Back to Journals » Journal of Pain Research » Volume 16
Percutaneous Transforaminal Endoscopic Surgery (PTES) for Treatment of Lumbar Degenerative Disease in Patients with Underlying Diseases: A Retrospective Cohort Study of 196 Cases
Authors Zhou T, Ma T, Gu Y, Zhang L, Che W, Wang Y
Received 13 November 2022
Accepted for publication 7 March 2023
Published 31 March 2023 Volume 2023:16 Pages 1137—1147
DOI https://doi.org/10.2147/JPR.S396993
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Krishnan Chakravarthy
Tianyao Zhou,1,2,* Tianle Ma,1,2,* Yutong Gu,1,2 Liang Zhang,1 Wu Che,1 Yichao Wang1
1Department of Orthopedic Surgery, Zhongshan Hospital Fudan University, Shanghai, 200032, People’s Republic of China; 2Shanghai Southwest Spine Surgery Center, Shanghai, 200032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yutong Gu, Email [email protected]
Objective: To evaluate the postoperative outcomes, safety and feasibility of percutaneous transforaminal endoscopic surgery (PTES) for the treatment of lumbar degenerative disease (LDD) in the patients with underlying diseases.
Methods: From June 2017 to April 2019, PTES was performed to treat 226 patients of single-level LDD. According to clinical background, the patients were divided into two groups. A total of 102 patients with underlying diseases were included in group A. The other 124 LDD patients without underlying diseases were included in group B. The occurrence of postoperative complications was recorded. Leg pain was assessed before, immediately, 1 month, 2 months, 3 months, 6 months, 1 year, and 2 years after PTES using VAS, and ODI before PTES and 2 years after PTES were recorded. The therapeutic quality (Excellent, Good, Moderate or Poor) was defined according to MacNab grade at 2-year follow-up.
Results: No aggravation of underlying diseases or serious complications was observed in all patients within 6 months after the operation. Altogether, 196 patients were followed up for more than 2 years, 89 patients in group A and 107 patients in group B. The VAS score of leg pain and ODI dropped significantly after surgery (P< 0.001) in both groups. One case of group B received PTES again due to recurrence 52 months after surgery. According to MacNab, the excellent and good rate was 97.75% (87/89) in group A and 96.26% (103/107) in group B. In operative duration, frequency of intraoperative fluoroscopy, blood loss, incision length, hospital stay, VAS, ODI, and the excellent and good rate, there was no statistical difference between the two groups.
Conclusion: PTES is safe, effective and feasible for the treatment of LDD with underlying diseases, which is comparable to PTES for LDD without underlying diseases. The entrance point of PTES (Gu’s Point) is located at the corner of the flat back turning to the lateral side. PTES is not only a minimally invasive surgical technique but also includes a postoperative care system for preventing LDD recurrence.
Keywords: lumbar degenerative disease, the underlying disease, transforaminal, endoscopic discectomy, minimally invasive surgery
Introduction
Lumbar degenerative disease (LDD) is maintaining growth within the recent years with the development of society and aging population. Conservative treatments, such as bed-resting and oral medication application, are generally firstly introduced to the patients, while surgical treatments will be subsequently considered for those patients with severe disease progression. The efficacy and safety of surgical treatment remain to be a major clinical challenge for patients suffering from underlying diseases such as vital organ failure, post-cardiac surgery, post-live or kidney transplantation, diabetes, and hypertension. So far, few studies have been reported in this field. In addition, traditional open surgical procedures also have certain disadvantages, including wide dissection of paraspinal muscles, bone structures destruction, massive blood loss, long operative duration and slow recovery.1 Comparatively, a transforaminal endoscopic technique such as Yeung endoscopic spine surgery (YESS) and Thomas Hoogland endoscopic spine surgery (TESS), becoming a widely performed approach gradually,2,3 shows great advantages such as smaller incision, less damage of soft tissue and bone structure, and faster recovery.4–7
Based on a lot of practice, we successfully developed a practical and easy-handled transforaminal endoscopic technique named percutaneous transforaminal endoscopic surgery (PTES).8–10 The surgical procedure of PTES is known as simple orientation, easy puncture, reduced steps, less fluoroscopic X-ray exposure and shorter operative duration compared with the conventional endoscopic techniques, thus providing an effective way to treat LDD. In this study, we used PTES to treat the patients of LDD with or without underlying diseases and evaluated the postoperative outcomes, safety and feasibility of PTES for the treatment of LDD in the patients with underlying diseases.
Materials and Methods
From June 2017 to April 2019, PTES was performed to treat 226 single-level LDD patients in day surgery ward. A total of 102 patients with underlying diseases were included in group A. The other 124 LDD patients without underlying diseases were included in group B. The underlying diseases included hypertension, diabetes, chronic bronchitis, emphysema, coronary heart diseases, arrhythmia, post-cardiac valve surgeries, post-cardiac stenting, old cerebral infarction, post liver cancer resection, post liver transplantation, renal insufficiency undergoing hemodialysis, post kidney transplantation, rheumatism, Sjogren’s syndrome and lupus erythematosus. The detailed grouping criteria are shown in Figure 1. This retrospective cohort study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Zhongshan Hospital Fudan University. In the patients of post liver transplantation or post kidney transplantation, all their organs were donated voluntarily with written informed consent and this was conducted in accordance with the Declaration of Istanbul.
 |
Figure 1 Flowchart demonstrating the grouping criteria of this study. |
Inclusion criteria: ① The neurologic symptoms of patients with or without underlying diseases were unilateral or bilateral legs pain, or intermittent claudication with no symptom of legs when rest and pain, numbness, discomfort or tiredness of unilateral or bilateral leg after walking 50 to 100 meters, unable to walk, relieved after rest; ② MRI and CT showed single-level lumbar degeneration including lumbar disc herniation, lateral recess stenosis, intervertebral foramen stenosis or central spinal stenosis from L1 to S1, which were consistent with the clinical symptoms of corresponding neurologic compression (Figure 2A and B); ③ The patients had received systematic and formal conservative treatment for at least 3 months, but the prognosis was poor and symptoms severely affected work and daily life; ④ Underlying diseases were under control, the patients were in good physiological condition and could tolerate local anesthesia; ⑤ Patients were in stable psychological condition and able to understand and think independently; ⑥ Patients obtained follow-up at least for 2 years.
Exclusion criteria: ① Laboratory examination indicated operative contraindications such as coagulation dysfunction; ② Patients had vital organ failure such as heart failure; ③ Patients had lumbar spondylolisthesis degree above II; ④ Patients with mental illness were not able to cooperate with the surgeon during prone position surgery; ⑤ Patients had local tissue wound or infection in the operative region.
Pre- and Postoperative Imaging
All patients are evaluated before the procedure by CT and MRI imaging to determine the involved segment or to determine if there is calcification. Posteroanterior and lateral radiographs are obtained to detect spondylolisthesis, scoliosis or high iliac crest when the lower plate of L4 vertebral body is not higher than the line between the highest points of the bilateral iliac crest. After the treatment, MRI images are obtained to assess neurologic decompression or exclude dural cyst, myelomeningocele, dural tears or spinal fluid leaks, reherniation. A postoperative CT scan is used to assess the facet joint bone dissection that occurred during intervertebral foramen enlargement, and lateral radiographs of lumbar hyperextension and hyperflexion are used to see if there is any lumbar instability related to the facet joint bone dissection.
Surgical Procedure
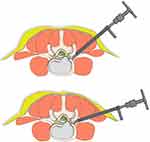
The patient is placed in a prone position on an arch-shaped soft waist pillow with sponge or silica gel pad, promoting hip flexion and keeping the lower back in a horizontal position. The corresponding intervertebral space is determined by C-arm on a posteroanterior view (Figure 2C), and its intersection with the lower back midline indicates the body surface projection of the anatomical center of the intervertebral disc. The entrance point, named “Gu’s point”,8–10 is located at the corner of the flat back turning to lateral side (Figures 2D and 3). The operation is performed under local infiltration anesthesia with conscious sedation. Aiming at the vertical line passing through the body surface projection of the anatomical center of the intervertebral disc, the puncture needle is inserted with an angle of 25° to 85° horizontally (Figure 3). After successful puncture, the C-arm is used to confirm that the needle reaches the posterior one-third of the intervertebral space or intracanal area close to the posterior wall of the disc on lateral view and near the outer edge of the pedicle on posteroanterior view (Figure 2E and F). Once the soft tissue gets stepwise dilated, the 8.8-mm protective cannula is inserted over the thick guiding rod of 6.3mm in diameter to dock at the facet joint and pressed down further to make the angle of cannula to horizontal plane smaller, and a 7.5-mm trephine is introduced to cut the ventral bone of articular process for the enlargement of intervertebral foramen, which is “press-down enlargement of foramen8–10”.(Figure 4) When resistance disappears, the posteroanterior view confirms that the tip of trephine exceeds the inner edge of the pedicle and its tip is close to the posterior edge of the target intervertebral space on lateral view (Figure 2G and H). The surgical instruments are used to remove the hypertrophic ligamentum flavum and herniated disc under transforaminal endoscopy to expose the ipsilateral traversing and exiting nerve root, and the lateral recess is enlarged. The compressed ipsilateral and contralateral traversing nerve root are exposed for bilateral neurologic decompression, and the central spinal canal is enlarged (Figure 2I and J). Then the incision is closed (Figure 2K).
Postoperative Treatment
After the operation, patients were advised to stay in bed till the next day. Functional exercise begins on the third day after operation, and the patient returns to work 1 week after operation. The flexible brace should be worn for 2 weeks. Special treatment for underlying diseases should continue after surgery such as controlling blood pressure, blood glucose and vital organ function. Anticoagulation therapy could begin 3 days after surgery.
Clinical Follow-Up
Leg pain was assessed using visual analogue scale (VAS) before operation, immediately, and 1 month, 2 months, 3months, 6 months, 1 year and 2 years after surgery. ODI before PTES and 2 years after PTES were recorded. The clinical efficacy was evaluated using MacNab 2 years after surgery and scored as excellent (painless with unlimited activity), good (occasionally lumbar or leg pain, which could interfere with the normal life or entertainment), fair (improved functionality but intermittent pain, as patients usually had to change their work and lifestyle) and poor (no improvement in symptoms and acquiring further surgical treatment).
Statistical Analysis
SPSS, version 25.0 (IBM Corp., Armonk, New York, USA) was used to perform data analysis. Student’s t-test is used for intergroup analysis of normal distributed continuous variables including age, BMI, operative duration, incision length, follow-up and ODI. The Mann–Whitney U-test is used for intergroup analysis of discrete variables, rating variables and not normally distributed continuous variables such as fluoroscopy frequency, blood loss, hospital stay, VAS. Pearson’s Chi-squared test is used for intergroup analysis of unordered categorical variables such as gender, lumbar level and rates of herniation calcified, scoliosis, spondylolisthesis and high iliac crest, and Fisher’s exact test for ordered categorical variables such as rate of excellent and good outcomes. Intragroup comparison of leg pain VAS at different time points is performed using Kruskal–Wallis test followed by the Dunn procedure with Bonferroni correction. Preoperative and postoperative ODI are compared using Student’s t-test. P<0.05 was considered a significant difference.
Results
In group A, 89 patients received a follow-up of 2 years, and all patients had no aggravation of underlying diseases during half a year after the operation. There were 107 patients with the follow-up of 2 years in group B, and only one patient had recurrence 52 months postoperatively and underwent another PTES. No serious complications such as nerve injury, wound infection, abdominal organ injury and blood vessel rupture were observed.
Table 1 summarizes the baseline data of the two groups. There was no statistical in age, gender, BMI, involved lumbar level, rates of herniation calcified, scoliosis, spondylolisthesis, high iliac crest and follow-up time between the two groups. Table 2 manifests the proportion of various underlying disease in group A. Among these cases, 2 patients had hypertension combined with diabetes and coronary heart disease treated with cardiac stenting, 17 patients had hypertension with diabetes, 3 patients had hypertension with coronary cardiac disease, 1 patient had hypertension with old cerebral infarction, 3 patients had diabetes with chronic bronchitis and 1 patient had diabetes with coronary cardiac disease.
 |
Table 1 The Demographic Data of LDD Patients with (A) and without (B) Underlying Diseases |
 |
Table 2 The Demographic Data of Underlying Diseases in Group a |
All patients underwent single-level surgery. The operative duration from setting of body position to the end of operation was 55.91±6.48 min in group A and 55.29±6.73 min in group B. The projection of X-ray was 6(5–9) times in group A and 6(6–9) times in group B. The blood loss was 13 (5–32) mL in group A and 12 (4–30) mL in group B. The length of incision was 9.71±1.79 mm in group A and 9.64±1.63 mm in group B. The hospital stay was 3 (2–4) days in both groups. No significant difference between the two groups was shown in the operative duration, projection of X-ray, blood loss, incision length and hospital stay (Table 3).
 |
Table 3 The Perioperative Data of Both Two Groups |
The VAS of leg pain is exhibited in Table 4. In group A, the VAS score of 8(7–10) dropped to 1(0–2) immediately (P<0.001) and 0(0–1) 2 years after operation (P<0.001). VAS score of group B dropped from 9(7–10) before operation to 1(0–3) immediately (P<0.001) and 0(0–1) 2 years after operation (P<0.001). There was no statistical difference in the VAS before or at different time after surgery between the two groups. However, 2 patients (2.2%, 2/89) in group A and 3 patients (2.8%, 3/107) in group B had rebounding leg pains about 1 week after surgery, which got alleviated during 2 months.
 |
Table 4 The VAS Pain Assessment and ODI Score of Both Groups |
The ODI was 70.20±9.01% in group A and 66.70±8.84% in group B before operation, which significantly dropped to 15.50±4.91% and 13.20±4.82%, respectively, 2 years after surgery (P<0.001) (Table 4). According to the MacNab classification, the excellent and good rate was 97.75% (87/89) in group A and 96.26% (103/107) in group B. There was no statistical difference between the two groups (Table 5).
 |
Table 5 The MacNab Classification Data at 24-Month Follow-Up |
The postoperative lumbar hyperextension and hyperflexion lateral radiographs revealed no new intervertebral instability although the facet joint was involved in some cases.(Figure 2L–N) There were 5 cases of nerve root sleeves rupture without cerebrospinal fluid leakage or other abnormal clinical symptoms, 2 cases in group A and 3 cases in group B. No other complications such as wound infection, permanent nerve injury, abdominal organ injury and rupture of large vessels occurred.
Discussion
For those LDD patients with underlying diseases such as diabetes, cardiovascular or cerebrovascular diseases, lung diseases, traditional open surgery has high risk because of the relatively larger surgical incision, more dissection of the soft tissue and longer recovery time11 With the development of minimally invasive spine surgery, transforaminal endoscopic surgery has become popular in recent decades. Transforaminal endoscopic surgery can provide similar outcomes with smaller damage of soft tissue, less blood loss, hardly influence on spinal stability, fewer complications and faster recovery,12–14 which is considered the most optimized treatment option for patients of LDDs with underlying diseases. This is supported by the results of this study. In both groups undergoing PTES, the mean blood loss was less than 15mL, and the average length of surgical incision was less than 10 mm. All patients were hospitalized in day surgery ward and the hospital stay was 3(2–4) days, which helped to reduce patients’ psychological pressure, decrease the incidence of nosocomial infections and save medical resources, conforming to ERAS (Enhanced Recovery After Surgery) protocol15,16 No major complications such as nerve damage, wound infection, abdominal organ damage, or great vessel rupture were found.
TESS was a widely used transforaminal endoscopic technique, but it had a complexity of C-arm guided orientation, difficulty to find the optimal trajectory for the target, and more steps of surgical manipulation, which led to much exposure to X-ray, long duration of operation, and steep learning curve.15 In 2017, we first introduced the PTES technique.8 For the orientation before puncture in PTES, only posteroanterior fluoroscopy is required to determine the target disc. The entrance point is located at the corner of the flat back turning to the lateral side, named “Gu’s Point”,8–10 which is determined not depending on C-arm fluoroscopy and distance measurement from the midline (Figures 2I and 3). Our entrance point is more medial than those used by other transforaminal endoscopic techniques, which has four advantages: (1) Avoid injuring the exiting nerve root. The exiting nerve root leaves the foramen from superomedial to inferolateral, which is easy to meet during enlargement of the foramen if the entrance point locates laterally. (2) Avoid blockage by the high iliac crest for the L5/S1 level. The height of the iliac crest at “Gu’s Point” is lower than the peak of the iliac crest located at the lateral side of the waist, which makes puncture and subsequent operation easier for L5/S1. (3) Shorten the manipulation path especially in obesity patients. The more lateral from the midline the entrance point is, the longer the path for the surgical target is. More subcutaneous adipose tissue makes the puncture point more distal from the surgical target, which needs a very long working channel for transforaminal endoscopic surgery if the entrance point is more lateral. (4) Avoid injuring abdominal viscera and great vessels. Even if puncture in a large horizontal angle from “Gu’s Point”, the tip of the needle could be blocked by the bony structure of the spine, which prevents the needle from penetrating into the abdomen and vessels. During the puncture procedure of PTES, the target is not a fixed point, making the puncture angle more flexible and reducing the difficulty of puncture. Simple orientation and easy puncture are achievable because of “press-down enlargement of foramen”. When enlarging the foramen, pressing down the cannula docked at the articular process to decrease the horizontal angle of the trephine could remove more bones in the ventral part of the articular process, the working channel can be inserted into the spinal canal even if the puncture angle was 85° to the horizontal plane. It was “press-down enlargement of foramen”8–10 (Figure 4), which made it possible to remove the herniated nucleus compressing contralateral nerve root. Additionally, a 7.5 mm trephine is used in PTES to enlarge the intervertebral foramen instead of a stepwise procedure. Reduced steps, simple orientation and easy puncture could significantly decrease the exposure of X-ray and the operative duration. In this study, PTES required mean intraoperative fluoroscopy of 6 times in both groups. The operative duration from the setting of body position to the end of the operation was 55.91±6.48 minutes in group A of patients with underlying diseases and 55.29±6.73 minutes in group B.
Can PTES successfully relieve the symptoms of patients suffering LDD combined with multiple underlying diseases? Most LDDs present clinically as unilateral or bilateral leg pain, and sometimes there is intermittent claudication that unilateral or bilateral leg pain, numbness, discomfort or tiredness occurs after walking for 50 to 100 meters, which could be relieved after a few minutes of rest. When lumbar disc herniation, lateral recess stenosis or intervertebral foramen, most patients have one leg pain and few have pain in different position of both legs coming from nerve root compression of different levels. When lumbar central spinal canal stenosis, there is bilateral pain in the symmetric position of both legs resulting from the compression of bilateral traversing nerve roots of same level. During the PTES procedure, press-down enlargement of foramen8–10 is performed, and the hypertrophic ligamentum flavum and the protruding nucleus are removed under endoscopy to enlarge the lateral recess and decompress the ipsilateral exiting and traversing nerve root (Figure 2I). The central and contralateral posterior longitudinal ligament, annulus fibrosus or nucleus pulposus can be removed using flexible bipolar radiofrequency and angled nucleus pulposus forceps to expose the contralateral traversing nerve root and enlarge the central spinal canal. Bilateral traversing nerve roots are decompressed from one side through a small incision. The results of this study showed that the VAS of leg pain and ODI significantly dropped after operation in both groups (P<0.001), and the excellent and good rate at 2-year follow-up was 97.75% (87/89) in group A and 96.26% (103/107) in group B. There was no statistical significance in clinical outcomes of PTES for LDD between the patients with and without underlying diseases.
Although the facet joint was involved in some cases, no new intervertebral instability was found in lumbar hyperextension and hyperflexion lateral radiographs. There were 5 cases of nerve root sleeves rupture but no cerebrospinal fluid leakage or other abnormal clinical symptoms were observed. Two patients (2.2%, 2/89) in group A and 3 patients (2.8%, 3/107) in group B had rebounding leg pains about 1 week after surgery, which were relieved during 2 months. One patient (0.9%, 1/107) experienced a recurrence 52 months after surgery in group B, whereas group A experienced none. The low recurrence rate is closely related to postoperative care. Repeatedly remind patients: 1. Avoiding frequent bending; 2. Avoiding lifting heavy objects; 3. Avoiding maintaining the same posture for a long time; 4. Avoid focusing strength on the waist when coughing and sneezing.8,9 The remaining portion in the intervertebral disc could keep stable after the protruded nucleus pulposus been removed. Bad postoperative care would make the remaining nucleus pulposus broken, and the fragments would protrude and compress neurologic elements.
Preoperative preparation should be made for different underlying diseases. In brief, comprehensive examination is conducted to evaluate the severity of underlying diseases. For example, blood pressure should be controlled at a systolic pressure 90–180mm Hg, diastolic pressure 60–110mm Hg,17,18 and blood glucose should be adjusted during 6–10mmol/L.19,20 Oral anticoagulants including Aspirin, clopidogrel and dipyridamole need to be suspended at least 7 days preoperatively.21,22 Sometimes specialized experts should be invited to assess the risk of underlying diseases such as stroke, heart diseases. The sedative and analgesic medicine such as dexmedetomidine23–25 could be used during PTES under local anesthesia to improve the compliance of patients, easily awakened if needed, achieving the MAC (Monitor Anesthesia Care)25 III level standard. ECG monitor is extremely important for safety during surgery. In addition, PTES has the advantages of small invasion, little bleeding and fast recovery. The underlying diseases should continuously be treated postoperatively. All these factors significantly decreased the influence on the vital organs of patients undergoing operation. In our study, no patients had aggravation of underlying diseases half a year after surgery.
There are also some limitations in this study. It is a single-center retrospective study with a relatively small number of patients. There is no comparison of PTES with other spinal endoscopic techniques. Therefore, we will perform a multicenter prospective controlled study to compare PTES with YESS, TESS, Unilateral Biportal Endoscopy (UBE) for the treatment of LDD.
In conclusion, PTES is safe, effective and feasible for the surgical treatment of LDD with underlying diseases, which is comparable to PTES for LDD without underlying diseases. The entrance point of PTES (Gu’s Point) is located at the corner of the flat back turning to the lateral side. PTES is not only a minimally invasive surgical technique but also includes a postoperative care system for preventing LDD recurrence.
Abbreviations
PTES, Percutaneous Transforaminal Endoscopic Surgery; VAS, Visual Analogue Scale; YESS, Yeung Endoscopic Spine Surgery; TESS, Thomas Hoogland Endoscopic Spine Surgery.
Data Sharing Statement
All the data and materials concerning this research are available within the article.
Acknowledgments
We gratefully acknowledge Shengyuan Zhou of Changzhen Hospital affiliated to the Naval Medical University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
This retrospective study and the access to clinical data of patients involved in this study were approved by Ethics Committee of Zhongshan Hospital. Written informed consents to participate were obtained from all the patients.
Funding
Technical Standards Project of 2023 Shanghai “Innovation Action Plan of Science and Technology” supported by Science and Technology Commission of Shanghai Municipality, China (Grant No. 23DZ2201900).
Disclosure
The authors declared that they have no conflict of interest.
References
1. Yuan C, Zhou Y, Pan Y, Wang J. Curative effect comparison of transforaminal endoscopic spine system and traditional open discectomy: a meta-analysis. ANZ J Surg. 2020;90(1–2):123–129. doi:10.1111/ans.15579
2. Choi KC, Kim JS, Ryu KS, Kang BU, Ahn Y, Lee SH. Percutaneous endoscopic lumbar discectomy for L5-S1 disc herniation: transforaminal versus interlaminar approach. Pain Physician. 2013;16(6):547–556. doi:10.36076/ppj.2013/16/547
3. Li K, Zhang T, Gao K, Lv CL. The utility of diagnostic transforaminal epidural injection in selective percutaneous endoscopic lumbar discectomy for multilevel disc herniation with monoradicular symptom: a prospective randomized control study. World Neurosurg. 2019;126:e619–e24. doi:10.1016/j.wneu.2019.02.102
4. Li J, Cui H, Liu Z, Sun Y, Zhang W. Utility of diffusion tensor imaging for guiding the treatment of lumbar disc herniation by percutaneous transforaminal endoscopic discectomy. Sci Rep. 2019;9(1). doi:10.1038/s41598-019-55064-3
5. Pan M, Li Q, Li S, et al. Percutaneous endoscopic lumbar discectomy: indications and complications. Pain Physician. 2020;23(1):49–56.
6. Yu P, Qiang H, Zhou J, Huang P. Percutaneous transforaminal endoscopic discectomy versus micro-endoscopic discectomy for lumbar disc herniation. Med Sci Monit. 2019;25:2320–2328. doi:10.12659/MSM.913326
7. Chen Z, Zhang L, Dong J, Xie P, Liu B, Wang Q. Percutaneous transforaminal endoscopic discectomy versus microendoscopic discectomy for lumbar disc herniation: two-year results of a randomized controlled trial. Spine. 2020;45(8):493–503. doi:10.1097/BRS.0000000000003314
8. Gu YT, Cui Z, Shao HW, Ye Y, Gu AQ. Percutaneous transforaminal endoscopic surgery (PTES) for symptomatic lumbar disc herniation: a surgical technique, outcome, and complications in 209 consecutive cases. J Orthop Surg Res. 2017;12(1):25. doi:10.1186/s13018-017-0524-0
9. Wang H, Zhou TY, Gu YT, Yan ZQ. Evaluation of efficacy and safety of percutaneous transforaminal endoscopic surgery (PTES) for surgical treatment of calcified lumbar disc herniation: a retrospective cohort study of 101 patients. BMC Musculoskelet Disord. 2021;22(1):1–9. doi:10.1186/s12891-020-03938-3
10. Zhou T, Ma T, Gu Y, Zhang L, Che W, Wang Y. How to predict the culprit segment in percutaneous transforaminal endoscopic surgery under local anesthesia for surgical treatment of lumbar degenerative diseases? Radiologic images or clinical symptoms. Front Surg. 2023;9:1060318. doi:10.3389/fsurg.2022.1060318
11. Kapetanakis S, Chaniotakis C, Kazakos C, Papathanasiou JV. Cauda equina syndrome due to lumbar disc herniation: a review of literature. Folia Med. 2017;59(4):377. doi:10.1515/folmed-2017-0038
12. Ruan W, Feng F, Liu Z, Xie J, Cai L, Ping A. Comparison of percutaneous endoscopic lumbar discectomy versus open lumbar microdiscectomy for lumbar disc herniation: a meta-Analysis. Int J Surg. 2016;31:86–92. doi:10.1016/j.ijsu.2016.05.061
13. Liu C, Zhou Y. Percutaneous endoscopic lumbar diskectomy and minimally invasive transforaminal lumbar interbody fusion for recurrent lumbar disk herniation. World Neurosurg. 2017;98:14–20. doi:10.1016/j.wneu.2016.10.056
14. Gibson JN, Cowie JG, Iprenburg M. Transforaminal endoscopic spinal surgery: the future ‘gold standard’ for discectomy? - a review. Surgeon. 2012;10(5):290–296. doi:10.1016/j.surge.2012.05.001
15. Zhou C, Zhang G, Panchal RR, et al. Unique complications of percutaneous endoscopic lumbar discectomy and percutaneous endoscopic interlaminar discectomy. Pain Physician. 2018;21(2):E105–E12.
16. Rasouli MR, Rahimi-Movaghar V, Shokraneh F, Moradi-Lakeh M, Chou R. Minimally invasive discectomy versus microdiscectomy/open discectomy for symptomatic lumbar disc herniation. Cochrane Database Syst Rev. 2014;9:CD010328. doi:10.1002/14651858.CD010328.pub2
17. Ackland G, Brudney C, Cecconi M, et al. Perioperative quality initiative consensus statement on the physiology of arterial blood pressure control in perioperative medicine. Br J Anaesth. 2019;122(5):542–551. doi:10.1016/j.bja.2019.01.011
18. Hartle A, Mccormack T, Carlisle J, et al. The Measurement of adult blood pressure and management of hypertension before elective surgery. Anaesthesia. 2016;71. doi:10.1111/anae.13348
19. Dhatariya K, Levy N, Kilvert A, et al. Nhs diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med. 2012;29(4):420–433. doi:10.1111/j.1464-5491.2012.03582.x
20. Barker P, Creasey PE, Levy N, et al.; Membership of the Working Party. Peri-operative management of the surgical patient with diabetes 2015: association of anaesthetists of Great Britain and Ireland. Anaesthesia. 2015;70(12):1427–1440. doi:10.1111/anae.13233
21. Llau JV, Lopez-Forte C, Sapena L, Ferrandis R. Perioperative management of antiplatelet agents in noncardiac surgery. Eur J Anaesthesiol. 2009;26(3):181–187. doi:10.1097/eja.0b013e328324b79f
22. Pereira JV, Sanjanwala RM, Mohammed MK, Le ML, Arora RC. Dexmedetomidine versus propofol sedation in reducing delirium among older adults in the ICU: a systematic review and meta-analysis. Eur J Anaesthesiol. 2020;37(2):121–131. doi:10.1097/EJA.0000000000001131
23. Kang JR, Yao JJJo HS. Perioperative management of diabetic patients undergoing hand surgery. J Hand Surg. 2015;40(5):1028–1031. doi:10.1016/j.jhsa.2015.02.024
24. Harned ME, Owen RD, Steyn PG, Hatton KW. Novel use of intraoperative dexmedetomidine infusion for sedation during spinal cord stimulator lead placement via surgical laminectomy. Pain Physician. 2010;13(1):19–22. doi:10.36076/ppj.2010/13/19
25. Ghisi D, Fanelli A, Tosi M, Nuzzi M, Fanelli G. Monitored anesthesia care. Minerva Anestesiol. 2005;71(9):533.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.