Back to Journals » Journal of Pain Research » Volume 13
Perioperative Analgesic Effects of Preemptive Ultrasound-Guided Rectus Sheath Block Combined with Butorphanol or Sufentanil for Single-Incision Laparoscopic Cholecystectomy: A Prospective, Randomized, Clinical Trial
Authors Fu H, Zhong C, Fu Y , Gao Y, Xu X
Received 8 March 2020
Accepted for publication 12 May 2020
Published 25 May 2020 Volume 2020:13 Pages 1193—1200
DOI https://doi.org/10.2147/JPR.S252952
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Huimin Fu,1 Chaochao Zhong,2 Yu Fu,1 Yongtao Gao,2 Xingguo Xu2
1Nantong University, Nantong, Jiangsu, People’s Republic of China; 2Department of Anesthesiology, Affiliated Hospital of Nantong University, Nantong, Jiangsu, People’s Republic of China
Correspondence: Xingguo Xu
Department of Anesthesiology, Affiliated Hospital of Nantong University, No. 20 Xisi Road, Nantong, Jiangsu 226001, People’s Republic of China
Tel +86 15162771638
Fax +86 051381160318
Email [email protected]
Purpose: Pain after single-incision laparoscopic cholecystectomy (SILC), especially visceral pain, often troubles patients and doctors. Whether preemptive butorphanol can relieve visceral pain in patients undergoing SILC remains unknown. The goal of this study was to assess the efficacy of ultrasound-guided bilateral rectus sheath block (RSB) and butorphanol for perioperative analgesia in patients undergoing SILC.
Patients and Methods: Fifty-eight patients who met the criteria were randomly divided into two groups, both of which were given preemptive RSB. Patients were given either butorphanol 0.02mg/kg (group B, n=29) or sufentanil 0.1 μg/kg (group S, n=29) as preemptive analgesia. The primary outcome was the cumulative frequency of rescue analgesic request within 24 hours after operation. Secondary outcomes were numeric rating scale (NRS) scores (from 0 to 10) of incisional pain and visceral pain, the length of hospital stay and the incidence of postoperative adverse events.
Results: The frequency of postoperative rescue analgesic request of group S was significantly higher than that of group B (P=0.021). The NRS scores for visceral pain were lower in group B at 2, 6 and 12 hours after surgery than in group S (both P< 0.001). The occurrence of postoperative nausea and vomiting (PONV) was significantly higher in group S. There were no significant differences between two groups for other outcomes.
Conclusion: Butorphanol can provide sufficient visceral pain treatment after SILC than the dose of sufentanil in equal analgesic effect.
Keywords: rectus sheath block, butorphanol, single-incision laparoscopic cholecystectomy, incisional pain, visceral pain, preemptive analgesia
Introduction
Currently, opioids are widely used for postoperative analgesia, and postoperative pain management has been suggested to be insufficient.1–4 First, opioids are associated with side effects, such as somnolence, postoperative nausea and vomiting (PONV), constipation, uroschesis, pruritus and respiratory depression, resulting in delayed discharge; 5 Second, even though opioid drugs are a primary choice for the management of patients experiencing severe visceral pain;6 Third, with the extensive use of opioids, more and more opioid tolerance and opioid-induced hyperalgesia have made severe effects. Sufentanil, as a kind of opioids, is a μ receptor agonist with highly fat solubility and easy to pass through the blood-brain barrier, yet it cannot produce adequate pain relief.7 If the acute postsurgical pain is not well treated, it may develop into chronic pain. To improve postoperative pain management, multimodal analgesic regimens that include regional block and non-steroidal anti-inflammatory drugs (NSAIDs) are increasingly used. With the application of ultrasound technology in analgesia, body pain can be controlled effectively, but visceral pain is not satisfactory.8 Visceral pain is a complex disorder, that can be caused by mechanical traction, dilation, spasm, inflammation, ischemia and chemical stimulation.9 Some studies have suggested that butorphanol, a κ-agonist, produces profound visceral analgesia.10 Schleich first used rectus sheath block (RSB) in 1899 to provide muscle relaxation and analgesia. Formerly, RSB was not extensively used because its non-visualization leads to a high incidence of complications, such as neurologic injury, inadvertent peritoneal injury, visceral trauma, and block failure. Nevertheless, with the introduction of ultrasound into regional anaesthesia practice, tissue planes, the bowel and the spread of local anaesthetics can be seen, which may decrease accidental puncture. RSB is mainly used for postoperative analgesia after abdominal surgery.11,12 Studies have shown that 10 mL of 0.5% ropivacaine is usually appropriate.13 Recently, an increasing number of studies have emphasized the clinical value of RSB for pain relief related to midline abdominal incisions and laparoscopic and umbilical surgery.14 The efficacy of RSB has been reported for postoperative analgesia after single-incision laparoscopic cholecystectomy (SILC).14 The efficacy of RSB has been reported for postoperative analgesia after SILC, according to our clinical experience, the incision pain can be relieved by RSB, but the effect of visceral pain is depressing. To mitigate visceral pain in patients undergoing SILC has not been studied. Accordingly, we decided to assess the efficiency of ultrasound-guided RSB with butorphanol for incisional pain and visceral pain in patients undergoing SILC.
Methods
Patients and Study Design
Patients undergoing elective SILC were enrolled in this study from February 2019 to April 2019 at the Affiliated Hospital of Nantong University. The study was carried out in accordance with the Declaration of Helsinki, registered prospectively with the Chinese Clinical Trial Registry (reg no.ChiCTR1900020738) and approved by the ethics committee of Affiliated Hospital of Nantong University (approval number: 2018-K067), and written informed consent was obtained. This study adheres to the CONSORT 2010 statement.
The inclusion criteria were as follows: male and female patients between 18 and 59 years of age with an American Society of Anesthesiology (ASA) score of I or II and a body mass index (BMI) of 18–30 kg/m2. Patients with preexisting neuropathy, coagulopathy, local skin infection, hepatic, renal or cardiorespiratory failure, local anaesthetic allergy, pregnancy, complications of gallstones with gallbladder perforation, diffuse peritonitis or acute pyogenic cholangitis were excluded.
Visceral pain is different from incisional pain, the incisional pain was defined as superficial pain on the abdominal wall, the visceral pain was defined as pain inside the abdomen, which may be deep, dull, and more difficult to localize. During a preoperative visit, patients were adequately informed about the concept of the visceral pain, incisional pain and NRS.
Randomization and Blinding
All patients scheduled for elective SILC were randomly divided into two groups using a computer-generated random sequence concealed in consecutively numbered, opaque, sealed envelopes, which were opened on the morning of surgery. All patients were randomly allocated to two groups: group B (n=29) patients were given butorphanol (Jiangsu Hengrui Medicine Co., Ltd, Lianyungang, Jiangsu, China) as preemptive analgesia; group S (n=29) patients were given sufentanil (Yichang Humanwell Pharmaceutical Co., Ltd, Yichang, Hubei, China) for preemptive analgesia. It is worth noting that the two groups underwent RSB 30 minutes before anesthetic induction.
All patients were treated by the same experienced anaesthesiologist, who specialized in ultrasound-guided regional anaesthesia and did not participate in the data collection. Another anesthesiologist, blinded to the group allocation, performed the postoperative data collection.
Group assignment and types of pain killer were also blinded to patients, surgeons and nurses.
Anesthesia
In the anesthesia preparation room, heart rate (HR), blood pressure (BP), electrocardiogram (ECG) and blood oxygen saturation (Spo2) were monitored, the vein access was opened, then all patients were underwent RSB 30 minutes before anesthesia induction, the probe (HFL38x/13–6MHzTransducer, SonoSite Inc., Bothell, WA, USA) was transversely placed at the lateral level of the umbilicus [Figure 1A]. Using the in-plane technique, the needle was advanced until the posterior aspect of the rectus muscle was penetrated. No blood and no gas were drawn back; furthermore, a small volume of saline (<2 mL) was initially injected to ensure that the needle tip was correctly positioned. When the needle was located between the posterior rectus muscle and posterior sheath, 20 mL of 0.5% ropivacaine (LBKL, AstraZeneca AB, Sweden) was injected bilaterally [Figure 1B]. After entering into the operating room, patients in group B and group S were administered butorphanol 0.02 mg/kg and sufentanil 0.1 µg/kg, respectively. Then anesthesia was induced with intravenous midazolam 0.1 mg/kg, propofol 2 mg/kg, sufentanil 0.2 µg/kg and cisatracurium 0.15 mg/kg. Anaesthesia was maintained with 10 mg/mL propofol at 4 mg/kg/h, 50 µg/mL remifentanil at 0.2 µg/kg/min and sevoflurane at 1%. To ensure an adequate depth of anaesthesia, response entropy indexes were kept between 40 and 60 during the entire anaesthesia period by adjusting the rate of infusion of propofol and concentration of sevoflurane.
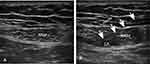 |
Figure 1 Ultrasound images (A) before and (B) after rectus sheath block. Abbreviations: RAM, rectus abdominal muscle; LA, local anaesthetic. |
After surgery, butorphanol 1 mg was administered intravenously as rescue analgesia in patients with a NRS score>3 in group B, or sufentanil 5 µg in group S.
Data Collection
Primary outcome: In both groups, a blinded investigator who was not involved in patient recruitment or the anaesthesia procedure recorded the incisional pain at rest and during cough and the visceral pain using a NRS score (NRS; 0 =no pain; 10 =worst pain) at 2, 6,12 and 24 h after the operation.
Secondary outcomes: PONV, somnolence, constipation, uroschesis, pruritus, and respiratory depression were separately assessed by a blinded observer. Butorphanol 1 mg was administered intravenously as rescue analgesia in patients with a NRS score>3 in group B, or sufentanil 5 µg in group S. The blinded observer recorded the frequency of postoperative rescue analgesic request. Vital signs, such as BP, HR, Spo2, and ECG, were recorded during the operation. The operative duration, haemorrhage volume and consumption of remifentanil and propofol were also recorded.
Statistical Analyses
The results of preliminary study were used as a reference standard of calculation of sample size. Assuming a power of 80% and α=0.05 (2-tailed), formula n = [Zα/2+Zβ]2/ (P1-P2)2 was estimated that at least 25 patients would be required for each group. Finally, we chose 30 people in each group to prevent drop-outs.
Statistical analysis was performed using SPSS 21.0 (IBM Corporation, Armonk, NY, USA). Continuous data (age, BMI, NRS scores, duration of the operation, bleeding amount, length of stay, frequency of analgesic request) were presented as the mean ± standard deviation (SD) if they are normally distributed, otherwise, they will be presented as the mean and interquartile range; Categorical data (sex, ASA, adverse events) were expressed as frequency and analyzed by the chi-squared (χ2) test. The patient characteristics, duration of the operation, bleeding amount and length of stay were compared by Student t test. NRS scores of incisional or visceral pain and frequency of analgesic request are compared by Mann–Whitney U-test. P<0.05 was considered statistically significant.
Results
Patients
The study flow diagram is presented in Figure 2. A total of 60 participants (31 males, 29 females) were recruited into the study, two of them were excluded from the study, including one patients due to complications involving gallstones with gallbladder perforation during surgery, one patients due to BMI>30 kg/m2. Other patients met the inclusion criteria. Individual characteristics of patients are expressed in Table 1. There were no significant differences between two groups.
 |
Table 1 Patient Characteristics |
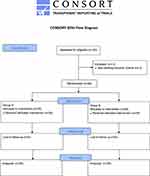 |
Figure 2 CONSORT flow diagram. |
Postoperative Outcomes
Frequency of postoperative rescue analgesic request in the group S was 1.4±1.05 compared with 0.76±0.69 in group B (P<0.05). The occurrence of postoperative nausea and vomiting (PONV) was significantly higher in group S. No significant differences were noted in duration of the operation, bleeding amount, length of stay, constipation, uroschesis, Somnolence, Pruritus and respiratory depression between two groups (Tables 2 and 3).
 |
Table 2 Comparison of Postoperative Outcomes |
 |
Table 3 Adverse Events During the First 24 h After Surgery |
Postoperative Pain
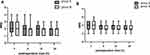
There were no significant differences in the time needed for the block procedure or the quality of ultrasound images. The pain scores during first 24 hours after the operation is shown in Figures 3 and 4. By comparisons between two groups at each time point, we found no significant difference in NRS scores of incisional pain at rest or during cough. Group B had significantly lower NRS scores of visceral pain at 2, 6 and 12 hours compared with group S (P=0.000, P=0.000 and P=0.002, respectively)
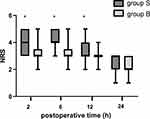 |
Figure 4 NRS scores of visceral pain during 24 hours after operation. The results were represented by bar graph. *P<0.05 compared with group B. Abbreviation: NRS, numeric rating scale. |
Discussion
Cholecystolithiasis is a common and frequently occurring disease. Laparoscopic cholecystectomy is the “gold standard” for treating cholecystolithiasis. Nevertheless, Progression to minimally invasive surgery has occurred from open surgery to laparoscopic surgery, single-incision surgery and robotic surgery, and surgeons have embraced the concepts of less invasiveness, less pain, earlier recovery, and shorter operations.15 Compared with laparoscopic cholecystectomy, SILC has an outstanding cosmetic effect.16 SILC is becoming increasingly popular.17,18 SILC involves only a 2-cm incision into the umbilicus between the T7 and T11 intercostal nerves, incisional pain predominates over visceral pain just at the time of wake up.10 RSB mainly blocks the sheath nerve plexus between the rectus abdominis and posterior sheath of the rectus muscles, which is dominated by the ventral rami of the 6th to 11th intercostal nerves, providing analgesia for the peritoneum, muscle and skin involved in anterior abdominal wall incisions. In the postanesthesia care unit (PACU), we found that the range of sensory blockade was measured as a circular area with a radius of 5 cm centred on the umbilicus. Ropivacaine wears off after 12 hours, perhaps RSB can effectively relieve incisional pain last for 12 hours.
Theoretically, RSB block should provide excellent analgesia for the abdominal wall, but unfortunately, visceral pain was still evident in group S.5 Visceral pain is mainly transmitted by unmyelinated C fibres, is a complex sensory experience caused by trauma and inflammation, and is generally described as dull, diffuse and poorly localized.19 Visceral pain is difficult to manage effectively, largely due to the complexities of visceral innervation, which leads to the visceral sensory mechanisms and factors that contribute to the pathogenesis of visceral pain are poorly understood.20 Visceral hyperalgesia and central sensitization have been suggested to be part of the pathophysiology.21,22 At present, some studies have shown that the management of visceral pain can be achieved by activating κ-receptors.5 Opioids have been reported to have a small effect on visceral pain, which agrees with our data: the NRS scores of visceral pain were lower in group II at 2, 6 h after surgery than in group I and lower in group IV than in group III.9 Butorphanol, a mixed agonist-antagonist opioid, induces analgesia by opioid pathways.5,23 Some studies have shown that butorphanol relieves visceral pain by indirectly suppressing cyclooxygenase activity and thus preventing prostaglandin formation in response to injury. In addition, the main metabolite of butorphanol activates K-receptors and has dual effects of excitation and antagonism on μ-receptors.9 In contrast to μ-receptor agonists (such as sufentanil), which cause side effects, such as respiratory depression, nausea and vomiting, butorphanol alleviated pruritus, and the incidence of side effects was low. Based on the data we got, by limiting postoperative opioid use in group B, patients have fewer adverse biological reactions.
In addition to the effectiveness of the nerve block and drug treatment, timing of intervention itself is also important, butorphanol and sufentanil take effect about 3 min. The complete onset time of ropivacaine as a local anesthetic for RSB is about 30 min.23,24 RSB and intravenous administration of butorphanol or sufentanil were started before anesthesia induction, all of which provides preemptive analgesia and avoids central sensitization caused by nociceptive stimuli before surgery.
RSB and butorphanol are useful for multimodal postoperative pain management in SILC patients. This combination also facilitates earlier mobilization and discharge and follows the trend of enhanced recovery after surgery (ERAS). Indeed, analgesia management has a far-reaching impact on the perioperative period.
Limitations
Like all research, there are a number of limitations to this study. First, this study did not examine whether prolonged postoperative analgesia could be achieved with continuous infusion through rectus sheath catheter placement; 25 Second, a link has been found between cytokines (IL-1β, IL-2, IL-4, IL-5) and pain severity, in our research the level of inflammatory factors have not been checked, and the conclusion can be better confirmed by combining with laboratory examination in the future; Third, the sample size used in the experiment is limited, so it may not be able to make a definite answer to these questions, but according to the existing results, we can draw a preliminary conclusion, which needs to be further increased for further study.
Conclusions
Preemptive butorphanol administration can reduce the frequency of postoperative rescue analgesic request in patients undergoing SILC compared with preemptive sufentanil administration. Butorphanol may play an important role in relieving visceral pain.
Abbreviations
RSB, rectus sheath block; SILC, single-incision laparoscopic cholecystectomy; NRS, numeric rating scale; HR, heart rate; BP, blood pressure; ECG, electrocardiogram; Spo2, blood oxygen saturation; PONV, postoperative nausea and vomiting; NSAIDs, non-steroidal anti-inflammatory drugs; ASA, American Society of Anesthesiology; BMI, body mass index; PACU, postanesthesia care unit; ERAS, enhanced recovery after surgery.
Data Sharing Statement
All necessary data supporting our findings have been presented within the manuscript. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Approval for this study was obtained from the ethics committee of the Affiliated Hospital of Nantong University (approval number: 2018-K067), and each patient provided a written informed consent.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the Social Development Foundation of Nantong City (MS12019023).
Disclosure
The authors declare that they have no competing interests.
References
1. McNicol ED, Ferguson MC, Hudcova J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 2015;6:Cd003348.
2. Webster LR. Risk factors for opioid-use disorder and overdose. Anesth Analg. 2017;125(5):1741–1748. doi:10.1213/ANE.0000000000002496
3. Kane-Gill SL, Rubin EC, Smithburger PL, Buckley MS, Dasta JF. The cost of opioid-related adverse drug events. J Pain Palliat Care Pharmacother. 2014;28(3):282–293. doi:10.3109/15360288.2014.938889
4. Arthur J, Hui D. Safe opioid use: management of opioid-related adverse effects and aberrant behaviors. Hematol Oncol Clin North Am. 2018;32(3):387–403. doi:10.1016/j.hoc.2018.01.003
5. Johnson AC, Greenwood-van Meerveld B. The pharmacology of visceral pain. Advan Pharmacol. 2016;75:273–301.
6. Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet. 2019;393(10180):1558–1568. doi:10.1016/S0140-6736(19)30430-1
7. Chapman CR, Vierck CJ, American Pain Society. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain. 2017;18(4):
8. Baranidharan G, Simpson KH, Dhandapani K. Spinal cord stimulation for visceral pain-a novel approach. Neuromodulation. 2014;17(8):753–758. doi:10.1111/ner.12166
9. Tsang BK, He Z, Wongchanapai W, Ho IK, Eichhorn JH. Visceral analgesic tolerance to intrathecal butorphanol in rats. Can J Anaesthesia. 1998;45(10):1019–1023. doi:10.1007/BF03012311
10. Sviggum HP, Niesen AD, Sites BD, Dilger JA. Trunk blocks 101: transversus abdominis plane, ilioinguinal-iliohypogastric, and rectus sheath blocks. Int Anesthesiol Clin. 2012;50(1):74–92. doi:10.1097/AIA.0b013e31823bc2eb
11. Yakoshi C, Hashimoto H, Niwa H, et al. [Analgesic efficacy and clinical safety of intraperitoneal instillation combined with rectus sheath block using ropivacaine for pain relief after laparoscopic gynecological surgery]. Masui. 2014;63(3):296–302.
12. Chung W, Yoon Y, Kim JW, et al. Comparing two different techniques of rectus sheath block after single port laparoscopic surgery in benign adnexal mass patients: surgical versus ultrasonography guidance-a randomized, single-blind, case-controlled study. Eur J Obstet Gynecol Reprod Biol. 2017;217:29–33. doi:10.1016/j.ejogrb.2017.08.020
13. Jeong H-W, Kim CS, Choi KT, Jeong S-M, Kim D-H, Lee J-H. Preoperative versus postoperative rectus sheath block for acute postoperative pain relief after laparoscopic cholecystectomy: a randomized controlled study. J Clin Med. 2019;8(7):7. doi:10.3390/jcm8071018
14. Kamei H, Ishibashi N, Nakayama G, Hamada N, Ogata Y, Akagi Y. Ultrasound-guided rectus sheath block for single-incision laparoscopic cholecystectomy. Asian J Endosc Surg. 2015;8(2):148–152. doi:10.1111/ases.12178
15. Lirici MM, Tierno SM, Ponzano C. Single-incision laparoscopic cholecystectomy: does it work? A systematic review. Surg Endosc. 2016;30(10):4389–4399. doi:10.1007/s00464-016-4757-5
16. Omar MA, Redwan AA, Mahmoud AG. Single-incision versus 3-port laparoscopic cholecystectomy in symptomatic gallstones: a prospective randomized study. Surgery. 2017;162(1):96–103. doi:10.1016/j.surg.2017.01.006
17. Rizzuto A, Serra R, Mignogna C, Palaia I, Zittel FU, Sacco R. Single incision laparoscopic cholecystectomy in geriatric patients. Int J Surg. 2016;35:83–87. doi:10.1016/j.ijsu.2016.09.075
18. Kim JS, Choi JB, Lee SY, et al. Pain related to robotic cholecystectomy with lower abdominal ports: effect of the bilateral ultrasound-guided split injection technique of rectus sheath block in female patients A prospective randomised trial. Medicine. 2016;95:31. doi:10.1097/MD.0000000000004864
19. Gebhart GF, Bielefeldt K. Physiology of visceral pain. Compr Physiol. 2016;6(4):1609–1633.
20. B-E MR, ϕrding H, Andersen C, Licht PB, Toft P. Early visceral pain predicts chronic pain after laparoscopic cholecystectomy. Pain. 2014;155(11):2400–2407. doi:10.1016/j.pain.2014.09.019
21. Davis MP. Drug management of visceral pain: concepts from basic research. Pain Res Treat. 2012;2012:265605. doi:10.1155/2012/265605
22. Vanderah TW. Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain. 2010;26(1):S10–S15. doi:10.1097/AJP.0b013e3181c49e3a
23. Reddi D, Curran N. Chronic pain after surgery: pathophysiology, risk factors and prevention. Postgrad Med J. 2014;90(1062):
24. Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52(3):259–285. doi:10.1016/0304-3959(93)90161-H
25. Fazzari J, Sidhu J, Motkur S, et al. Applying serum cytokine levels to predict pain severity in cancer patients. J Pain Res. 2020;13:313–321. doi:10.2147/JPR.S227175
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.