Back to Journals » Infection and Drug Resistance » Volume 12
Performance evaluation of the (1,3)-β-D-glucan detection assay in non-intensive care unit adult patients
Authors Murri R, Camici M, Posteraro B, Giovannenze F , Taccari F , Ventura G, Scoppettuolo G , Sanguinetti M , Cauda R, Fantoni M
Received 27 July 2018
Accepted for publication 6 September 2018
Published 20 December 2018 Volume 2019:12 Pages 19—24
DOI https://doi.org/10.2147/IDR.S181489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Rita Murri,1 Marta Camici,1 Brunella Posteraro,2 Francesca Giovannenze,1 Francesco Taccari,1 Giulio Ventura,1 Giancarlo Scoppettuolo,1 Maurizio Sanguinetti,3 Roberto Cauda,1 Massimo Fantoni1
1Institute of Infectious Diseases, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy; 2Institute of Medical Pathology and Semeiotics, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy; 3Institute of Microbiology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy
Objectives: To assess the performance of the (1,3)-β-D-glucan (BDG) detection assay in a large cohort of patients with suspected candidemia who were admitted to non-intensive care unit hospital wards.
Methods: This observational, retrospective cohort study was conducted in a 1,100-bed university hospital in Rome, where an infectious disease consultation team has been operational. Two groups of patients were included in the analysis: Group 1, patients with Candida bloodstream infection (BSI) who had at least one BDG test performed ±48 hours from the first positive blood culture (Candida BSI Group) and Group 2, patients with risk factors for candidemia who had at least one BDG test but had negative blood cultures (Control Group). Both Group 1 and Group 2 did not receive prior antifungal therapy. Different BDG cutoff values were considered: 80, 200, 300, 400, and ≥500 pg/mL. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the receiver operating characteristic curve were calculated.
Results: A total of 1,296 patients were studied. Of them, 100 patients (candidemic) were in Group 1 and the remaining 1,196 patients (controls) were in Group 2. There were no differences in demographic characteristics between patients of the two groups. According to the above cutoff values, sensitivity (%) and specificity (%) of the BDG assay ranged from 91 to 60.7 and 87.7 to 97.8, respectively, whereas the PPV (%) and NPV (%) ranged from 38.2 to 68.3 and 99.1 to 97.0, respectively.
Conclusion: Serum BDG has a very high NPV in a population with~10% prevalence of candidemia. This NPV may support decisions to discontinue antifungal therapy in those patients who were empirically treated because of the suspect of candidemia.
Keywords: β-glucan, candidemia, Candida, bloodstream infections, antimicrobial stewardship, diagnostic biomarkers
Background
Bloodstream infections (BSIs) due to Candida spp. are a frequent clinical condition in hospitalized patients, which significantly contributes to their morbidity and mortality. Candida spp. account for 9% of all BSIs and up to 25% of BSIs associated with central venous catheter (CVC).1 The mortality rate of candidemia has been found to be high in the past two decades, being estimated as 5%–71%.2–4
The gold standard method for the diagnosis of Candida BSIs are blood cultures, which usually take about 2 days to yield positive results, but their sensitivity is as low as 50%.5,6 Many studies have investigated the risk factors for candidemia; however, the list of risk factors is large7 and most of the hospitalized patients shared at least one of these risk factors. Several scores have been evaluated to help in identifying patients at the highest risk of Candida BSIs, but they are often difficult to calculate in nonintensive care unit (non-ICU) settings.8,9 Therefore, when suspecting candidemia, criteria that allow physicians to start antifungal therapy remain controversial. Anyway, appropriate antifungal therapy, especially when early administered, was seen to correlate with a better survival.10
To reduce unnecessary antifungal empirical therapy, many efforts have been devoted to improve the diagnostic sensitivity of culture-independent tests.11 Among them, the T2Candida nanodiagnostic panel (T2 Biosystems, Lexington, MA, USA) and a (1,3)-β-D-glucan (BDG) assay (Fungitell; Associates of Cape Cod, East Falmouth, MA, USA) were cleared by the US Food and Drug Administration for the diagnosis of candidemia and invasive fungal infections, respectively.12 As Bayesian biomarkers, these tests assign a probability of infection; thence, management decisions based on test results will be left to the judgment of physicians .12 However, both molecular and nonmolecular diagnostic tests could be a strong support for more rapidly identifying patients who should start empirical antifungal treatment.13
With regard to BDG, studies conducted in the ICU setting showed that BDG assay can be used to specifically improve the early discrimination between patients with culture-documented candidemia and patients with suspected candidemia.14–16 Particularly, high negative predictive values (NPVs) of BDG (≥97%) in patients at risk for candidemia12 could be useful for an early discontinuation of antifungal therapy. To the best of our knowledge, very few studies were conducted in non-ICU wards until now. In the present study, we aimed to assess the performance of the BDG assay in a large cohort of patients with suspected candidemia admitted to non-ICU hospital wards.
Methods
This observational, retrospective, case–control study was conducted at a 1,100-bed university hospital (Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy), where an inpatient infectious disease consultation team (IDCT) has operated since November 2012. The team is made up of four infectious disease specialists. Any hospital physician operating in medical and surgical units can request an infectious disease consultation via the hospital’s computerized information system. The IDCT takes charge of patients at the bedside within 24 hours of the request. The service is not active in Hematology unit and ICU, so patients from these wards were not included in the study. Follow-up consultations are provided only after a request by the ward physician. An alert system for blood cultures is active from Monday to Saturday: a microbiologist informs the in-charge infectious disease specialist of any positive blood culture at the time of identification. Data from every consultation is daily added into a standardized database.
To calculate the sensitivity, specificity, positive predictive value (PPV), and NPV of BDG assay, we identified two groups of people. Group 1 consisted of all patients with culture-documented candidemia who had at least one BDG test performed ±48 hours from the first positive blood culture and had never been treated before with antifungal therapy (ie, Candida BSI Group). Group 2 consisted of all patients with at least one risk factor for candidemia (ie, surgery in the previous 30 days, presence of CVC, antibiotic therapy in the last 90 days, dialysis, or an immunosuppression status) who had at least one BDG test performed but had negative blood cultures and had never been treated with antifungal therapy (ie, Control Group). All consecutive patients from January 2013 to November 2016 who met one of the two study group criteria were enrolled in Candida BSI Group or Control Group, respectively. Candida BSI was defined as the isolation of Candida spp. in at least one blood culture drawn from a patient. Candida catheter-related (CR)-BSI was defined when the time of positivity of blood culture from a peripheral vein was at least 2 hours more than time of positivity of blood culture from a CVC.17 Septic shock at the time of blood culture was also assessed. During the study period, the rate of Candida BSIs was 16% of all BSIs identified at our Institution.
The Fungitell assay (Associates of Cape Cod) was used for BDG measurement. BDG results were evaluated using different cutoff values: 80, 200, 300, 400, and ≥500 pg/mL. Candida spp. were isolated from blood using the Bactec (Thermo Fisher Scientific, Waltham, MA, USA) or BacT/Alert (bioMérieux, Marcy l’Etoile, France) system and were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.18 For clinical use, the BDG test was considered positive if BDG level was above the manufacturer’s recommended cutoff (80 pg/mL).
No informed consent was required because the activity of the IDCT constitutes routine clinical practice and only anonymized data were analyzed. The need for informed consent was waived by the local Institutional Review Board of the Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, that approved the study (protocol number 49/18).
Statistical analysis
Demographic, epidemiological, and clinical characteristics of patients in Candida BSI Group were compared to those of Control Group. Normally distributed values were expressed as mean (±SD), and nonnormally distributed values as median (interquartile range). Chi-squared or Fisher’s exact test was used to compare the distribution of categorical variables, and Student’s t-test or Mann–Whitney U test was used to compare quantitative variables. A two-sided P-value <0.05 was considered statistically significant. To calculate the diagnostic performance of BDG assay, the sensitivity, specificity, PPV, and NPV of the test and their 95% CIs were calculated.
The 30-day crude mortality rate (death from any cause within 30 days from the first positive blood cultures in Candida BSI Group and from the BDG assay in Control Group) was also calculated for both the study groups stratifying for the BDG result. The discriminatory power of the BDG assay was assessed by the area under the receiver operating characteristic (ROC) curve (AUROC) calculation. All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
We identified 1,296 patients with at least one BDG result, of which 100 patients were in Candida BSI Group and 1,196 patients were in Control Group. The mean age was 65 (±20) years, and 774 patients (59.7%) were males. Approximately two-thirds of the study population was admitted to Medicine wards and a third to General Surgery (and other surgical specialties) wards. The clinical characteristics of the study population are shown in Table 1. Patients in Candida BSI Group were more likely to have septic shock (9.1% vs 0.7%; chi-squared test, P<0.001). Risk factors for Candida infection, such as the presence of CVC or a previous hospital stay, were significantly more common in Candida BSI Group. In 38 cases (38%) of candidemia, a CR-BSI was identified. Sixty-two percent of Candida BSIs were caused by Candida albicans, 24.2% by Candida parapsilosis, and 5% by Candida glabrata. The 30-day mortality rate was significantly higher in Candida BSI Group than in Control Group (30.3% vs 8.6%; chi-squared test, P<0.0001).
  | Table 1 Characteristics of the study population Abbreviation: BSI, bloodstream infection. |
The mean of risk factors for candidemia was 0.86 (SD 0.80). It was higher in people with positive BDG result (1.13 [SD 0.84]) than in people with negative BDG result (0.80 [SD 0.78]) (t-test, P<0.001). Only few patients (33, 2.5%) had three or more risk factors.
Using the 80 pg/mL cutoff value, a negative BDG result was found in nine (9%) of the 100 patients in Candida BSI Group, and in 1,049 (87.7%) of the 1,196 patients in Control Group. Only one of the nine patients with candidemia and negative BDG result had a BSI due to C. parapsilosis. The number of patients who had candidemia despite a negative BDG result was nine out of 1,058 (0.85%). In Control Group, 34 (2.8%) patients had a BDG cutoff of ≥500 pg/mL. The sensitivity, specificity, PPV, and NPV of BDG test at different cutoff values are shown in Table 2. Time to BDG test did not correlate with the BDG value (linear regression, P=0.34).
With the increase of the cutoff value (80 to ≥500 pg/mL), sensitivity of the biomarker decreased (from 89.8% to 60.7%), whereas specificity remained >90% (up to 97.8%). The AUROC was ≥0.79 for all the cutoff values of BDG. Figure 1 shows the AUROC calculated using the cutoff of 80 pg/mL as a diagnostic value.
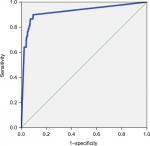  | Figure 1 Area under the ROC curve showing the diagnostic sensitivity and specificity of the BDG detection assay. Abbreviations: BDG, (1,3)-β-D-glucan; ROC, receiver operating characteristic. |
Overall, the 30-day mortality of patients with a positive BDG result was higher than that of patients with a negative BDG result (19.8% vs 8.0%; chi-squared test, P<0.001). Only in Control Group, patients with a positive BDG result trended toward higher mortality compared to patients with a negative BDG result (12.2% vs 8.0%; chi-squared test, P=0.08). In particular, 18 of 34 patients with a positive BDG result died within 30 days without starting antifungal therapy (mean of survival, 7.9 days [SD 7.3]), and six of these patients had a BDG value of ≥500 pg/mL (mean of survival, 8 days [SD 8.6]).
Discussion
In the present study that involves non-ICU patients, we show that the BDG detection assay (cutoff, 80 pg/mL) had a high NPV (>99%), thus confirming the good accuracy of the assay for identifying patients without Candida BSI. Our findings may support strategies aimed at discontinuing empirical antifungal therapy when BDG results are negative.
Several studies have evaluated the performance of BDG assay in different clinical contexts. Studying a small population of ICU patients in 2011, Posteraro et al showed that the NPV of BDG was higher (98.7%) than that of Candida score (≥3, 97.2%) or colonization index (≥0.5, 91.7%).14 In a study on 89 surgical ICU patients with abdominal candidiasis, Tissot et al found an NPV of 78%,15 whereas in a study on 100 patients, most of them admitted to ICU, the NPV of BDG was 86%–90%.16 Giacobbe et al recently found a high PPV (96%) and a high NPV (93%–95%) when using both BDG and procalcitonin for the diagnosis of Candida BSI in ICU patients.19 In two meta-analyses,20,21 the AUC for BDG was 0.88 and 0.84. In a study on application of mass spectrometry in diagnosis of fungal infections, Mery et al showed that mass spectrometry disaccharide index potentially complements BDG detection.22 Although it is difficult to compare our results with those of previous reports because of differences in the study design, invasive candidiasis definition, patient populations, and negative controls, the NPV of BDG was always excellent. Unlike previous studies, we included a very large population and, to maximize the stringency of the study, we used as controls those patients with risk factors for candidemia but who did never receive antifungal therapy. Some authors highlighted that BDG test result could be more frequently negative in patients with C. parapsilosis BSI.23 However, in our population only one of nine patients with a negative BDG result had a C. parapsilosis BSI.
The pretest probability in the present cohort was 8%, slightly lower than in other published cohorts. This is probably due to the non-ICU setting of the study. It should be considered that in extremely high-risk patients (such as those in ICU with >30% of pretest likelihood), NPV could be lower than that in our study.
Confirming previously published results, the role of BDG in the diagnosis of Candida BSI is probably less relevant than excluding it. In fact, sensitivity of BDG is suboptimal, ranging from 77% to 91%.24,25 In our study, even at the highest cutoff (≥500 pg/mL), sensitivity was 61%. BDG can be found in many biological compounds, and several factors, such as antimicrobial therapy, nutritional intake, albumin, surgical sponges and/or gauze, and hemodialysis products, are known to be possible causes of false positivity.26 According to some studies, even systemic bacterial infections may result in false-positive BDG results.27 However, since 18 patients in Control Group (1.5%) died within 30 days, we cannot definitively rule out the presence of Candida infection considering that the blood culture sensitivity is suboptimal.28
Empirical antifungal therapy accounts for about 40% of antifungal use.16,29–31 Hence, efforts should be focused on improving the diagnostic sensitivity and specificity of available tests. Considering the well-recognized limitations of blood cultures in diagnosing invasive candidiasis,32,33 an accurate and rapid biomarker could be very useful in treatment decision for patients with suspected candidemia. BDG results can be available in 12–24 hours. Considering the NPV of our study, clinicians could stop treatment based on negative result rapidly but not before the results of negative blood cultures are known (if possible, never before the first 48–72 hours). Determination of BDG is costly, and there are no cost-benefit studies of BDG determination in non-ICU patients. For these reasons, in our opinion, its use should be limited to professionals with a great expertise in the infectious diseases field and the management of invasive fungal infections.
People with positive BDG result had a higher mortality in our study. This result confirms previous findings.34–36 It should be noted that the mean of survival in people with positive BDG result within the Control Group was very short. We do not know whether an earlier initiation of empirical antifungal therapy in these patients could have modified the survival.
The study has several limitations. First, since it is a monocentric study, the generalizability of the results must be demonstrated. Second, this is not an intervention study. The real efficacy of a BDG-based strategy to control overtreatment with antifungal regimens is not widely demonstrated. Unfortunately, only retrospective studies were published on the use of BDG in daily practice and almost all of them were done in ICU patients. Third, the usefulness of BDG in uncertain Candida infections is unknown. Fourth, no data on invasive candidiasis in the absence of candidemia (ie, abdominal candidiasis) are reported and the performance of BDG in such a situation was not evaluated.
Conclusion
The NPV value of BDG result was optimal even in a real-world setting, possibly supporting prospective studies on discontinuation of empirical antifungal therapy when BDG results are negative. The control of overtreatment is a cornerstone of antimicrobial stewardship: cost-effectiveness studies on the use of BDG in the context of a suspected invasive candidiasis are strongly warranted as well as studies on a BDG-based therapeutic approach.
Disclosure
The authors report no conflicts of interest in this work.
References
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. | ||
Falagas ME, Apostolou KE, Pappas VD. Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur J Clin Microbiol Infect Dis. 2006;25(7):419–425. | ||
Tumbarello M, Fiori B, Trecarichi EM, et al. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS One. 2012;7(3):e33705. | ||
Bassetti M, Merelli M, Righi E, et al. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J Clin Microbiol. 2013;51(12):4167–4172. | ||
Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284–1292. | ||
Avni T, Leibovici L, Paul M. PCR diagnosis of invasive candidiasis: systematic review and meta-analysis. J Clin Microbiol. 2011;49(2):665–670. | ||
Scudeller L, Viscoli C, Menichetti F, et al. An Italian consensus for invasive candidiasis management (ITALIC). Infection. 2014;42(2):263–279. | ||
León C, Ruiz-Santana S, Saavedra P, et al. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34(3):730–737. | ||
Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994;220(6):751–758. | ||
Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54(12):1739–1746. | ||
Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM, Mylonakis E. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev. 2014;27(3):490–526. | ||
Clancy CJ, Nguyen MH. Non-culture diagnostics for invasive candidiasis: promise and unintended consequences. J Fungi. 2018;4(1):27. | ||
Clancy CJ, Nguyen MH. Diagnosing Invasive Candidiasis. J Clin Microbiol. 2018;56(5):e01909–17. | ||
Posteraro B, de Pascale G, Tumbarello M, et al. Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1→3)-β-D-glucan assay, Candida score, and colonization index. Crit Care. 2011;15(5):R249. | ||
Tissot F, Lamoth F, Hauser PM, et al. β-glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasis. Am J Respir Crit Care Med. 2013;188(9):1100–1109. | ||
Martínez-Jiménez MC, Muñoz P, Valerio M, Vena A, Guinea J, Bouza E. Combination of Candida biomarkers in patients receiving empirical antifungal therapy in a Spanish tertiary hospital: a potential role in reducing the duration of treatment. J Antimicrob Chemother. 2015;70(11):3107–3115. | ||
Park KH, Lee MS, Lee SO, et al. Diagnostic usefulness of differential time to positivity for catheter-related candidemia. J Clin Microbiol. 2014;52(7):2566–2572. | ||
de Carolis E, Vella A, Vaccaro L, et al. Development and validation of an in-house database for matrix-assisted laser desorption ionization-time of flight mass spectrometry-based yeast identification using a fast protein extraction procedure. J Clin Microbiol. 2014;52(5):1453–1458. | ||
Giacobbe DR, Mikulska M, Tumbarello M, et al. Combined use of serum (1,3)-β-D-glucan and procalcitonin for the early differential diagnosis between candidaemia and bacteraemia in intensive care units. Crit Care. 2017;21(1):176. | ||
Hou TY, Wang SH, Liang SX, Jiang WX, Luo DD, Huang DH. The screening performance of serum 1,3-beta-D-glucan in patients with invasive fungal diseases: a meta-analysis of prospective cohort studies. PLoS One. 2015;10(7):e0131602. | ||
Lamoth F, Cruciani M, Mengoli C, et al. β-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin Infect Dis. 2012;54(5):633–643. | ||
Mery A, Sendid B, François N, et al. Application of mass spectrometry technology to early diagnosis of invasive fungal infections. J Clin Microbiol. 2016;54(11):2786–2797. | ||
Mikulska M, Giacobbe DR, Furfaro E, et al. Lower sensitivity of serum (1,3)-β- d -glucan for the diagnosis of candidaemia due to Candida parapsilosis. Clin Microbiol Infec. 2016;22(7):646.e5–646.e8. | ||
Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1→3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41(5):654–659. | ||
Persat F, Ranque S, Derouin F, Michel-Nguyen A, Picot S, Sulahian A. Contribution of the (1→3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2008;46(3):1009–1013. | ||
Marty FM, Koo S. Role of (1→3)-beta-D-glucan in the diagnosis of invasive aspergillosis. Med Mycol. 2009;47(Suppl 1):S233–S240. | ||
Albert O, Toubas D, Strady C, et al. Reactivity of (1→3)-β-d-glucan assay in bacterial bloodstream infections. Eur J Clin Microbiol Infect Dis. 2011;30(11):1453–1460. | ||
Friedrich R, Rappold E, Bogdan C, Held J. Comparative Analysis of the Wako β-Glucan test and the Fungitell® assay for the diagnosis of Candidemia and Pneumocystis jirovecii pneumonia. J Clin Microbiol. 2018:e00464-18. | ||
León C, Ostrosky-Zeichner L, Schuster M. What’s new in the clinical and diagnostic management of invasive candidiasis in critically ill patients. Intensive Care Med. 2014;40(6):808–819. | ||
Valerio M, Rodriguez-Gonzalez CG, Muñoz P, et al. Evaluation of antifungal use in a tertiary care institution: antifungal stewardship urgently needed. J Antimicrob Chemother. 2014;69(7):1993–1999. | ||
Ruhnke M. Antifungal stewardship in invasive Candida infections. Clin Microbiol Infect. 2014;20(Suppl 6):11–18. | ||
Berenguer J, Buck M, Witebsky F, Stock F, Pizzo PA, Walsh TJ. Lysis-centrifugation blood cultures in the detection of tissue-proven invasive candidiasis. Disseminated versus single-organ infection. Diagn Microbiol Infect Dis. 1993;17(2):103–109. | ||
Groll AH, Shah PM, Mentzel C, Schneider M, Just-Nuebling G, Huebner K. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect. 1996;33(1):23–32. | ||
Bassetti M, Merelli M, Ansaldi F, et al. Clinical and therapeutic aspects of candidemia: a five year single centre study. PLoS One. 2015;10(5):e0127534. | ||
Sbrana F, Sozio E, Bassetti M, et al. Independent risk factors for mortality in critically ill patients with candidemia on Italian Internal Medicine Wards. Intern Emerg Med. 2018;13(2):199–204. | ||
Posteraro B, Tumbarello M, de Pascale G, et al. (1,3)-β-d-Glucan-based antifungal treatment in critically ill adults at high risk of candidaemia: an observational study. J Antimicrob Chemother. 2016;71(8):2262–2269. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

