Back to Journals » Journal of Pain Research » Volume 10
Perineural versus intravenous dexamethasone as an adjuvant in regional anesthesia: a systematic review and meta-analysis
Authors Zhao WL, Ou XF, Liu J, Zhang WS
Received 28 March 2017
Accepted for publication 2 June 2017
Published 4 July 2017 Volume 2017:10 Pages 1529—1543
DOI https://doi.org/10.2147/JPR.S138212
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Wen-Ling Zhao,1 Xiao-Feng Ou,1 Jin Liu,1,2 Wen-Sheng Zhang1,2
1Laboratory of Anesthesia and Critical Care Medicine, Translational Neuroscience Centre, 2Department of Anesthesiology, West China Hospital, Sichuan University, Chengdu, China
Background: Dexamethasone is a common adjuvant for local anesthetics in regional anesthesia, but the optimal route of administration is controversial. Therefore, we did a systematic review and meta-analysis of randomized controlled trials to assess the effect of perineural versus intravenous dexamethasone on local anesthetic regional nerve-blockade outcomes.
Materials and methods: Medline (through PubMed), Embase, Cochrane, Web of Science, and Biosis Previews databases were systematically searched (published from inception of each database to January 1, 2017) to identify randomized controlled trials. The data of the selected trials were statistically analyzed to find any significant differences between the two modalities. The primary outcome was the duration of analgesia. Secondary outcomes included duration of motor block, postoperative nausea and vomiting, and postoperative analgesic dose at 24 hours. We conducted a planned subgroup analysis to compare the effects between adding epinephrine or not.
Results: Ten randomized controlled trials met the inclusion criteria of our analysis, with a total of 749 patients. Without the addition of epinephrine, the effects of perineural and intravenous dexamethasone were equivalent concerning the duration of analgesia (mean difference 0.03 hours, 95% CI –0.17 to 0.24). However, with the addition of epinephrine, the analgesic duration of perineural dexamethasone versus intravenous dexamethasone was prolonged (mean difference 3.96 hours, 95% CI 2.66–5.27). Likewise, the impact of epinephrine was the same on the duration of motor block. The two routes of administration did not show any significant differences in the incidence of postoperative nausea and vomiting, nor on postoperative analgesic consumption at 24 hours.
Conclusion: Our results show that perineural dexamethasone can prolong the effects of analgesic duration when compared to the intravenous route, only when epinephrine is coadministered. Without epinephrine, the two modalities show equivalent effect as adjuvants on regional anesthesia.
Keywords: anesthesia adjuvants, dexamethasone, regional anesthesia
Introduction
Uncontrolled pain after surgery may produce a range of detrimental acute and chronic effects.1 In some surgical procedures under regional anesthesia, commonly used local anesthetics cannot provide analgesia for a sufficiently prolonged time. Therefore, local anesthetic–opioid combinations or continuous catheterization are chosen to prolong analgesic duration. However, unintentional opioid-associated side effects and several problems associated with carrying a catheter, especially for day-care patients, make these treatment options unsatisfactory. Since 1982, anesthesiologists have been using a variety of adjuvants added to local anesthetics to enhance regional anesthesia.2 Dexamethasone is one of these additives.3
Dexamethasone has been proved to be an effective adjuvant for extending the duration of sensory and motor block for peripheral nerve block when given perineurally.4–6 Intravenous injection can also be used for alleviating postoperative pain.7 However, there are conflicting reports about which of the two routes of administration is the best or if both exert an equivalent effect.8–12
To solve this query, a series of randomized controlled trials (RCTs) were conducted to compare the effect of prolongation of analgesia between perineural and intravenously administered dexamethasone. We carried out a systematic review and meta-analysis to assemble all the associated individual clinical trials to assess the two modalities on the main outcomes: prolonging sensory-block duration, decreasing postoperative nausea and vomiting, and sparing analgesic consumption at 24 hours after surgery.
Materials and methods
This systematic review and the meta-analysis results were reported following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Search strategy
Two authors (WLZ and XFO) independently searched the following databases: Medline (through PubMed), Embase, Cochrane Central Register of Controlled Trials, Web of Science, and Biosis Previews, from inception of each database to January 1, 2017. There were no language restrictions. The full literature-search strategies for PubMed and Embase are presented in Table S1. In brief, we used MeSH terminology for dexamethasone, regional anesthesia, and their synonyms to search the five databases to obtain relevant literature. Then, all the titles and abstracts were screened and full texts of eligible RCTs retrieved. The references of key English reviews and eligible studies were also manually searched.
Selection criteria
When the titles and abstracts were screened, four selection criteria were applied: 1) population – adult patients (aged ≤19 years) under surgery who received regional anesthesia; 2) intervention – dexamethasone given perineurally as an adjuvant to local anesthetics; 3) control – same-dose dexamethasone given intravenously; and 4) design – RCTs. Studies that satisfied these criteria were retrieved for full texts to be assessed. Meeting abstracts were eliminated due to their incomplete information.
Data extraction
Two authors (WLZ and XFO) independently extracted the following raw data from the selected studies: main authors, year of publication, country, number of patients, type of nerve block, ultraguided localization technique, type and dose of local anesthetics (including dexamethasone and other adjuvants), and main outcomes. Then, concrete data of the following outcomes were extracted: duration of analgesia or sensory block, duration of motor block, incidence of postoperative nausea and vomiting, and postoperative analgesic use (morphine equivalents) at 24 hours. For the continuous type of data, we emailed the corresponding author to obtain the raw data when the variables in full text were not reported as means and SD. If authors did not respond, we used a method of data conversion. For calculation of given means, 95% CIs, SD ((√n × [upper – lower]/2t), given medians, and interquartile ranges, the method reported by Hozo et al was used.13 Each of the two aforementioned authors checked these data at least three times. Any disagreements on the results were resolved independently by a third experienced author (JL).
Assessment for risk of bias in included studies
Risks of included studies were assessed by the Cochrane risk-of-bias tool. We classified risk of bias as low, unclear, or high for each of random-sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias (such as missing participant data). To grade the qualities of evidence and the strength of recommendations, we used GradePro version 3.6.1 for our four outcomes.
Statistical analysis
Continuous variables, analgesic duration, duration of motor block, and postoperative analgesic use at 24 hours were reported as mean differences (MDs) with 95% CIs. We analyzed postoperative nausea and vomiting as the categorical variable and expressed relative risk with 95% CIs. Heterogeneity of the four outcomes was evaluated by using the c2 and I2 tests. A fixed-effect model was used when c2 P-value was >0.1 and I2<50%. When P<0.1 and I2>50%, we chose a random-effect model, and the planned subgroup analysis was performed to compare adding epinephrine or not to the local anesthetic mixture. Potential publication biases were assessed by funnel-plot analysis. All analyses were performed with RevMan (version 5.3; Cochrane Library, Oxford, UK).
Results
Study selection and characteristics
A flowchart of the study-selection procedure is shown in Figure 1. In order to avoid any missing studies, we did not restrict the design of RCTs in searching Web of Science and Biosis Previews or define the route of administration. A total of 2,939 studies were identified during our first search: 638 articles were removed due to duplication, and 2,301 titles and abstracts were eliminated after screening. Finally, ten studies were included in our ultimateanalysis.14–23 One study was eliminated due to its high standard deviation, which was even larger than the mean value, possibly due to the small-study effect.24,25 The basic information of the ten studies is presented in Table 1. Patients of seven studies received brachial nerve block, one study used a nerve simulator to localize the nerve, and the rest were ultrasound-guided. The remaining studies were on perianal block, sciatic nerve block, and ankle block. Two of the studies were multicenter clinical trials, which were conducted in Canada and Thailand. The dose of dexamethasone ranged from 4 to 10 mg. Three trials added epinephrine with local anesthetics and dexamethasone, and hence they constituted the planned subgroup analysis in this study.
  | Figure 1 Flowchart of the search strategy. Abbreviation: RCTs, randomized controlled trials.
|
  | Table 1 Characteristics of included studies Abbreviations: NRS, numeric rating scale; VAS, visual analog scale. |
The risks of bias for the ten studies included here were discussed by all the authors; the risks discussed are summarized in Figure S1. One study16 did not present the process they used to generate random sequences for group allocation. They also unveiled the allocation results to the anesthesiologists who participated in that trial, and did not refer to any blinding of the outcome assessment, as with another one.14 For one of the ten studies,22 the methods section did not clearly show the approach used for blinding the participants.
Duration of analgesia
The duration of sensory block or analgesia was reported by nine trials. One19 was eliminated due to its definition of median analgesia time, namely time to first analgesic request in >50% of patients, which was far different to other studies’ definitions (Table S2). In a random-effect model, a pooled analysis of nine trials showed that the MD between perineural and intravenous dexamethasone was 1.62 hours (95% CI –0.05 to 3.29, I2=80%; P=0.06; Figure 2A), which indicated that dexamethasone given perineurally or intravenously had a similar effect on the duration of sensory block or analgesia. Due to the high heterogeneity, we conducted a subgroup analysis comparing the addition or exclusion of epinephrine. When epinephrine was used, perineural dexamethasone prolonged analgesic duration by 3.96 hours (95% CI 2.66–5.27, P<0.00001) in comparison to the intravenous dexamethasone group, with no heterogeneity (I2=0). In the absence of epinephrine, both groups had equal analgesic duration (MD 0.03 hours, I2=7%, 95% CI –0.17 to 0.24; P=0.75; Figure 2B). There were no observations of publication biases when constructing the funnel plots (Figure S2).
  | Figure 2 Forest plots showing the duration of analgesia. Notes: Analysis of all data in the associated studies (A); subgroup analysis by differentiating addition or not of epinephrine (B). |
Duration of motor block
Four trials reported on duration of motor block. The effects on prolonging duration of motor block between the two modalities was statistically insignificant (MD 1.67 hours, I2=94%, 95% CI –2.88 to 6.22; P=0.47; Figure 3A) as revealed by the random-effect model. However, subgroup analysis showed that in the presence of epinephrine, the perineural dexamethasone group had duration prolonged by 4 hours (95% CI 2.82–5.20, I2=3%; P<0.00001; Figure 3B) when compared to the intravenous dexamethasone group.
  | Figure 3 Forest plots showing the duration of motor block. Notes: Analysis of four studies (A) and subgroup analysis by differentiating addition or not of epinephrine (B).
|
Postoperative nausea and vomiting
Six studies assessed the incidence of postoperative nausea and vomiting. The pooled analysis demonstrated that there was no difference between the perineural and intravenous dexamethasone groups (risk ratio 0.87, 95% CI 0.35–2.11; P=0.75; Figure 4A), without heterogeneity (I2=0) or publication bias (Figure S3).
  | Figure 4 Forest plots comparing perineural and intravenous groups at 24 hours. Notes: Incidence of postoperative nausea and vomiting (A); postoperative analgesic consumption (B). |
Postoperative analgesic use at 24 hours
Postoperative opioid consumption (morphine equivalents) at 24 hours was evaluated by three trials. In this analysis, analgesic use was the same between the two groups (MD –3.66 mg, I2=0, 95% CI –8.67 to 1.34; P=0.15; Figure 4B).
Grade quality
GradePro evaluation of the confidence of evidence is shown in Table 2.
Discussion
The results of our review and meta-analysis show that the two modalities of perineural and intravenous dexamethasone as local anesthetic additives can produce a similar effect on the duration of analgesia or sensory block. From the subgroup analysis, we found that the high heterogeneity in the data analysis stemmed from adding epinephrine or not, a well-known adjuvant in local anesthetics to prolong block duration.26,27 With epinephrine, prolongation of duration of analgesia and motor block were observed in the perineural dexamethasone group when compared to the intravenous dexamethasone method. Without epinephrine, both groups showed an equivalent effect on analgesia duration. In addition, the incidence of postoperative nausea and vomiting and postoperative analgesic consumption at 24 hours exhibited no statistical significant differences when comparing the two routes of administration.
Such adjuvants as midazolam,28 ketamine,29 clonidine,30 dexmedetomidine,31 epinephrine, or dexamethasone are coadministered with local anesthetics in order to enhance the effect of single-shot peripheral nerve block. Although several meta-analyses4–6 have demonstrated that dexamethasone given perineurally can extend the analgesic duration of common local anesthetics for brachial plexus block, the effectiveness of intravenous dexamethasone has been controversial.12 Actually, in a meta-analysis of 38 studies, systemic dexamethasone (>0.1 mg·kg–1) reduced postoperative pain and analgesic consumption.7 As such, the current paramount issue is to demonstrate that perineural dexamethasone has an extra effect on the duration of analgesia through a direct mechanism on nerve blocking.32 In this meta-analysis, the pooled results from ten RCTs revealed that dexamethasone (4–10 mg) can produce similar duration of sensory or motor block when administered perineurally or intravenously without epinephrine. In other words, the viewpoint that dexamethasone has a direct inhibition of peripheral nerves needs to be considered carefully and examined again. However, limited evidence in our review and meta-analysis elucidates that there may be a synergistic effect between dexamethasone and epinephrine when given locally.
On the one hand, whether or not dexamethasone has a direct effect on nerve conduction has been disputed. In isolated rat sciatic nerves, neither dexamethasone nor buprenorphine can inhibit the compound action potentials from A and C fibers.33 In vivo, an animal study demonstrated that bupivacaine plus 67 μg dexamethasone did not increase block duration more than bupivacaine alone.34 In contrast, in a mouse sciatic nerve-blockade model, high-dose (0.5 mg·kg–1) perineural dexamethasone added to bupivacaine prolonged the duration of sensory and motor block, while low-dose (0.14 mg·kg–1) dexamethasone did not. However, these results should be considered with skepticism, because of the unblinded procedure in that study.35 Despite the fact that sciatic nerves are commonly used to evaluate the effect of local anesthetic, we cannot directly extrapolate the results from rodents to humans. In addition, the period of block conduction can differ due to the neurobiology of acute postoperative pain in clinical patients. Acute postoperative pain includes not the conduction of nociception, but direct nerve damage and inflammatory mediator release, which activate peripheral nociceptors to deliver information to the central nervous system.1 Dexamethasone, a long-effective glucocorticoid, has the appropriate anti-inflammatory property by increasing the production of anti-inflammatory substances and decreasing the release of inflammatory mediators.36 This characteristic may be responsible for its systematic mechanism in prolonging block duration. Overall, further studies are needed to find and prove the precise indirect mechanism of dexamethasone and also its interaction with epinephrine perineurally.
On the other hand, dexamethasone is prescribed “off-label” for perineural administration. Neurotoxicity is a serious problem for local anesthetics and additives that we have to consider. Dexamethasone 133 μg·mL–1 combined with ropivacaine increased neurotoxicity for isolated sensory neurons. As such, the author advised that much attention be paid to the time- and concentration-dependent toxicity when dexamethasone is combined with ropivacaine.37 In agreement with previous results,38,39 in a preliminary animal experiment, we found that solutions of commonly used local anesthetics (bupivacaine, ropivacaine) in combination with nonparticulate dexamethasone sodium phosphate could crystallize, even in physiological pH (unpublished data). Therefore, the patient’s safety might be compromised when crystalliferous solution is unintentionally injected into the subarachnoid space or into the blood vessels.
Considering potential neurotoxicity or hazards from crystallization, the unknown mechanism of action, and the fact that both methods have a similar effect on block duration, controlling nausea and vomiting, and sparing opioid consumption, we conclude that intravenous dexamethasone is preferable to perineural dexamethasone, as it carries fewer risks to the patient. Moreover, a recent published meta-analysis40 proved that a combination of intravenous dexamethasone with other antiemetics showed more efficacy than a single antiemetic in preventing nausea and vomiting after laparoscopic cholecystectomy.
There are some limitations of this analysis. Firstly, postoperative blood glucose levels and long-term neurological sequelae were not analyzed. Only two of the ten trials15,19 chosen here reported blood glucose concentrations after surgery. In these two studies, data demonstrated that there were no significant differences in blood glucose levels between the two routes of administration. Also, no serious relevant neurologic symptoms were found in these clinical trials. Secondly, although heterogeneity was low or inexistent among the four outcomes, there was a risk of bias in some of the studies. Thirdly, this review did not evaluate the interaction between the dosage of dexamethasone and block duration. However, Albrecht et al41 elucidated that no inconclusive evidence was found between different concentrations of dexamethasone (4–10 mg) and analgesic duration by subgroup analysis.
Knowledge on many aspects of the two modalities is not clear, and further clinical studies are required to explore and determine their mechanism of action. A large-scale, multicenter, prospective, double-blinded RCT is needed to be performed to prove which is the most effective adjuvant, the best method of delivery (local anesthetic, perineural, or intravenous dexamethasone), and whether dexamethasone has a synergistic or additive effect with epinephrine when administered locally. If the most efficient route of administration proves to be additive, then the optimal dose of dexamethasone has to be determined. Finally, prospective studies should investigate the safety of dexamethasone at higher doses (>133 μg·mL–1), when administered perineurally with local anesthetics.
Conclusion
Our systematic review and meta-analysis suggests that local epinephrine and dexamethasone have a synergistic effect. However, without epinephrine, intravenous dexamethasone and perineural dexamethasone share similar effects on block duration, postoperative nausea and vomiting, and postoperative analgesic consumption at 24 hours. At present, and considering the potential risk of the off-label use of dexamethasone perineurally and its as yet unknown mechanism of action, the route of intravenous administration is thus preferable. Further animal and human studies are needed to explore the definite relationship and the potential synergistic mechanism between local dexamethasone and epinephrine and to select the most effective route of administration required to guide clinical practice.
Acknowledgment
The authors show much gratitude to Robert McCarthy for supplying the raw data presented in their studies.
Disclosure
The authors report no conflicts of interest in this work.
References
Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia: Churchill Livingstone; 2009. | ||
Candido KD, Knezevic NN. All adjuvants to local anesthetics were not created equal: animal data evaluating neurotoxicity, thermal hyperalgesia, and relevance to human application. Reg Anesth Pain Med. 2011;36(3):211–212. | ||
Kirksey MA, Haskins SC, Cheng J, Liu SS. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: a systematic qualitative review. PloS One. 2015;10(9):e0137312. | ||
Arulkumar S, Elnagar I, Hamilton C, Fox C. Dexamethasone as an adjunct for brachial plexus blockade: meta-analysis. 2014. Available from: http://www.lsuhscshreveport.edu/Assets/uploads/LSUHealthShreveport/Documents/Anesthesiology/Posters/DexamethasoneRegionalAnesthesiaIARS2014.pdf. Accessed June 13, 2017. | ||
Huynh TM, Marret E, Bonnet F. Combination of dexamethasone and local anaesthetic solution in peripheral nerve blocks: A meta-analysis of randomised controlled trials. Eur J Anaesthesiol. 2015;32(11):751–758. | ||
Choi S, Rodseth R, McCartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2014;112(3):427–439. | ||
De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588. | ||
Lee S, Choi S. Intravenous and perineural dexamethasone in peripheral nerve block: are they truly equivalent? Anesth Analg. 2015;121(1):251. | ||
Sondekoppam RV, Uppal V, Ganapathy S. Intravenous or perineural dexamethasone for interscalene brachial plexus block: the equivalence not yet proven. Br J Anaesth. 2014;112(1):175–176. | ||
Tighe SQ. Perineural or intravenous dexamethasone: do we still need catheters? Anaesthesia. 2016;71(8):983–984. | ||
Bolin ED, Wilson S. Perineural versus systemic dexamethasone: questions remain unanswered. Reg Anesth Pain Med. 2015;40(4):393–394. | ||
Albrecht E, Kern C, Kirkham KR. Perineural vs intravenous administration of dexamethasone: more data are available. Br J Anaesth. 2015;114(1):160. | ||
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. | ||
Abdelmonem A, Rizk SN. Comparative study between intravenous and local dexamethasone as adjuvant to bupivacaine in perianal block. Egypt J Anaesth. 2011;27(3):163–168. | ||
Desmet M, Braems H, Reynvoet M, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study. Br J Anaesth. 2013;111(3):445–452. | ||
Kawanishi R, Yamamoto K, Tobetto Y, et al. Perineural but not systemic low-dose dexamethasone prolongs the duration of interscalene block with ropivacaine: a prospective randomized trial. Local Reg Anesth. 2014;7:5–9. | ||
Rahangdale R, Kendall MC, McCarthy RJ, et al. The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: a randomized, double-blind, placebo-controlled study. Anesth Analg. 2014;118(5):1113–1119. | ||
Abdallah FW, Johnson J, Chan V, et al. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: a randomized, triple-arm, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2015;40(2):125–132. | ||
Chun EH, Kim YJ, Woo JH. Which is your choice for prolonging the analgesic duration of single-shot interscalene brachial blocks for arthroscopic shoulder surgery? Intravenous dexamethasone 5 mg vs. perineural dexamethasone 5 mg randomized, controlled, clinical trial. Medicine (Baltimore). 2016;95(23):e3828. | ||
Dawson RL, McLeod DH, Koerber JP, Plummer JL, Dracopoulos GC. A randomised controlled trial of perineural vs intravenous dexamethasone for foot surgery. Anaesthesia. 2016;71(3):285–290. | ||
Leurcharusmee P, Aliste J, Van Zundert TC, et al. A multicenter randomized comparison between intravenous and perineural dexamethasone for ultrasound-guided infraclavicular block. Reg Anesth Pain Med. 2016;41(3):328–333. | ||
Rosenfeld DM, Ivancic MG, Hattrup SJ, et al. Perineural versus intravenous dexamethasone as adjuncts to local anaesthetic brachial plexus block for shoulder surgery. Anaesthesia. 2016;71(4):380–388. | ||
Aliste J, Leurcharusmee P, Engsusophon P, et al. A randomized comparison between intravenous and perineural dexamethasone for ultrasound-guided axillary block. Can J Anaesth. 2017;64(1):29–36. | ||
Morales-Muñoz C, Sánchez-Ramos JL, Diaz-Lara MD, González-González J, Gallego-Alonso I, Hernández-del-Castillo MS. Analgesic effect of a single-dose of perineural dexamethasone on ultrasound-guided femoral nerve block after total knee replacement. Rev Esp Anestesiol Reanim. 2017;64(1):19–26. | ||
Rücker G, Carpenter JR, Schwarzer G. Detecting and adjusting for small-study effects in meta-analysis. Biom J. 2011;53(2):351–368. | ||
Niemi G, Breivik H. Adrenaline markedly improves thoracic epidural analgesia produced by a low-dose infusion of bupivacaine, fentanyl and adrenaline after major surgery: a randomised, double-blind, cross-over study with and without adrenaline. Acta Anaesthesiol Scand. 1998;42(8):897–909. | ||
Sakaguchi Y, Sakura S, Shinzawa M, Saito Y. Does adrenaline improve epidural bupivacaine and fentanyl analgesia after abdominal surgery? Anaesth Intensive Care. 2000;28(5):522–526. | ||
Boussofara M, Carles M, Raucoules-Aime M, Sellam MR, Horn JL. Effects of intrathecal midazolam on postoperative analgesia when added to a bupivacaine-clonidine mixture. Reg Anesth Pain Med. 2006;31(6):501–505. | ||
Kazemeini A, Rahimi M, Fazeli MS, et al. The effect of local injections of bupivacaine plus ketamine, bupivacaine alone, and placebo on reducing postoperative anal fistula pain: a randomized clinical trial. ScientificWorldJournal. 2014;2014:424152. | ||
Trifa M, Engelhardt T, Khalifa SB. The addition of clonidine to bupivacaine in saphenous/sciatic nerve blocks in children. Paediatr Anaesth. 2016;26(3):321–322. | ||
Xiang Q, Huang DY, Zhao YL, et al. Caudal dexmedetomidine combined with bupivacaine inhibit the response to hernial sac traction in children undergoing inguinal hernia repair. Br J Anaesth. 2013;110(3):420–424. | ||
Martinez V, Fletcher D. Dexamethasone and peripheral nerve blocks: on the nerve or intravenous? Br J Anaesth. 2014;113(3):338–340. | ||
Yilmaz-Rastoder E, Gold MS, Hough KA, Gebhart GF, Williams BA. Effect of adjuvant drugs on the action of local anesthetics in isolated rat sciatic nerves. Reg Anesth Pain Med. 2012;37(4):403–409. | ||
Buvanendran A, Kroin JS, Li J, Moric M, Tuman KJ. Relative contribution of adjuvants to local anesthetic for prolonging the duration of peripheral nerve blocks in rats. Reg Anesth Pain Med. 2016;41(5):589–592. | ||
An K, Elkassabany NM, Liu J. Dexamethasone as adjuvant to bupivacaine prolongs the duration of thermal antinociception and prevents bupivacaine-induced rebound hyperalgesia via regional mechanism in a mouse sciatic nerve block model. PloS One. 2015;10(4):e0123459. | ||
Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond). 1998;94(6):557–572. | ||
Williams BA, Hough KA, Tsui BY, Ibinson JW, Gold MS, Gebhart GF. Neurotoxicity of adjuvants used in perineural anesthesia and analgesia in comparison with ropivacaine. Reg Anesth Pain Med. 2011;36(3):225–230. | ||
Watkins TW, Dupre S, Coucher JR. Ropivacaine and dexamethasone: a potentially dangerous combination for therapeutic pain injections. J Med Imaging Radiat Oncol. 2015;59(5):571–577. | ||
Hwang H, Park J, Lee WK, et al. Crystallization of local anesthetics when mixed with corticosteroid solutions. Ann Rehabil Med. 2016;40(1):21–27. | ||
Awad K, Ahmed H, Abushouk AI, et al. Dexamethasone combined with other antiemetics versus single antiemetics for prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy: an updated systematic review and meta-analysis. Int J Surg. 2016; 36(Pt A):152–163. | ||
Albrecht E, Kern C, Kirkham KR. A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia. 2015;70(1):71–83. |
Supplementary materials
  | Table S1 Search strategy |
  |
  | Table S2 Definitions Abbreviation: VAS, visual analog scale. |
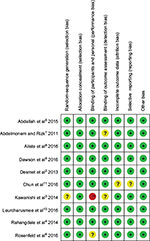  | Figure S1 Risks of bias for the ten included studies by all authors’ judgment. Notes: Red, high risk of bias; yellow, unclear risk of bias; green, low risk of bias. |
  | Figure S2 Funnel plots of duration of analgesia: adding epinephrine (left) and not adding epinephrine (right). |
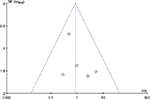  | Figure S3 Funnel plot of postoperative nausea and vomiting. |
References
Abdelmonem A, Rizk SN. Comparative study between intravenous and local dexamethasone as adjuvant to bupivacaine in perianal block. Egypt J Anaesth. 2011;27(3):163–168. | ||
Desmet M, Braems H, Reynvoet M, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study. Br J Anaesth. 2013;111(3):445–452. | ||
Kawanishi R, Yamamoto K, Tobetto Y, et al. Perineural but not systemic low-dose dexamethasone prolongs the duration of interscalene block with ropivacaine: a prospective randomized trial. Local Reg Anesth. 2014; 7:5–9. | ||
Rahangdale R, Kendall MC, McCarthy RJ, et al. The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: a randomized, double-blind, placebo-controlled study. Anesth Analg. 2014;118(5):1113–1119. | ||
Abdallah FW, Johnson J, Chan V, et al. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: a randomized, triple-arm, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2015;40(2):125–132. | ||
Rosenfeld DM, Ivancic MG, Hattrup SJ, et al. Perineural versus intravenous dexamethasone as adjuncts to local anaesthetic brachial plexus block for shoulder surgery. Anaesthesia. 2016;71(4):380–388. | ||
Chun EH, Kim YJ, Woo JH. Which is your choice for prolonging the analgesic duration of single-shot interscalene brachial blocks for arthroscopic shoulder surgery? Intravenous dexamethasone 5 mg vs. perineural dexamethasone 5 mg randomized, controlled, clinical trial. Medicine (Baltimore). 2016;95(23):e3828. | ||
Aliste J, Leurcharusmee P, Engsusophon P, et al. A randomized comparison between intravenous and perineural dexamethasone for ultrasound-guided axillary block. Can J Anaesth. 2017;64(1):29–36. | ||
Dawson RL, McLeod DH, Koerber JP, Plummer JL, Dracopoulos GC. A randomised controlled trial of perineural vs intravenous dexamethasone for foot surgery. Anaesthesia. 2016;71(3):285–290. | ||
Leurcharusmee P, Aliste J, Van Zundert TC, et al. A multicenter randomized comparison between intravenous and perineural dexamethasone for ultrasound-guided infraclavicular block. Reg Anesth Pain Med. 2016;41(3):328–333. | ||
Chun EH, Kim YJ, Woo JH. Which is your choice for prolonging the analgesic duration of single-shot interscalene brachial blocks for arthroscopic shoulder surgery? Intravenous dexamethasone 5 mg vs. perineural dexamethasone 5 mg randomized, controlled, clinical trial. Medicine (Baltimore). 2016;95(23):e3828. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

