Back to Journals » Drug Design, Development and Therapy » Volume 17
Perioperative Administration of Pregabalin and Esketamine to Prevent Chronic Pain After Breast Cancer Surgery: A Randomized Controlled Trial
Authors Wang M, Xiong HP, Sheng K, Sun XB, Zhao XQ, Liu QR
Received 20 March 2023
Accepted for publication 2 June 2023
Published 8 June 2023 Volume 2023:17 Pages 1699—1706
DOI https://doi.org/10.2147/DDDT.S413273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Miao Wang,1,* Hua-Ping Xiong,2,* Kai Sheng,1 Xing-Bing Sun,1 Xiao-Qian Zhao,3 Qing-Ren Liu1
1Department of Anesthesiology, Xishan People’s Hospital of Wuxi City, Wuxi, 214105, People’s Republic of China; 2Department of Anesthesiology, Wuxi Maternal and Child Health Hospital, Wuxi School of Medicine, Jiangnan University, Jiangsu, 214002, People’s Republic of China; 3Department of Breast Diseases, Wuxi Maternal and Child Health Hospital, Wuxi School of Medicine, Jiangnan University, Jiangsu, 214002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qing-Ren Liu, Department of Anesthesiology, Xishan People’s Hospital of Wuxi City, No. 1128 Dacheng Road, Wuxi, 214105, People’s Republic of China, Email [email protected] Xiao-Qian Zhao, Department of Breast Diseases, Wuxi Maternal and Child Health Hospital, Wuxi School of Medicine, Jiangnan University, No. 48 Huaishu Alley, Wuxi, 214002, People’s Republic of China, Email [email protected]
Background: Perioperative multimodal analgesia can prevent chronic pain after breast cancer surgery. This study aimed to investigate the efficacy of combined perioperative oral pregabalin and postoperative esketamine in preventing chronic pain after breast cancer surgery.
Methods: Ninety patients undergoing elective breast cancer surgery were randomized into the combined pregabalin and esketamine group (EP group) and the general anesthesia alone group (Control group). The EP group received 150 mg of oral pregabalin 1 h before surgery and twice daily for seven days postoperatively, and a patient-controlled analgesia pump after surgery that delivered 100 μg sufentanil + 1.25 mg/kg esketamine + 4 mg tropisetron in 100 mL saline solution intravenously. The Control group received placebo capsules before and after the surgery and routine postoperative analgesia (100 μg sufentanil + 4 mg tropisetron in 100 mL saline solution). The primary outcome was the incidence of chronic pain three and six months after surgery. Secondary outcomes included acute postoperative pain, postoperative opioid consumption, and incidence of adverse events.
Results: The incidence of chronic pain in the EP group was significantly lower than in the Control group three (14.3% vs 46.3%, P = 0.005) and six (7.1% vs 31.7%, P = 0.009) months postoperatively. The rest numerical rating scale (NRS) pain scores 1– 3 days postoperatively and coughing NRS pain scores 1– 7 days postoperatively in the EP group were significantly lower than in the Control group (all P ˂ 0.05). The cumulative sufentanil consumption in the EP group during postoperative 0– 12, 12– 24, and 24– 48, 0– 24, and 0– 48 hours were significantly lower than in the Control group (all P ˂ 0.05).
Conclusion: Combined perioperative oral pregabalin and postoperative esketamine effectively prevented chronic pain after breast cancer surgery, improved acute postoperative pain, and reduced postoperative opioid consumption.
Keywords: pregabalin, esketamine, chronic pain, breast cancer surgery
Introduction
Breast cancer is one of the most important health concerns for women worldwide, accounting for approximately 11% of cancer cases in China and increasing gradually in prevalence.1 Breast cancer management differs depending on the cancer type, but surgery remains the main treatment.2 Chronic postsurgical pain is a common complication that significantly affects patients’ quality of life and is closely related to mortality.3 A large-scale cross-sectional study reported that 47% of the patients had chronic pain after breast cancer surgery, 39% with moderate pain, and 13% with severe pain.4 Therefore, it is important to prevent the development of chronic pain after breast cancer surgery.
Low-quality evidence has suggested that paravertebral blocks could reduce the risk of chronic pain 3–12 months after breast cancer surgery. Moderate evidence indicated that intravenous infusion of lidocaine could reduce the incidence of chronic pain 3–6 months after breast cancer surgery.5 However, evidence on the effect of other medications in preventing chronic pain after breast cancer surgery is insufficient.
Pregabalin, which inhibits the tissue damage-induced dorsal horn neuronal hyperexcitability and prevents central sensitization, was shown to effectively treat neuropathic pain6 and prevent chronic pain after surgery.7,8 However, the evidence of its efficacy in preventing chronic pain after breast cancer surgery when applied alone perioperatively is weak.9–12
Ketamine, an N-methyl-D-aspartate receptor inhibitor, can effectively treat chronic pain.13 As an adjuvant for perioperative multimodal analgesia, it assists in preventing the progression from acute to chronic postoperative pain.14 However, the effect of ketamine on preventing chronic pain after breast cancer surgery remains debated.15,16 Esketamine, the S-enantiomer of ketamine, has twice the analgesic potency of racemic ketamine and fewer psychiatric side effects.
There has been no report on the efficacy of combined pregabalin and esketamine in preventing chronic pain after breast cancer surgery. We hypothesized that combined perioperative oral pregabalin and postoperative esketamine could reduce the incidence of chronic pain after breast cancer surgery. The primary outcome of this study was the incidence of chronic pain three and six months after surgery, and the secondary outcomes included postoperative pain at rest or during coughing, analgesic consumption, and adverse events.
Methods
Study Design
This was a single-center, prospective, double-blind, randomized controlled trial. The Independent Ethics Committee for Clinical Research of Xishan People’s Hospital of Wuxi City approved the study (xs2020ky003), and the Consolidated Standards of Reporting Trials guidelines were followed. The study was registered in the Chinese Clinical Trial Registry (ChiCTR2000038124). All participants signed written informed consent and all procedures followed the tenets of the Declaration of Helsinki.
Participants
The study enrolled 90 patients who underwent elective breast cancer surgery under general anesthesia between September 15, 2020, and August 14, 2021. The inclusion criteria were age between 18 and 80 years, body mass index between 15 and 30 kg/m2, and American Society of Anesthesiologists classes I to III. The exclusion criteria were severe cardiac and pulmonary diseases, hepatic and renal insufficiency, history of chronic pain, and psychiatric diseases. Patients were randomized to receive combined pregabalin and esketamine (EP group) or general anesthesia only (Control group) using a randomized number table A pharmacist prepared capsules containing either pregabalin or a placebo and syringes containing esketamine or a normal saline solution. These were sealed in sequentially-numbered envelopes and delivered to the post-anesthesia care unit (PACU). The nurse opened the envelopes following the numerical sequence. Each envelope contained a capsule and syringe. All participants and investigators remained blinded to their randomization assignment.
Anesthetic Procedure
All patients underwent surgery without sedation. One hour before surgery, the EP group received 150 mg of oral pregabalin, while the control group received a placebo capsule. Blood pressure (BP), electrocardiogram, heart rate (HR), and pulse oxygen saturation were routinely measured upon arrival at the operating room. Induction for tracheal intubation included intravenous 0.4 μg/kg sufentanil, 1.5–2.5 mg/kg propofol, and 0.6 mg/kg rocuronium. The tidal volume was set at 8–10 mL/kg and the respiratory rate at 10–12 bpm. General anesthesia was maintained through inhalation of 2.0–3.0% sevoflurane and intravenous remifentanil 0.05–0.2 μg/kg/h. Intraoperative HR was maintained at 50–80 beats/min, and BP variations did not exceed 20% of the baseline value. Intravenous atropine (0.5 mg) was administered if the HR dropped below 50 beats/min, and intravenous ephedrine or phenylephrine was given if the BP was below 80% of the baseline value. Cisatracurium (3 mg) was given intraoperatively at 1-h intervals to maintain muscle relaxation, and 0.1 μg/kg sufentanil and 2 mg tropisetron were administered intravenously ten minutes before the end of the surgery. The patients were transferred to the PACU after the surgery with the tracheal tube in place. Anesthesia was reversed with neostigmine and atropine, and the tracheal tube was removed once the patient was awake and muscle strength was fully restored.
Analgesic Procedure
While at the PACU, patients with an NRS pain score >3 received 2 μg sufentanil intravenously, which was repeated at 5-minute intervals as needed until the patient’s NRS pain score was ≤3. Once the patient’s pain was under control, the analgesic pump was activated. In the EP group, the analgesic pump delivered 100 μg sufentanil, 1.25 mg/kg esketamine, and 4 mg tropisetron in 100 mL saline solution. In the Control group, the pump delivered 100 μg sufentanil and 4 mg tropisetron in 100 mL saline solution. The pump settings were: basal rate, 1 mL/h; bolus, 2 mL; lock-out time, 15 min; dosage limit, 7 mL/h. Patients with an NRS score ≥ 4 were eligible to receive intravenous nonsteroidal anti-inflammatory drugs such as parecoxib or ketorolac for pain relief, even after exceeding the dosage limit. Patients in the EP group received oral pregabalin 150 mg twice daily for seven days after the surgery, while those in the Control group received oral placebo capsules.
Outcomes
The primary outcome was the incidence of chronic pain three and six months after surgery. The secondary outcomes included acute postoperative pain, postoperative opioid consumption, and the incidence of adverse events. The total intraoperative remifentanil dose was recorded, and the NRS score at rest and when coughing was assessed 1, 3, 6, 12, 24, and 48 hours postoperatively. The cumulative sufentanil consumption was recorded for postoperative 0–12, 12–24, and 24–48 hours and calculated for 0–24 and 0–48 hours after surgery. Postoperative adverse events, such as nausea, vomiting, dizziness, pruritus, hallucinations, and nightmares, were recorded during the 48 hours after surgery. Chronic postsurgical pain was defined as pain in the surgical incision site and its surroundings with an NRS score at rest of ≥1 that persisted for more than two months postoperatively. The occurrence of chronic pain was evaluated three and six months postoperatively.
Sample Size
The reported incidence of chronic pain after breast cancer surgery is 47%.4 We anticipated that the combination of esketamine and pregabalin would reduce this incidence by 60%. To achieve a statistical power of 80% at a significance level of 0.05 using Pearson’s chi-squared test to compare the rates between the two groups, a sample of 80 patients was required, 40 per group. Considering a loss to follow-up of 10%, 45 patients per group were needed.
Statistical Methods
IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
The distribution of continuous variables was assessed using a normal probability plot. Normally distributed variables are reported as mean ± standard deviation, while non-normally distributed variables are presented as the median and interquartile range (IQR). The independent samples t-test or Mann–Whitney U-test assessed differences between groups for continuous variables, except for the NRS pain scores. NRS pain scores were analyzed using repeated measures analysis of variance (ANOVA). Count data are reported as numbers (%) and were compared using the chi-squared or Fisher’s exact test. Statistical significance was set at P < 0.05.
Results
As shown in the study flow diagram (Figure 1), 106 patients with breast cancer were recruited, of which 12 did not meet the inclusion criteria, and four declined to participate, leaving 90 patients for randomization. The basic patient characteristics are shown in Table 1. The two groups were similar in their demographic and perioperative clinical characteristics.
 |
Table 1 Demographic and Clinical Characteristics of Patients |
 |
Figure 1 CONSORT flow diagram. Notes: CONSORT figure adapted from Schulz KF, Altman DG, Moher D, CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3): e1000251. Copyright: © 2010 Schulz et al. Creative Commons Attribution License.17 Abbreviations: EP, combined pregabalin and esketamine; CONSORT, Consolidated Standards of Reporting Trials. |
One patient was lost to follow-up in the EP group and two in the Control group by the 3-month assessment, and none by the 6-month assessment. The incidence of chronic pain in the EP group was significantly lower than in the Control group three (14.3% vs 46.3%, P = 0.005) and six (7.1% vs 31.7%, P = 0.009) months postoperatively (Table 2).
 |
Table 2 Outcome Measurement of Patients |
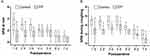
The postoperative rest NRS pain scores on days 1–3 and coughing NRS pain scores on days 1–7 in the EP group were significantly lower than in the Control group (all P ˂ 0.05). However, the two groups had similar resting NRS scores on postoperative days 4–7 (all P > 0.05; Figure 2). As shown in Figure 3, the cumulative sufentanil consumption during the 0–12, 12–18, 18–24, 0–24, 24–48, and 0–48 hours after surgery in the EP group was significantly lower than in the Control group (all P ˂ 0.05).
The intraoperative remifentanil consumption was similar in the EP and Control groups (814.5 ± 294.6 vs 937.1 ± 314.3 μg, respectively; P = 0.161). Two patients in the EP group experienced postoperative hallucinations, and two experienced nightmares, but the differences between the groups were statistically insignificant (both P = 0.152). The rates of nausea (20.9%) and vomiting (9.3%) after surgery in the EP group were insignificantly lower than the respective values in the Control group (30.2% and 14.0%; P = 0.323 and P = 0.501). Furthermore, the groups had similar rates of dizziness and pruritus (P = 0.501, P = 0.557; Table 2).
Discussion
Our study presented an effective protocol for preventing chronic pain three and six months after breast cancer surgery. The study results demonstrated that administering combined perioperative oral pregabalin and postoperative esketamine effectively reduced the incidence of chronic postsurgical pain in these patients. Furthermore, this combination therapy significantly improved postoperative pain and reduced opioid consumption.
Previous studies have shown that the effectiveness of perioperative oral pregabalin in preventing chronic pain varied with dose and duration. Oral pregabalin for 5–9 days postoperatively did not reduce the incidence of chronic pain,10,18,19 while administration for 14 days prevented chronic pain from occurring.7,14 However, compliance in patients taking long-term oral pregabalin was poor, and long-term use was associated with dizziness and sedation.7,20
In our study, we administered 150 mg pregabalin orally one hour before surgery and twice daily for seven days postoperatively, similarly to the dose used in the study by Reyad et al.11 We combined this treatment with low-dose esketamine as controlled intravenous analgesia for 48 hours postoperatively. The dose of esketamine was referred from the study by Bornemann-Cimenti and colleagues.21 We found a significant reduction in the incidence of chronic pain three and six months after surgery. Similar results were observed in other studies, including the one by Anwar et al14 that evaluated the preventive effect of combined perioperative oral pregabalin and postoperative intravenous ketamine on chronic pain after cardiac surgery. Their results showed that the combination therapy significantly reduced the incidence of chronic pain.
It is worth noting that perioperative oral pregabalin was not very effective in reducing acute postoperative pain in patients after breast cancer surgery.12 Reyad and colleagues11 found that daily oral pregabalin (150 mg) before and for seven days after surgery did not reduce the visual analog scale (VAS) pain scores at rest and during movement on postoperative days 1–7. Similarly, Khan et al10 found that daily postoperative oral pregabalin (150 mg) for nine days did not reduce the VAS pain scores at rest and during movement on postoperative days 1–9. Additionally, previous studies have shown that preoperative and intraoperative administration of ketamine did not reduce acute postoperative pain.22 However, in our study, we observed a reduction in rest pain on postoperatively days 1–3 and coughing pain on postoperative days 1–7. It is suggested that combined perioperative oral pregabalin and postoperative intravenous esketamine optimized their analgesic efficacy.
Preoperative and 12-h postoperative oral pregabalin did not reduce postoperative opioid consumption in patients after breast cancer surgery,23 nor did preoperative and daily postoperative oral pregabalin for 7–9 days.10,11 In our study, the postoperative addition of esketamine significantly reduced the cumulative opioid consumption over the 24 and 48 h after surgery. Furthermore, a previous study demonstrated that combined oral pregabalin and ketamine reduced the 24-h morphine consumption after cardiac surgery.14
An early meta-analysis showed that perioperative oral pregabalin reduced the incidence of postoperative nausea and vomiting (PONV).24 However, a recent meta-analysis on breast cancer showed that pregabalin did not reduce the incidence of PONV.12 Our study found that the incidence of PONV in the EP group was insignificantly lower than in the Control group. Regrettably, we did not evaluate the severity of nausea and vomiting further. A previous study has shown that combined pregabalin and ketamine could reduce the severity of nausea after cardiac surgery.14 Our study found two cases each of postoperative hallucinations and nightmares, with an incidence of 4%, significantly lower than the incidence reported by Avidan et al25 for a single preoperative dose of ketamine (0.5 or 1.0 mg/kg). Dizziness is a common adverse effect of pregabalin. A meta-analysis showed that pregabalin led to a higher incidence of postoperative dizziness than the placebo.26 However, the incidence of dizziness was similar in both groups in this study. This could be due to the lower postoperative sufentanil consumption in the EP group compared to the Control group.
Limitations
First, this study did not investigate the effect of pregabalin alone on chronic pain in patients after breast cancer surgery. Previous studies showed that daily oral pregabalin for 5–9 days after surgery did not reduce the incidence of chronic pain.10,18,19 Consequently, this study focused on the effect of combining pregabalin with the adjuvant esketamine. Second, the oral pregabalin dose was identical for all patients; it was not adjusted according to body weight. This might have resulted in under- or overdose for some patients. Third, postoperative sufentanil use might have been influenced by non-surgically-induced pain such as headache and back pain. Fourth, follow-up was limited to three and six months postoperatively; longer-term effects were not investigated. Finally, the primary outcome was qualitative rather than quantitative as a chronic pain scale, such as the Brief Pain Inventory, was unavailable.
Conclusions
Our study highlighted the potential benefits of administering combined perioperative oral pregabalin and postoperative esketamine in preventing chronic pain after breast cancer surgery. The combination therapy also improved acute postoperative pain and reduced opioid use. However, the drugs’ dose and timing and duration of their administration should be further confirmed in multi-center randomized controlled clinical trials.
Data Sharing Statement
The data generated during the current study are available from the corresponding author (Qing-Ren Liu) upon reasonable request. The study protocol, statistical analysis plan, and clinical study report will also be available.
Acknowledgments
We are grateful to Xue Song and Yun-Hui Zhang for their kind assistance. This work was supported by the Top Talent Support Program for Young and Middle-Aged People of Wuxi Health Committee (HB2020112) and the Medical and Health Science and Technology Development Program of Wuxi (NZ2021035).
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat. 2016;159(3):395–406. doi:10.1007/s10549-016-3947-0
2. Jatoi I, Kemp Z. Surgery for breast cancer prevention. JAMA. 2021;325(17):1804. doi:10.1001/jama.2021.1647
3. Yin M, Wang C, Gu K, et al. Chronic pain and its correlates among long-term breast cancer survivors. J Cancer Surviv. 2022;17:460–467. doi:10.1007/s11764-022-01241-9
4. Gärtner R, Jensen M-B, Nielsen J, et al. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985–1992. doi:10.1001/jama.2009.1568
5. Weinstein EJ, Levene JL, Cohen MS, et al. Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Syst Rev. 2018;4(4):CD007105. doi:10.1002/14651858.CD007105.pub3
6. Derry S, Bell RF, Straube S, et al. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1(1):CD007076. doi:10.1002/14651858.CD007076.pub3
7. Buvanendran A, Kroin JS, Della Valle CJ, et al. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010;110(1):199–207. doi:10.1213/ANE.0b013e3181c4273a
8. Yu Y, Liu N, Zeng Q, et al. The efficacy of pregabalin for the management of acute and chronic postoperative pain in thoracotomy: a meta-analysis with trial sequential analysis of randomized-controlled trials. J Pain Res. 2019;12:159–170. doi:10.2147/JPR.S183411
9. Rai AS, Khan JS, Dhaliwal J, et al. Preoperative pregabalin or gabapentin for acute and chronic postoperative pain among patients undergoing breast cancer surgery: a systematic review and meta-analysis of randomized controlled trials. J Plast Reconstr Aesthet Surg. 2017;70(10):1317–1328. doi:10.1016/j.bjps.2017.05.054
10. Khan JS, Hodgson N, Choi S, et al. Perioperative pregabalin and intraoperative lidocaine infusion to reduce persistent neuropathic pain after breast cancer surgery: a multicenter, factorial, randomized, controlled pilot trial. J Pain. 2019;20(8):980–993. doi:10.1016/j.jpain.2019.02.010
11. Reyad RM, Omran AF, Abbas DN, et al. The possible preventive role of pregabalin in postmastectomy pain syndrome: a double-blinded randomized controlled trial. J Pain Symptom Manage. 2019;57(1):1–9. doi:10.1016/j.jpainsymman.2018.10.496
12. Chang -C-C, Yen W-T, Lin Y-T, et al. Perioperative pregabalin for preventive analgesia in breast cancer surgery: a meta-analysis of randomized controlled trials. Clin J Pain. 2020;36:968–977. doi:10.1097/AJP.0000000000000883
13. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American society of regional anesthesia and pain medicine, the American academy of pain medicine, and the American society of anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521–546. doi:10.1097/AAP.0000000000000808
14. Anwar S, Cooper J, Rahman J, et al. Prolonged perioperative use of pregabalin and ketamine to prevent persistent pain after cardiac surgery. Anesthesiology. 2019;131(1):119–131. doi:10.1097/ALN.0000000000002751
15. Kang C, Cho A-R, Kim K-H, et al. Effects of intraoperative low-dose ketamine on persistent postsurgical pain after breast cancer surgery: a prospective, randomized, controlled, double-blind study. Pain Physician. 2020;23(1):37–47.
16. Steyaert A, Forget P, Dubois V, Lavand’homme P, De Kock M. Does the perioperative analgesic/anesthetic regimen influence the prevalence of long-term chronic pain after mastectomy? J Clin Anesth. 2016;33:20–25. doi:10.1016/j.jclinane.2015.07.010
17. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3):e1000251.
18. Fassoulaki A, Melemeni A, Tsaroucha A, et al. Perioperative pregabalin for acute and chronic pain after abdominal hysterectomy or myomectomy: a randomised controlled trial. Eur J Anaesthesiol. 2012;29(11):531–536. doi:10.1097/EJA.0b013e32835800e0
19. Konstantatos AH, Howard W, Story D, et al. A randomised controlled trial of peri-operative pregabalin vs. Placebo for video-assisted thoracoscopic surgery. Anaesthesia. 2016;71(2):192–197. doi:10.1111/anae.13292
20. Dong J, Li W, Wang Y. The effect of pregabalin on acute postoperative pain in patients undergoing total knee arthroplasty: a meta-analysis. Int J Surg. 2016;34:148–160. doi:10.1016/j.ijsu.2016.08.521
21. Bornemann-Cimenti H, Wejbora M, Michaeli K, et al. The effects of minimal-dose versus low-dose S-ketamine on opioid consumption, hyperalgesia, and postoperative delirium: a triple-blinded, randomized, active- and placebo-controlled clinical trial. Minerva Anestesiol. 2016;82(10):1069–1076.
22. Zhao Z, Xu Q, Chen Y, et al. The effect of low-dose ketamine on postoperative quality of recovery in patients undergoing breast cancer surgery: a randomised, placebo-controlled trial. Int J Clin Pract. 2021;75(12):e15010. doi:10.1111/ijcp.15010
23. Koyuncu T, Oğuz G, Akben S, et al. the effects of pregabaline on postoperative pain and opioid consumption used perioperatively in patients undergoing modified radical mastectomy. Agri. 2013;25(4):169–178. doi:10.5505/agri.2013.19970
24. Grant MC, Betz M, Hulse M, et al. The effect of preoperative pregabalin on postoperative nausea and vomiting: a meta-analysis. Anesth Analg. 2016;123(5):1100–1107. doi:10.1213/ANE.0000000000001404
25. Avidan MS, Maybrier HR, Abdallah AB, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet. 2017;390(10091):267–275. doi:10.1016/S0140-6736(17)31467-8
26. Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth. 2015;114(1):10–31. doi:10.1093/bja/aeu293
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.