brachiocephalic vein
Introduction
The totally implantable venous access port (TIVAP) is a completely closed central venous infusion system that is buried under the skin and can be retained in the body for a long time.1 The TIVAP comprises a piercing injection port and a central venous catheter system. It is frequently used for the infusion of various materials, such as chemotherapeutic drugs, fluid supplements, and parenteral nutrition support.2–5 Compared with the peripherally inserted central catheter (PICC) or central venous catheter (CVC), the TIVAP has many advantages and is more readily accepted by patients because of its long service life, ease of nursing, and lack of impact on the quality of life.6–12 TIVAP has been widely used in the clinic in recent years, and clinicians have gradually observed a series of clinical complications, such as arterial puncture, pneumothorax, thrombosis, and catheter rupture.6 These complications not only cause additional pain to patients but also delay treatment and even endanger patients’ lives. Thus, clinicians have focused more on these complications.
Many methods have been reported to implant TIVAPs into patients, among which the subclavian vein (SCV) and internal jugular vein (IJV) approaches are the two most commonly used clinically.2,6,13 However, few studies have investigated the brachiocephalic vein (BCV) as a central venous access in adult patients. In recent years, with the advances in the application of ultrasound technology, ultrasound-guided TIVAP implantation via the BCV has also been used clinically. However, retrospective multicentre studies are lacking regarding its associated complications and safety. In this study, we collected the clinical data of 433 adult patients who had undergone TIVAP implantation through ultrasound-guided BCV puncture at four hospitals in China. We retrospectively analysed the perioperative and postoperative complications to objectively evaluate the safety of supraclavicular, ultrasound-guided TIVAP implantation using the BCV approach.
Patients and Methods
From March 2018 to May 2019, the clinical data of patients who had undergone ultrasound-guided TIVAP via the BCV were collected from four Chinese hospitals. The operation process, use and maintenance were recorded in detail, and, excluding patients with incomplete data and who were lost to follow-up, 433 patients were included in this study (236 male and 197 female, aged 23 to 91 years; mean age: 60 ± 11 years).
This study was approved by the Ethics Committee of The Second Affiliated Hospital of Soochow University, and all the patients signed informed consent forms. The operating procedures were carried out in accordance with the relevant guidelines and regulations. The TIVAP used was purchased from BARD Medical (BardPort,8,806,061, 6F; UT, USA) and B. BRAUN Medical (B. BRAUN, 04436946, 6.5F; France). The operation was performed by two trained interventional doctors.
Implantation Procedures
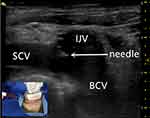
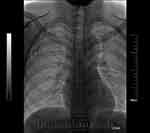
Each patient should show favourable outcomes on preoperative blood routine, blood coagulation and other related tests; if necessary, correct blood coagulation by the transfusion of platelets was performed for those with poor blood coagulation function (INR ≥ 2; platelets < 50×109). The operation was performed according to standard aseptic procedures. During the operation, the patient was placed in the supine position, with the head turned to the opposite side, and the posterior foot of the sternocleidomastoid muscle was exposed. Routine disinfection and towel laying were performed within 15 cm of the surgical incision. Under ultrasound guidance, the puncture point was anaesthetized with lidocaine diluted by 5 mL, and then BCV puncture was performed (Figure 1). The guide wire was placed after successful venepuncture, and it was confirmed by fluoroscopy that the guide wire entered the superior vena cava. A 5-mm incision was made at the puncture site, the detachable sheath was introduced through the guide wire, and the catheter was introduced through the sheath. A 3-cm skin incision was made at 2 transverse fingers below the clavicle on the ipsilateral side, and the subcutaneous tissue was bluntly separated to make a pouch. The pouch, located at the subcutaneous depth of 0.5–1.0 cm, should not exceed the superficial fascia of the pectoralis major muscle, and the size should be sufficient to accommodate the body of the port. A subcutaneous tunnel was made with a subcutaneous tunnel needle to guide the catheter to connect with the port body through the subcutaneous tunnel. Under the fluoroscopy method of digital subtraction angiography (DSA), we confirmed that the end of the catheter was placed at the junction between the superior vena cava and right atrium (Figure 2). The catheter was cut with scissors vertically in the appropriate position to connect the catheter and port. The port was positioned in the pouch and properly fixed, and the incision was sutured after local haemostasis. A non-invasive needle was punctured into the port body; blood was drawn unobstructed, and normal saline was injected to confirm the absence of an exudate. The incision was covered with sterile gauze after disinfection, and butterfly-shaped harmless needles and dressings were properly fixed. The entire X-ray image of the TIVAP was retained to ensure no sharp angle of the catheter and that the position of the end of the catheter was good (Figure 2).
Because the thoracic duct converges into the BCV at the confluence of SCV and IJV on the left, the right BCV approach was used to avoid lymphatic leakage caused by the thoracic duct injury. When the right BCV puncture failed, the left BCV was selected. Every time TIVAP was utilized, the skin at the port was disinfected with Iodophor. Before the infusion of any drug, the nurse flushed the catheter with 10 mL of 50~100 IU/mL of heparin saline or saline to detect catheter obstruction or subcutaneous leakage, and immediately flushed again after completing each drug infusion. The clinical data were recorded, including the times of puncture, operation time, and management of complications.
The first successful puncture indicates that the puncture needle entered the BCV, the guide wire and catheter were introduced smoothly, and there was no secondary skin puncture.
Catheter-related infection indicates that the patient developed fever, chills and blood culture was positive (usually Staphylococcus aureus) after the TIVAP was used.
Port-related infection indicates that the skin of the port body appeared to be broken, red and swollen, and bacterial infection was considered.
Results
In total, 433 patients had undergone ultrasound-guided totally TIVAP via the BCV with a success rate of 100%. The success rate of the first puncture was 94.92% (411/433); 18 patients were successful in the second puncture, and 4 patients were successful in the third puncture (Table 1). Among them, 15 patients had undergone left BCV puncture after the failure of right BCV puncture, and no serious complications occurred, such as chylothorax. The average operation time was 29.66 ±7.45 min (range: 18–60 min). The incidence of puncture-related complications was 1.15% (5/433). The SCA was accidentally punctured in four patients (0.92%), among whom 2 patients were subjected to the left BCV approach; finally, the BCV was punctured successfully. One case (0.23%) developed a small amount of pneumothorax due to accidental puncture of the pleura, and the second puncture of the right BCV was successful. The patient did not receive special treatment because he had shown no serious clinical symptoms (Table 2). The average TIVAP time was 318.15 ±44.22 days (range: 38–502 days). The incidence of postoperative complications was 5.08% (22/433). There were two cases of delayed incision healing, 3 cases of catheter-related infection, 6 cases of port-related infection, 2 cases of thrombosis and 8 cases of fibrin sheath formation. One patient had infusion disturbance 2 days after the operation, and chest X-ray revealed bending at the connection between the catheter and port. However, no other serious complications, such as catheter rupture and drug leakage, occurred during the follow-up. Unplanned removal of the TIVAP was caused by postoperative complications in 14 patients.
 |
Table 1 Details of the US-Guided BCV Puncture for TIVADs (N = 433) |
 |
Table 2 Incidence of Perioperative and Postoperative Complications and Processing Measures |
Discussion
In this study, we found that ultrasound-guided TIVAP implantation via the BCV not only has a high success rate of the first puncture but also has few perioperative and postoperative complications, improving the efficiency of TIVAP and providing a reliable implantation method that clinicians can choose.
Compared with the traditional method of blind puncture using clinical landmarks, ultrasound-guided percutaneous puncture can significantly improve the success rate of the first puncture, reduce puncture-related complications, and avoid the pain of repeated puncture.14,15 Although percutaneous IJV and SCV cannulation are the two most commonly used methods clinically, the TIVAP, as an invasive operation, will cause related complications during puncture and long-term retention in the body, and the risk of complications is closely related to the different implantation methods. The percutaneous IJV and SCV approaches usually lead to certain clinical complications, such as arterial puncture, pneumothorax, and ectopic catheters.16
Due to the superficial position and thin vascular wall of the BCV, under real-time ultrasound guidance, the BCV lumen remains open regardless of the haemodynamics and respiratory status and rarely overlaps with the carotid artery or brachiocephalic artery.17,18 The puncture site is distant from the nasobuccal area, reducing the probability of bacterial contamination in the nasopharynx. Additionally, Brass et al,19 found that clinicians can clearly identify different blood vessels and determine whether they are unobstructed under ultrasound guidance compared with blind puncture using clinical landmarks, thus reducing the overall incidence of complications by 71%, the incidence of arterial puncture by 72%, the time required for successful cannulation by 30.52 seconds, and the probability of successful cannulation for the first time by 57%. Many clinical guidelines also advocate central venous cannulation under the real-time ultrasound guidance.20,21
The IJV is close to the common carotid artery and to the top of the pleura and tip of the lung behind the sternoclavicular joint, and no reliable clinical landmarks are available. These factors lead to accidental injury to the common carotid artery and top of the pleura during puncture, resulting in cervical haematoma, pneumothorax, haemothorax and other complications.22 The puncture point of the IJV is high, causing the catheter to be folded back 180 degrees before it can connect to the TIVAP of the anterior upper chest wall, increasing the risk of ectopia, bending, blockage, and even fracture of the catheter.23,24 Compared with BCV, the path of IJV cannulation is longer. Both the high puncture point and long catheter path will lead to a significant decrease in comfort after cannulation, thereby increasing the risk of unplanned removal of the TIVAP, which not only causes pain to the patient but also affects the treatment of the disease. TIVAP implantation via the BCV approach was approximately 62% less likely to be operationally difficult than the IJV approach described by Beccaria et al.11
The SCV is just anterior to the subclavian artery. In the inner 1/3 segment of the clavicle, the top of the pleura and tip of the lung is behind the subclavian vein, a situation that can easily lead to cervical haematoma, pneumothorax and haemothorax as percutaneous SCV puncture.9,25 In one study, 2620 patients had undergone the percutaneous SCV method, and the risk of catheter rupture was as high as 2.56%, suggesting that the pinch-off syndrome (POS) is a high-risk factor of catheter rupture, which can lead to pulmonary embolism.23 An explanation may be that, when using the SCV approach, the catheter is placed between the clavicle and first rib and pressing the catheter for a long time causes POS.23 Additionally, the vascular path to the junction of the superior vena cava and right atrium is straight in the BCV approach; however, the connection between the SCV and superior vena cava is an acute angle.26 This difference in anatomical structure can easily lead to increased ectopic catheters when the percutaneous SCV approach is used. If the ectopic catheter is not corrected, it can result in blockage and damage of the catheter and even rupture of blood vessels during use.
After the ipsilateral IJV and SCV converge behind the sternoclavicular joint to form the BCV, the diameter of the blood vessel increases significantly. Compared with the IJV and SCV, the anatomical position of the BCV is more fixed, the position is superficial and the diameter is larger, making it convenient for clinicians to puncture the BCV for TIVAP implantation. Additionally, using a strict in-plane approach and placing the ultrasound probe on the supraclavicular area, the best ultrasonic long-axis section of the BCV can be obtained. During puncture of the BCV, the doctor can observe the entire trajectory of the needle because the position of the BCV is not disturbed by the bone.7,8 Additionally, the route of the puncture needle is parallel to the pleura, decreasing the occurrence of pneumothorax.
Compared with the SCV approach, the BCV approach punctures above the clavicle, and the catheter moves across the top of the clavicle to avoid POS and catheter rupture. Additionally, the IJV, SCV and BCV form a special Y-shaped vascular anatomical structure.27 After the TIVAP was implanted using the BCV approach, the range of motion of the catheter changed little with the movement of the neck and upper limbs of the patient, effectively reducing the ectopic guide wire in the IJV and SCV. Ectopic catheters and catheter fractures were not observed in this study. Hyun-Jung Shin et al,6 reported ectopic catheters in both percutaneous IJV and SCV pathways under ultrasound guidance, and the ectopic catheter rate in the latter approach was as high as 5.9%. In this study, one patient had infusion disturbance on the second day after the operation, and chest X-ray showed catheter bending at the junction between the catheter and port that was related to the operation technique of the surgeon. Improper handling at the junction between the catheter and port, as well as the large bag made by the surgeon so that the port has a large range of motion and even rotation, may be important factors leading to catheter bending at the junction.2
The BCV approach is often reported in newborns because the diameter and length of the blood vessels of the IJV and SCV are too diminutive; thus, it is difficult to perform vascular puncture. Nevertheless, in the BCV approach in newborns, the left side is used more than the right side, with greater success.7,11,28 However, in adult patients, the left BCV is deeper than the right, the position change is also greater, and the ultrasound display is poor. Additionally, because the thoracic duct flows into the central vein through the left BCV, the right BCV is preferred to avoid chylous leakage caused by injury to the thoracic duct.29 However, in 72 patients who had undergone left BCV cannulation, thoracic duct injury was not observed.11 In our study, 15 patients were implanted with the left BCV approach, 13 due to poor ultrasonography of the right BCV and 2 due to the failure of the right BCV puncture. None of the 15 patients had thoracic duct injury.
The thoracic duct30 is formed by the confluence of the left and right lumbar lymphatic trunks and intestinal lymphatic trunks, passing upward through the abdomen, chest and neck, collecting lymph from the left half of the body, abdomen and lower extremities, accounting for approximately 3/4 of the human body’s lymph and flowing into the angle of the left jugular vein. The remaining 1/4 of the lymph is collected by the right lymphatic duct and flows into the right venous angle. The diameter of the thoracic duct is only approximately 2 mm, the wall of the thoracic duct is thin and transparent, the shape is tortuous, the confluence point varies greatly, and the ultrasound display is weak.31 Injury of the thoracic duct can lead to chylothorax, malnutrition, impaired immune function, respiratory function damage and even death. Although it can be conservatively treated by diet control, most thoracic duct ruptures require further surgical treatment.32 Therefore, the right BCV approach is preferred in our study.
All 433 patients had undergone successful implantation of the TIVAP (100%). The success rate of the first puncture was 94.92% (411/433). The second puncture was successful in 18 patients (81.82%; 18/22), and the third puncture was successful in 4 patients (100%; 4/4) (Table 1). The central venous catheter (CVC) is very helpful to diagnose and treat patients, but there is a risk of arterial puncture and pneumothorax during cannulation; thus, the number of punctures should be reduced as much as possible. If the third puncture is not successful, we will use ultrasound to evaluate and puncture the right IJV or SCV; however, our study showed that the 433 patients were successfully punctured via the BCV. Compared with the success rate of the first puncture (86%; 611/709) via the IJV approach under ultrasound guidance reported by Beccaria et al,11 we obviously showed a greater advantage (94.92% (411/433)). The cause may be related to the larger diameter of the BCV than those of the IJV and SCV, and the superficial position of BCV, which guarantee successful puncture and explain why BCV is first used in central venous cannulation in infants.7,33
In this study, the incidence of puncture-related complications was 1.15% (5/433). The subclavian artery was accidentally punctured in 4 cases, among which one had undergone a second right BCV puncture and another case had undergone a third right BCV puncture; both procedures were successful. In the other two cases, due to the formation of local subcutaneous haematoma, the second puncture of the left BCV was also successful after evaluation by ultrasound. The incidence of artery puncture complications was 0.92% (4/433), it is within the complication threshold outlined in SIR guidelines.5 We think that TIVAP implantation via the BCV approach under ultrasound guidance is safer than other approaches, experienced doctors have a lower incidence of accidental arterial puncture. One case (0.23%) developed a small amount of pneumothorax due to accidental puncture of the pleura, and a second puncture of the right BCV was successful. The patient did not receive special treatment because he showed no serious clinical symptoms and other serious complications related to puncture did not occur. Breschan et al,7 reported that the success rate of ultrasound-guided TIVAP via the BCV in premature infants was 94%; accidental puncture (1%) of the right SCA occurred in one 2.1-kg infant, and no other serious complications related to puncture were found.
The postoperative complication rate was 5.08% (22/433; 0.111/1000 catheter-days) and the overall complication rate was 6.24% (27/433; 0.137/1000 catheter-days), which were lower than that of ultrasound-guided cannulation techniques of the IJV in the study of Tsuruta et al,2 (the postoperative complication rate of 9.1%; 0.201/1000 catheter-days) and Gebauer et al,34 (the overall complications accounted for 0.15 per 1000 catheter day).
Delayed incision healing occurred in 2 cases, and the skin of the incision was found to be broken in 1 case 2 weeks after the operation without swelling and exudation and healed well after debridement and suture. Another case had incision dehiscence with suppuration in the second month after operation and pain after constant pressing; the TIVAP was removed in advance after the failure of treatment with antibiotics. Skin ulcers caused by port-related infection were found in 6 patients, catheter-related infection occurred in 3 patients, and the TIVAP was removed in 6 patients after ineffective systemic antibiotics therapy. During the follow-up period, there were 9 cases of complications of port-related infection and catheter-related infection. In the total 197,694 catheter days, resulting in 0.045 infectious complications per 1000 catheter days in the total of 197,694 catheter days. However, the data reported by Gebauer et al,34 is as high as 0.15 per 1000 catheter days. The infectious complications were related to aseptic operation during operation and standardized nursing after operation. Careful operation, attention to catheter maintenance and regular care (once a month) are crucial factors to avoid unplanned TIVAP extraction.
The infusion was blocked in 2 patients due to thrombosis and in 8 patients due to fibrin sheath formation. The TIVAP was removed after ineffective thrombolytic therapy. Another patient had infusion disturbance 2 days after the operation, and chest X-ray showed bending at the connection between the catheter and port. However, no other serious complications occurred such as catheter rupture and drug leakage during follow-up. Finally, the TIVAP was removed in 14 patients (3.23%) ahead of schedule due to postoperative complications. The average TIVAP carrying time was 318.15 ± 44.22 days in this study (range: 35~521 days).
This study is a multicentre study, which strives to objectively evaluate the safety of supraclavicular, ultrasound-guided TIVAP via the BCV in adult patients. However, our study has some limitations. First, it is retrospective and non-random, and some useful information may be lost during the follow-up. Second, we did not study whether other factors such as the patient’s BMI and disease type influenced the occurrence of complications. Finally, we only studied TIVAP implantation via the BCV approach without comparing other commonly used venous approaches (IJV and SCV). Therefore, randomized controlled trials with large samples warrant further study.
Conclusion
In summary, supraclavicular, in-plane, real-time ultrasound-guided TIVAP implantation via the BCV is a safe and effective central venous cannulation method for adult patients. Our multicentre study revealed that the operation not only has a high success rate but also has low complications. Chemotherapy and parenteral nutrition can be provided for patients on the day of operation, and postoperative maintenance is simple and convenient.
Ethical Approval
The study was approved by the ethics committee of The Second Affiliated Hospital of Soochow University and consent to participate from the patient was available.
All procedures of this study were performed in accordance with the Declaration of Helsinki.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Tang TT, Liu L, Li CX, et al. Which is better for patients with breast cancer: Totally Implanted Vascular Access Devices (TIVAD) or Peripherally Inserted Central Catheter (PICC)? World J Surg. 2019;43(9):2245–2249. doi:10.1007/s00268-019-05022-x
2. Tsuruta S, Goto Y, Miyake H, et al. Late complications associated with totally implantable venous access port implantation via the internal jugular vein. Support Care Cancer. 2019;28:2761–2768. doi:10.1007/s00520-019-05122-3
3. Ignatov A, Hoffman O, Smith B, et al. An 11-year retrospective study of totally implanted central venous access ports: complications and patient satisfaction. Eur J Surg Oncol. 2009;35(3):241–246. doi:10.1016/j.ejso.2008.01.020
4. Biffi R, de Braud F, Orsi F, et al. Totally implantable central venous access ports for long-term chemotherapy. A prospective study analyzing complications and costs of 333 devices with a minimum follow-up of 180 days. Ann Oncol. 1998;9(7):767–773. doi:10.1023/A:1008392423469
5. Dariushnia SR, Wallace MJ, Siddiqi NH, et al. Quality improvement guidelines for central venous access. J Vasc Interv Radiol. 2010;21(7):976–981. doi:10.1016/j.jvir.2010.03.006
6. Shin HJ, Na HS, Koh WU, et al. Complications in internal jugular vs subclavian ultrasound-guided central venous catheterization: a comparative randomized trial. Intensive Care Med. 2019;45(7):968–976. doi:10.1007/s00134-019-05651-9
7. Breschan C, Graf G, Jost R, et al. A retrospective analysis of the clinical effectiveness of supraclavicular, ultrasound-guided brachiocephalic vein cannulations in preterm infants. Anesthesiology. 2018;128(1):38–43. doi:10.1097/ALN.0000000000001871
8. Sun X, Xu J, Xia R, et al. Efficacy and safety of ultrasound-guided totally implantable venous access ports via the right innominate vein in adult patients with cancer: single-centre experience and protocol. Eur J Surg Oncol. 2019;45(2):275–278. doi:10.1016/j.ejso.2018.07.048
9. Expert Panel on Interventional R, Shaw CM, Shah S, et al. ACR Appropriateness Criteria ((R)) radiologic management of central venous access. J Am Coll Radiol. 2017;14(11S):S506–S529.
10. Xia R, Sun X, Bai X, et al. Efficacy and safety of ultrasound-guided cannulation via the right brachiocephalic vein in adult patients. Medicine. 2018;97(50):e13661. doi:10.1097/MD.0000000000013661
11. Beccaria PF, Silvetti S, Lembo R, et al. The brachiocephalic vein as a safe and viable alternative to internal jugular vein for central venous cannulation. Anesth Analg. 2018;127(1):146–150. doi:10.1213/ANE.0000000000003357
12. Taxbro K, Hammarskjold F, Thelin B, et al. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth. 2019;122(6):734–741. doi:10.1016/j.bja.2019.01.038
13. Vezzani A, Manca T, Brusasco C, et al. A randomized clinical trial of ultrasound-guided infra-clavicular cannulation of the subclavian vein in cardiac surgical patients: short-axis versus long-axis approach. Intensive Care Med. 2017;43(11):1594–1601. doi:10.1007/s00134-017-4756-6
14. Ma LI, Liu Y, Wang J, Chang Y, Yu L. Totally implantable venous access port systems and associated complications: a single-institution retrospective analysis of 2996 breast cancer patients. Mol Clin Oncol. 2016;4(3):456–460. doi:10.3892/mco.2016.726
15. Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607–1612. doi:10.1097/CCM.0b013e318218a1ae
16. Ruesch S, Walder B, Tramèr, M R. Complications of central venous catheters: internal jugular versus subclavian access - A systematic review. Crit Care Med. 2002;30(2):454–460. doi:10.1097/00003246-200202000-00031
17. Breschan C, Graf G, Jost R, et al. A Retrospective Analysis of the Clinical Effectiveness of Supraclavicular, Ultrasound-guided Brachiocephalic Vein Cannulations in Preterm Infants. Anesthesiology. 2018;128(1):38–43.
18. Jordan JR, Moore EE, Haenel J. Ultrasound-guided supraclavicular access to the innominate vein for central venous cannulation. J Trauma Acute Care Surg. 2014;76(5):1328–1331. doi:10.1097/TA.0000000000000209
19. Brass P, Hellmich M, Kolodziej L, Schick G. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Systematic Rev. 2015.
20. Saugel B, Scheeren TWL, Teboul, J-L. Ultrasound-guided central venous catheter placement: a structured review and recommendations for clinical practice. Crit Care. 2017;21(1):225. doi:10.1186/s13054-017-1814-y
21. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the society of hospital medicine. J Hosp Med. 2019;14:E1–E22. doi:10.12788/jhm.3287
22. Jankovic RJ, Pavlovic MS, Stojanovic MM, et al. Risk factors associated with carotid artery puncture following landmark-guided internal jugular vein cannulation attempts. Med Princ Pract. 2011;20(6):562–566. doi:10.1159/000329788
23. Lin CH, Wu HS, Chan DC, Hsieh CB, Huang MH. The mechanisms of failure of totally implantable central venous access system: analysis of 73 cases with fracture of catheter. Eur J Surg Oncol. 2010;36(1):100–103. doi:10.1016/j.ejso.2009.07.011
24. Nagasawa Y, Shimizu T, Sonoda H, Chou H, Mekata E. Is catheter rupture rare after totally implantable access port implantation via the right internal jugular vein? Report of a case. Surg Today. 2014;44(7):1346–1349. doi:10.1007/s00595-013-0631-4
25. Kurul S, Saip P. Totally implantable venous-access ports: local problems and extravasation injury. Lancet Oncol. 2002;3(11):684–692.
26. Gibson F, Bodenham, A. Misplaced central venous catheters: applied anatomy and practical management. Br J Anaesth. 2013;110(3):333–346. doi:10.1093/bja/aes497
27. Aydin T, Balaban O, Koculu R. Where is the Guidewire? Confirmation of central catheter placement in the brachiocephalic vein using Y-shape visualization by ultrasound. Cureus. 2019;11(2):e4124.
28. Habas F, Baleine J, Milesi C, et al. Supraclavicular catheterization of the brachiocephalic vein: a way to prevent or reduce catheter maintenance-related complications in children. Eur J Pediatr. 2018;177(3):451–459. doi:10.1007/s00431-017-3082-x
29. Sun X, Bai X, Cheng L, et al. Comparison of ultrasound-guided right brachiocephalic and right subclavian vein cannulation in adult patients. J Ultrasound Med. 2019;38(10):2559–2564. doi:10.1002/jum.14947
30. Skandalakis JE, Skandalakis LJ, Skandalakis, P N. Anatomy of the lymphatics. Surg Oncol Clin N Am. 2007;16(1):1–16. doi:10.1016/j.soc.2006.10.006
31. Johnson OW, Chick JF, Chauhan NR, et al. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur Radiol. 2016;26(8):2482–2493. doi:10.1007/s00330-015-4112-6
32. Parvinian A, Mohan GC, Gaba RC, et al. Ultrasound-guided intranodal lymphangiography followed by thoracic duct embolization for treatment of postoperative bilateral chylothorax. Head Neck. 2014;36(2):E21–24. doi:10.1002/hed.23425
33. Breschan C, Platzer M, Jost R, et al. Consecutive, prospective case series of a new method for ultrasound-guided supraclavicular approach to the brachiocephalic vein in children. Br J Anaesth. 2011;106(5):732–737. doi:10.1093/bja/aer031
34. Gebauer B, El-Sheik M, Vogt M, Wagner H-J. Combined ultrasound and fluoroscopy guided port catheter implantation—High success and low complication rate. Eur J Radiol. 2009;69(3):517–522. doi:10.1016/j.ejrad.2007.10.018
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.