Back to Journals » Journal of Pain Research » Volume 17
Percutaneous Endoscopic Transforaminal Discectomy for the Treatment of Lumbar Disc Herniation with Different Migration Levels: A Retrospective Study
Authors Sun J , Yu X, Feng K, Zheng W, Lu Y, Bao B
Received 3 October 2023
Accepted for publication 21 January 2024
Published 26 January 2024 Volume 2024:17 Pages 367—375
DOI https://doi.org/10.2147/JPR.S437968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rushna Ali
Jiewei Sun, Xiaojun Yu, Kan Feng, Wujun Zheng, Yong Lu, Bin Bao
Department of Cardiothoracic Surgery, Fuyang First People’s Hospital, Hangzhou, Zhejiang Province, People’s Republic of China
Correspondence: Xiaojun Yu, Department of Cardiothoracic Surgery, Fuyang First People’s Hospital, No. 429, Beihuan Road, Fuyang District, Hangzhou, Zhejiang Province, People’s Republic of China, Tel +8613858020606, Email [email protected]
Objective: To investigate the surgical method and efficacy of percutaneous endoscopic transforaminal discectomy (PETD) for the treatment of lumbar disc herniation (LDH) with different migration levels by introducing the strategy of foramenoplasty with the “distal nucleus pulposus as the core”.
Methods: Clinical data of LDH patients who underwent single-segment PETD surgery were retrospectively analyzed. Three groups were categorized according to the degree of nucleus pulposus migration in the sagittal position: no migration group, mild migration group, and high migration group. Different sites of foramenoplasty were used for LDH with different degrees of migration. All patients were followed up for at least 12 months. The clinical and follow-up data of the three groups were compared.
Results: A total of 102 patients were included, of which 46 (45.1%) were in the no migration group, 36 (35.3%) in the mild migration group, and 20 (19.6%) in the high migration group. Encouraging treatment results were obtained in all three groups.
Conclusion: PETD is effective in the treatment of LDH with different degrees of migration, and the foramenoplasty concept of “distal nucleus pulposus as the core” can effectively guide the molding site of foramenoplasty and facilitate the accurate placement of the working trocar.
Keywords: PETD, nuclear migration, superior articular process, foraminoplasty, 3D slicer
Introduction
Percutaneous Endoscopic Transforaminal Discectomy (PETD) is a minimally invasive procedure for the treatment of Lumbar Disc Herniation (LDH) in current clinical practice.1 The PETD uses the body’s natural bony channel, the foramenoplasty, as the surgical channel and removes the nucleus pulposus from the inside out under direct visualization, which has the advantages of being less invasive, more accurate, more efficacious, and faster recovery.2,3 However, the learning curve of this procedure is steep,4 and the entry diameter of the puncture and the site of foramenoplasty will directly affect the operating field, and if the foramen is not properly molded, it will also increase the surgical complications,5 such as nucleus pulposus residue, nerve root injury, and dural tear.6,7 Accurate foramenoplasty is crucial for PETD techniques.8 However, the herniation of the nucleus pulposus is clinically independent in each patient, with some nuclei protruding only in situ, while others have a high degree of prolapse.9,10 Obviously, a single model of foraminoplasty cannot be used to cover all cases. Unfortunately, there have been no studies regarding PETD surgery for the treatment of LDH with different levels of migration. The purpose of this study was to investigate PETD for the treatment of LDH with different migration levels. The efficacy was evaluated at 12 months of follow-up to provide a reference for clinical foramenoplasty.
Patients and Study Methods
Study Population
Clinical data of LDH patients admitted to the Fuyang First People’s Hospital, Hangzhou, Zhejiang Province, China, from February 2020 to February 2022 were retrospectively analyzed. All patients underwent PETD surgery and were performed by the same surgical team of surgeons. The study was approved by the Ethics Committee of the First Hospital of Fuyang, Hangzhou, China. Written informed consent was obtained from the patients to receive treatment and to publish this report. The study was carried out in accordance with the ethical standards of the Declaration of Helsinki of the World Medical Association
Inclusion criteria: (1) clear diagnosis of LDH based on symptoms, signs, and imaging data; (2) ineffective after 3 months of strict conservative treatment; (3) unilateral LDH in a single segment; (4) clear leg symptoms; (5) the nucleus pulposus on transverse view is typed as central, paracentral, and foraminal; and (6) planned for PETD.
Exclusion criteria: (1) patients unsuitable for PETD treatment; (2) intraoperative foramenoplasty was not performed; (3) deviation of the proposed foramenoplasty site from the actual model; and (4) incomplete data or inability to follow up. (6) the nucleus pulposus is typed as extreme lateral in the transverse position.
Surgical Procedure
The patient is placed in the prone position with appropriate pillow pads placed on the abdomen to enlarge the size of the intervertebral foramina. The surgical approach is posterior lateral approach. The puncture site and target point are selected under fluoroscopy of C-arm machine, and the target point is usually set as the tip of the superior articular eminence of the affected segment, and the puncture point is usually 10–14 cm away from the median line and 1–3 cm towards the cephalic end. The patient is routinely sterilized and toweling is performed. Anesthesia with 1% lidocaine was applied layer by layer to the deep fascia. Under the guidance of C-arm machine, the 16G guide needle was inserted to the target point, and a 7–8mm transverse incision was made at the entrance of the skin, and the soft tissue was expanded step by step using a soft tissue dilatation tube, and a suitable intervertebral foramina molding tool was selected to mold the intervertebral foramina at the proposed molding site. After the intervertebral foramen was satisfactorily molded, the endoscope was placed. The nucleus pulposus is gradually removed with a grasping tool. Until the nerve root periphery is completely loosened. The patient was instructed to cough forcefully, and the procedure was ended if there was no significant back or leg discomfort. A negative straight leg raise test in the immediate postoperative period indicated that decompression was complete and effective.
With the Distal Nucleus Pulposus as the Core
Differences in foraminal molding for LDH with different levels of migration are mainly seen in the removal of the superior articular process (SAP) site. The nucleus pulposus usually migrates downward due to gravity, the main bony structures surrounding the intervertebral foramina are the SAP and the pedicles. After foraminal molding, the working trocar can usually only move slightly cephalad but not pedicle because it will be blocked by the bone of the SAP and pedicle. Therefore, the molding site limits the movement of the working trocar to the most pedicle of the foramen. Based on the idea of the “distal nucleus pulposus as the core”, precise foraminal molding is required so that the surgeon can remove the most distal nucleus pulposus under endoscopic visualization, avoiding nucleus pulposus retention.
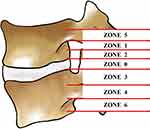
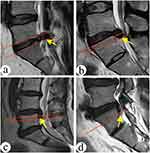
According to the degree of nucleus pulposus migration, the site of foramenoplasty was positioned at the appropriate location of the SAP. Based on the preoperative sagittal MRI images and the typing of the degree of nucleus pulposus migration, the nucleus pulposus migration in the sagittal position was classified into 7 zones.11,12 Zone 0: from the plane of the inferior border of the upper vertebral body to the plane of the superior border of the lower vertebral body; zone 1: from the inferior border of the upper pedicle to 3 mm below the inferior border of the upper pedicle; zone 2: from 3 mm below the inferior border of the upper pedicle to the inferior border of the upper vertebral body; zone 3: from the superior border of the lower vertebral body to the center of the lower pedicle; zone 4: from the center of the lower pedicle to the lower edge; zone 5: above the lower edge of the upper pedicle; zone 6: below the lower edge of the lower pedicle, (Figure 1). The zone in which the most distal part of the nucleus pulposus was located was designated as the zone of the degree of nucleus migration, and all cases in this study contained zones 0, 3, 4, and 6, (Figure 2). Nucleus migration in zone 0 was categorized as no migration group (Group-0), nucleus migration in zone 3 was categorized as mild migration group (Group-3), and nucleus migration in zones 4 and 6 was categorized as highly migrated group (Group-4).
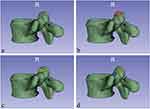
In order to further determine the authenticity of the foramenoplasty site, we modeled the preoperative and postoperative SAP using 3D slicer software, and compared the preoperative and postoperative 3D models to determine the authentic foramenoplasty site, and at the same time recorded the volume data of the SAP using this software. Under the concept of “distal nucleus pulposus as the core”, we successfully accomplished foraminal molding for LDH with different degrees of migration. The SAP was divided into three parts: superior, middle, and inferior, corresponding to tip, ventral, and basal (Figure 3a). In cases with no nucleus migration, the foramen was molded at the tip of the SAP (Figure 3b); in cases with mild nucleus migration, the foramen was molded ventrally on the SAP (Figure 3c); and in cases with high nucleus migration, the foramen was molded basally on the SAP (Figure 3d).
Evaluation of Clinical Outcomes
Clinical outcome was assessed by recording the Visual Analogue Scale (VAS) and Oswestry Disability Index (ODI) preoperatively and postoperatively at 1, 3, 6, and 12 months. Surgical satisfaction was assessed at the final follow-up using the modified MacNab criteria.
The VAS score was an 11-point scale from 0–10, with 0 degrees representing no pain and 10 degrees representing the most severe pain, and was recorded separately according to the patient’s leg pain.
The ODI score included 10 aspects of the patient’s lumbar and lower extremity pain level, sitting, standing, walking, and the degree of self-care, totaling 50 points to score the patient’s lumbar spine function, and the smaller the score represented the better the lumbar spine function.
Modified MacNab criteria: excellent: symptoms completely disappeared, resumption of work and life; good: slight symptoms, mild limitation of activities, no impact on work and life; fair: symptoms reduced, limitation of activities, affecting the normal work and life; poor: no difference before and after the treatment, or even aggravated.
Statistical Methods
SPSS 25.0 software was used for statistical analysis. Measurement information was expressed as xˉ±s, t-test was used for comparison of measurement information between groups, rank-sum test and chi-square test were used for comparison of count information between groups, and F was the test statistic in ANOVA, and the difference was considered statistically significant at P<0.05.
Results
The retrospective analysis included 102 patients (50 males and 52 females) with a mean follow-up of 15 months (range 12–18 months). There were 46 cases with nucleus pulposus migration in zone 0 (Group-0), 36 cases with nucleus pulposus migration in zone 3 (Group-3), 16 cases with medullary migration in zone 4, and 4 cases with medullary migration in zone 6. Since nucleus pulposus migration in zones 4 and 6 accounted for a small number of patients, they were included in the same group (Group-4). All cases were successfully molded and the molding site was consistent with the preoperative protocol. Group-0 was molded at the tip of the SAP, Group-3 was molded ventrally on the SAP, and Group-4 was molded at the base of the SAP. The mean age of the entire study cohort was 47.18±14.92 years. There was no statistically significant difference in all the remaining data including age, gender, symptomatic side, and diseased segment. (Table 1).
Before foramenoplasty, there was no significant difference in the volume of the SAP among the three groups. After foramenoplasty, the volume of the SAP in Group-4 (796.56 ± 371.97 mm³) was significantly less than that in the other two groups (Group-0, 999.51 ± 257.91 mm³; Group-3, 1020.48 ± 247.46 mm³), and the difference was statistically significant (P < 0.05).The molded and resected SAP volume of Group-4 (434.31 ± 301.78 mm³) was also significantly more than that of the other two groups (Group-0, 272.48 ± 247.46 mm³). The volume of the SAP (434.31 ± 301.78 mm³) was also significantly more than that of the other two groups (Group-0, 272.43 ± 245.71 mm³; Group-3, 281.07 ± 196.76 mm³), with a statistically significant difference (P < 0.05). The percentage of SAP resection was calculated based on the above data, and Group-4 molding had a higher percentage of SAP resection (34.95 ± 21.28%) than the other two groups (Group-0, 20.83 ± 16.87%; Group-0, 20.61 ± 12.09%). (Table 2).
 |
Table 2 Volume of the Superior Articular Process in Each Group of Intervertebral Foraminal Molding with the “Distal Nucleus Pulposus as the Core” |
Clinical efficacy was evaluated using VAS-leg pain scores, ODI scores, and modified MacNab criteria to demonstrate changes in clinical outcomes before and after surgery. For the entire study population (n=102), preoperative VAS scores averaged 6.80 ± 0.73 and decreased to 2.50 ± 0.61, 1.97 ± 0.83, 1.51 ± 0.50, and 1.17 ± 0.79 at 1, 3, 6, and 12 months postoperatively, respectively. Preoperative VAS leg scores of 7.20 ± 0.70 were higher in Group-4 than in the other two groups (Group-0,6.52 ± 0.66; Group-3,6.94 ± 0.72), (P=0.001). At one month postoperatively, VAS leg scores decreased significantly in all three groups, with the greatest decrease in Group-4, which was (2.45 ± 0.51) at one month postoperatively. The decreasing trend of VAS leg scores of the three groups in the following 3, 6, and 12 months became flat, but all of them were significantly different from the preoperative period, and there was no significant intergroup difference of VAS leg scores among the three groups after the operation (Figure 4a) (Table 3).
 |
Table 3 VAS-Leg Pain Scores in Each Group |
 |
Figure 4 (a) Trend graph of the change of VAS-leg scores for the three data sets. (b) Trend plot of ODI score changes for the three data sets. (c) Modified MacNab scores for all patients. |
In the whole study group (n=102), the preoperative ODI score of 44.19±3.17 decreased to 25.20±3.73, 17.38±1.54, 12.76±1.70, and 7.94±1.50 at 1, 3, 6, and 12 months postoperatively. The preoperative ODI scores of Group-0, Group-3, and Group-4 were: 43.26±2.82, 44.28±3.42, and 46.15±2.60, and the difference between the groups was statistically significant, (p<0.05). It decreased to: 25.76±4.16, 24.92±3.30, 24.40±3.36 respectively in the postoperative month, all of which were significantly different from the preoperative period in this group. Thereafter, the rate of decline in ODI scores slowed down at 3, 6, and 12 months postoperatively and basically stabilized after 6 months postoperatively (Figure 4b) (Table 4).
 |
Table 4 ODI Scores in Each Group |
At the final follow-up, 102 patients in this study were assessed for surgical satisfaction according to the modified MacNab criteria, with 64 patients indicating excellent, 34 patients indicating good, and 4 patients indicating less satisfactory (Figure 4c). No complications such as significant sensory disturbances or dural sac tears were observed in any of the three groups. One elderly female patient in Group-0, who did not wear a standardized lumbar girdle postoperatively and engaged in domestic labor for 1 month, underwent revision surgery at 6 weeks postoperatively for fusion internal fixation of the diseased segment.
Discussion
Lumbar disc herniation covers almost all age groups from adolescents to the elderly, which not only seriously affects the quality of life of patients, but also brings a huge economic burden to the society.13–15 There are various treatment modalities for this disease, among which the minimally invasive procedure represented by PETD has been widely recognized in clinical practice due to its advantages of small damage, fast recovery and good effect. Numerous physicians have also successfully treated various types of LDH, such as central protruding nucleus pulposus, extreme lateral protruding nucleus pulposus, and giant prolapsing nucleus pulposus, by PETD.9,16–18 The requirement of high precision for the establishment of the working channel of PETD is high, and the first endoscopic view often disoriented the operator because of the obstruction of soft tissues.19 If the intervertebral foramen molding site is targeted according to the degree of nucleus pulposus migration, then the inner opening of the working trocar can reach the designated position as expected. This is because in the sagittal position, after entering the skin, the working trocar is punctured from dorsal to ventral and cephalad to pedicle, and the puncture path passes through the molding area before reaching the nucleus pulposus, which also makes the inner opening of the working trocar more ventral and pedicle-oriented than the molding area of the SAP.20 A follow-up study showed that improper placement of the working trocar is an important cause of residual medulla, a large proportion of which occurs in cases with highly migrated medulla.5 In this study, for highly migrated nuclei (zones 4 or 6), the inner port of the working trocar was aligned with the distal part of the nucleus pulposus by using a “distal nucleus pulposus as the core” placement strategy, which corresponded to the foramenoplasty at the base of the SAP of the inferior vertebral body. This is the lowest point of bony structure that can be reached by molding the intervertebral foramina on the SAP. The base of the SAP moves downward into the pedicle, where there are many bony structures that make it difficult to enlarge the intervertebral foramina by molding. Therefore, foraminal molding at the base of the SAP is the optimal site for molding a highly migrated nucleus pulposus. In addition, for nuclei with no significant migration (zone 0) and mild migration (zone 3), we also select the site for foramen molding at the tip of the superior articular process or ventrally, respectively, based on the “distal nucleus pulposus as the core”.
In order to further clarify the site of foramenoplasty, we restored the real situation of foramen magnum molding by 3D modeling. 3D slicer software not only can model based on CT images, but also can directly obtain the volume of the model.21 After foramenoplasty, we found that there was no significant difference in the preoperative volume of the SAP among the three groups of patients (Group-0,1271.94±262.04 mm³; Group-3,1301.55±316.48 mm³; and Group-4,1230.87±421.03 mm³), but after foramenoplasty, the nucleus pulposus migrated to the SAP in the patients with zone 4 or zone 6 The volume of the SAP removed (434.31±301.78 mm³) was significantly larger than that of patients in zone 0 (272.43±245.71 mm³) and zone 3 (281.07±196.76 mm³). To analyze the reason, the direction of movement of the molding tool was from dorsal to ventral and from cephalad to pedicle, and the structure of the SAP was in the order of tip, ventral, and basal from cephalad to pedicle; therefore, wanting to mold the foramenoplasty at the basal would inevitably affect the bone of the ventral and or tip. By comparing the preoperative and postoperative models of the SAP, we also found that the foramenoplasty in cases with nucleus pulposus migration in zone 0 was mainly concentrated in the tip of the SAP, with some cases slightly affecting the ventral side; in cases with nucleus pulposus migration in zone 3, the foramenoplasty was mainly in the ventral side of the SAP, with the possibility that the tip or the basal would also be affected; and in cases with nucleus pulposus migration in zones 4 or 6, the foramenoplasty was often contained to the ventral and/or the tip. In a few cases of highly migrated nucleus pulposus in L5-S1, almost the base, ventral and tip of the SAP were molded. We believe that when LDH in L5-S1 undergoes PETD surgery, the working path is set up in a more inclined way in order to avoid the interference of the iliac spine, ie, the puncture point on the surface of the body is closer to the cephalic and the median line, and this, together with the factor of the highly migrated nucleus pulposus, makes it necessary to enlarge the area of foramen molding to a greater extent This, coupled with the high degree of nucleus pulposus migration, results in a larger enlargement area for foraminal molding, and ensures that the inner port of the working cannula is closer to the pedicle to meet the “distal nucleus pulposus as the core” molding concept.
Overall, all three groups of patients in this study achieved satisfactory treatment results, and both VAS leg scores and ODI scores were effectively reduced postoperatively. It is worth noting that patients with nucleus pulposus migration in zones 4 or 6 showed more pronounced leg pain symptoms as well as lumbar dysfunction preoperatively, which may be attributed to the greater degree of mechanical compression on the nerve root by the dislodged medulla, as well as to the induction of more relevant inflammatory factors, which resulted in more severe clinical symptoms. The VAS-leg scores and ODI scores of the three groups of patients at 1, 3, 6, and 12 months after surgery decreased significantly compared with the preoperative period, with the fastest decrease at 1 month after surgery, the downward trend slowing down at 3 months after surgery, and basically stabilizing at 6 and 12 months after surgery. At the final follow-up, 98 of 102 patients (96.1%) showed excellent or good results according to the modified MacNab criteria. Only 1 elderly female patient who had a recurrence, who did not wear a standardized postoperative girdle and engaged in domestic labor for 1 month, underwent revision surgery at 6 weeks postoperatively for fusion internal fixation of the diseased segment.
The current study also has some limitations. First, we investigated the degree of nucleus pulposus migration in the sagittal position and did not take into account the central, paracentral, intervertebral foraminal, and extreme lateral types of nucleus pulposus in the transverse position. Second, the intervertebral foraminal molding tools used in this study included both bone drills and circular saws, and the molding method and the damage to the bone can vary between tools. Third, in the short term, PETD for LDH has a satisfactory therapeutic effect, but it needs to be verified by long-term follow-up.
In conclusion, the “distal nucleus pulposus as the core” molding concept can effectively guide the intervertebral foramina molding site of PETD, which is conducive to the accurate placement of the working trocar, thus achieving satisfactory clinical results.
Informed Consent of the Patients
In this study, all patients gave informed consent and signed the informed consent form.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hoogland T, Schubert M, Miklitz B, Ramirez A. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine. 2006;31(24):E890–7. doi:10.1097/01.brs.0000245955.22358.3a
2. Gadjradj PS, Harhangi BS, Amelink J, et al. Percutaneous Transforaminal Endoscopic Discectomy Versus Open Microdiscectomy for Lumbar Disc Herniation: a Systematic Review and Meta-analysis. Spine. 2021;46(8):538–549. doi:10.1097/BRS.0000000000003843
3. Jitpakdee K, Liu Y, Kotheeranurak V, Kim JS. Transforaminal Versus Interlaminar Endoscopic Lumbar Discectomy for Lumbar Disc Herniation: a Systematic Review and Meta-Analysis. Global Spine J. 2023;13(2):575–587.
4. Li WS, Yan Q, Cong L. Comparison of Endoscopic Discectomy Versus Non-Endoscopic Discectomy for Symptomatic Lumbar Disc Herniation: a Systematic Review and Meta-Analysis. Global Spine J. 2022;12(5):1012–1026. doi:10.1177/21925682211020696
5. Choi KC, Lee JH, Kim JS, et al. Unsuccessful percutaneous endoscopic lumbar discectomy: a single-center experience of 10,228 cases. Neurosurgery. 2015;76(4):372–380. doi:10.1227/NEU.0000000000000628
6. Pan M, Li Q, Li S, et al. Percutaneous Endoscopic Lumbar Discectomy: indications and Complications. Pain Physician. 2020;23(1):49–56.
7. Yin J, Jiang Y, Nong L. Transforaminal approach versus interlaminar approach: a meta-analysis of operative complication of percutaneous endoscopic lumbar discectomy. Medicine. 2020;99(25):e20709. doi:10.1097/MD.0000000000020709
8. Zhao Y, Yuan S, Tian Y, Liu X. Necessity of routinely performing foraminoplasty during percutaneous endoscopic transforaminal discectomy (PETD) for lumbar disc herniation. Br J neurosurgery. 2023;37(3):277–283. doi:10.1080/02688697.2020.1817853
9. Ahn Y, Jang IT, Kim WK. Transforaminal percutaneous endoscopic lumbar discectomy for very high-grade migrated disc herniation. Clin Neurology Neurosurgery. 2016;147:11–17. doi:10.1016/j.clineuro.2016.05.016
10. Kim HS, Paudel B, Jang JS, Lee K, Oh SH, Jang IT. Percutaneous Endoscopic Lumbar Discectomy for All Types of Lumbar Disc Herniations (LDH) Including Severely Difficult and Extremely Difficult LDH Cases. Pain Physician. 2018;21(4):E401–e8.
11. Lee S, Kim SK, Lee SH, et al. Percutaneous endoscopic lumbar discectomy for migrated disc herniation: classification of disc migration and surgical approaches. Eur Spine J. 2007;16(3):431–437. doi:10.1007/s00586-006-0219-4
12. Zhao Y, Fan Y, Yang L, et al. Percutaneous Endoscopic Lumbar Discectomy (PELD) via a Transforaminal and Interlaminar Combined Approach for Very Highly Migrated Lumbar Disc Herniation (LDH) Between L4/5 and L5/S1 Level. Med Sci Monitor. 2020;26:e922777. doi:10.12659/MSM.922777
13. Martínez-Lage JF, Fernández Cornejo V, López F, Poza M. Lumbar disc herniation in early childhood: case report and literature review. Child’s Nervous System. 2003;19(4):258–260. doi:10.1007/s00381-003-0720-6
14. Strömqvist F, Strömqvist B, Jönsson B, Karlsson MK. The outcome of lumbar disc herniation surgery is worse in old adults than in young adults. Acta orthopaedica. 2016;87(5):516–521. doi:10.1080/17453674.2016.1205173
15. Kapetanakis S, Gkantsinikoudis N, Charitoudis G. Implementation of Percutaneous Transforaminal Endoscopic Discectomy in Competitive Elite Athletes With Lumbar Disc Herniation: original Study and Review of the Literature. Am J Sports Med. 2021;49(12):3234–3241. doi:10.1177/03635465211032612
16. Fiorenza V, Ascanio F. Percutaneous Endoscopic Transforaminal Outside-In Outside Technique for Foraminal and Extraforaminal Lumbar Disc Herniations-Operative Technique. World Neurosurg. 2019;130:244–253. doi:10.1016/j.wneu.2019.07.005
17. Yan Y, Wang Y, Yang J, et al. Percutaneous Endoscopic Lumbar Discectomy for Highly Upmigrated Disc Herniation Through the Transforaminal Isthmus Plasty Approach. World Neurosurg. 2018;120:511–515. doi:10.1016/j.wneu.2018.09.157
18. Yao Y, Qin R, Feng Q, et al. Percutaneous Endoscopic Transforaminal Decompression in the Treatment of Patients with Migrated Lumbar Disc Herniation: a Retrospective Study. World Neurosurg. 2019;128:e562–e9. doi:10.1016/j.wneu.2019.04.195
19. Li P, Yang F, Chen Y, Song Y. Percutaneous transforaminal endoscopic discectomy for different types of lumbar disc herniation: a retrospective study. J Int Me Res. 2021;49(10):3000605211055045. doi:10.1177/03000605211055045
20. Wang Y, Zhang W, Lian L, Xu J, Ding W. Transforaminal Endoscopic Discectomy for Treatment of Central Disc Herniation: surgical Techniques and Clinical Outcome. Pain Physician. 2018;21(2):E113–e23.
21. Cao L, Liu M, Wang M, et al. 3D slicer-based calculation of hematoma irregularity index for predicting hematoma expansion in intracerebral hemorrhage. BMC Neurol. 2022;22(1):452. doi:10.1186/s12883-022-02983-w
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.