Back to Journals » Journal of Pain Research » Volume 14
Percutaneous Endoscopic Interlaminar Discectomy with Modified Sensation-Motion Separation Anesthesia for Beginning Surgeons in the Treatment of L5-S1 Disc Herniation
Authors Kong M , Gao C, Cong W, Li G, Zhou C, Ma X
Received 11 February 2021
Accepted for publication 30 June 2021
Published 8 July 2021 Volume 2021:14 Pages 2039—2048
DOI https://doi.org/10.2147/JPR.S306319
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Krishnan Chakravarthy
Meng Kong,1 Changtong Gao,2 Wenbin Cong,3 Guanghui Li,1 Chuanli Zhou,1 Xuexiao Ma1
1Department of Spine Surgery, Affiliated Hospital of Qingdao University, Qing’dao, Shandong Province, 266000, People’s Republic of China; 2Minimally Invasive Interventional Therapy Center, Qingdao Municipal Hospital, Qing’dao, Shandong Province, 266000, People’s Republic of China; 3Department of Radiology, Affiliated Hospital of Qingdao University, Qing’dao, Shandong Province, 266000, People’s Republic of China
Correspondence: Xuexiao Ma; Chuanli Zhou
Department of Spine Surgery, Affiliated Hospital of Qingdao University, No. 59, Hai Er Road, Qing’dao, Shandong Province, 266000, People’s Republic of China
Tel +86 186 61807895
Email [email protected]; [email protected]
Purpose: To compare the clinical effects of local anesthesia (LA), general anesthesia (GA) and modified sensation-motion separation anesthesia (MA) in percutaneous endoscopic interlaminar discectomy (PEID) in the treatment of L5/S1 lumbar disc herniation (LDH) for the purpose of guiding junior surgeons.
Methods: Eighty-four patients with L5/S1 LDH underwent PEID using three anesthesia methods. Patients in the LA (26), GA (29) and MA (29) groups received a follow-up examination retrospectively. The general parameters, preparation and anesthesia duration, operative duration, recovery time, incidence of complications, ambulation time, length of hospital stay, incidence of severe complications, and reoperation rate were compared, and clinical outcomes were analyzed using a visual analog scale (VAS), the Oswestry Disability Index (ODI), and the Short-Form Health Survey 36 (SF-36).
Results: MA demonstrated obvious advantages over the other two methods with respect to operative duration and resulted in a better intraoperative experience than LA. The patients in the MA group required less time in bed postoperatively and shorter hospital stays than those in the GA group. The mean postoperative VAS, ODI and SF-36 scores were significantly better than the preoperative scores in all groups (P< 0.05), but no significant differences in these scores were found among the three groups (P> 0.05). Three cases (3/29) of nervous disorder occurred in the GA group. Two patients (one in the GA group (1/29) and one in the LA (1/26) group) underwent revision surgery, with a total recurrence rate of 2.4% (2/84).
Conclusion: Due to its high safety and good tolerance by patients, MA is a suitable method for spinal surgeons who are inexperienced with PEID in the treatment of L5/S1 disc herniation.
Keywords: anesthesia, LDH, sensation-motion separation, PEID
Introduction
Percutaneous endoscopic lumbar discectomy (PELD) is a minimally invasive technique for the treatment of a common spinal pathology, symptomatic lumbar disc herniation (LDH). The technique is effective for disc herniation at almost all locations; it has an overall failure rate of 4.3%, a reoperation rate of 2.4–8.5% and a recurrence rate of 0.8%.1–3 In cases of a high-riding iliac crest or local transverse process hypertrophy, lateral insertion into the working channel is difficult, and this poses a challenge in PELD.4,5 Hence, Rutten in 2005 and Choi in 2007 independently described a novel approach, percutaneous endoscopic interlaminar discectomy (PEID).6–9 The corridor used for PEID is similar to that used for traditional microendoscopic discectomy (MED) except that the working channel can be docked through the ligamentum flavum (LF) in the interlaminar space, whereas in MED, the working channel is docked upon the interlaminar space and does not touch the dura; this makes PEID a higher-risk procedure than MED. Traditionally, while the patient is under general anesthesia (GA), any procedure that requires a working channel that enters or exits the spinal canal can potentially injure the surrounding neural structures, especially when the surgeon is a beginner. Local anesthesia (LA) could be an alternative method of anesthesia in PEID due to the access it provides to intraoperative feedback from patients; such feedback can reduce the risk of iatrogenic nerve injury and postoperative cognitive dysfunction (POCD).10,11 However, significant pain induced by cutting the LF with scissors, manipulating the annulus fibrosus of the disc and rotating the working channel usually cannot be completely alleviated by local anesthetics, and this may cause extreme nervousness in patients and make surgery under LA a difficult process. One study reported that the procedure may have to be stopped because of severe pain.12 Since epidural anesthesia (EA, referred to here as MA, modified anesthesia) with ropivacaine can preserve motor function of the lower limbs and selectively block sensation during surgery,13 in the present study, we introduced a modified anesthesia technique based on the sensation-motion separation effect of ropivacaine and the synergistic effect of sufentanil14 as a compromise method in an attempt to create a situation in which both patients and surgeons could achieve favorable intraoperative experiences.
To the best of our knowledge, no relevant studies investigating the applicability of various anesthesia methods when the surgeon is a novice have been conducted. The purpose of this study was to evaluate the safety, efficacy and intraoperative experience of PEID when conducted under three different types of anesthesia, namely, LA, GA and MA, and provide an excellent strategy through which beginning surgeons can reduce the risk of neurological complications and associated anesthesia-related accidents and improve the efficiency of the operation.
Methods
Patients
The clinical data of 84 standard candidates who underwent PEID performed by the same surgeon between October 2017 and December 2018 were retrospectively analyzed. The subjects were divided into three groups, the LA (26), GA (29), and MA (29) groups, according to the method of anesthesia, which was determined by preoperative randomization. The inclusion criteria were as follows: 1) diagnosis of LDH at L5/S1 based on an imaging examination (computed tomography [CT] and magnetic resonance imaging [MRI] scans) and no lateral recess stenosis radiologically; 2) varying degrees of lower back pain and a positive reaction to the sciatic nerve stretch test; and 3) no treatment with steroids or nonsteroidal drugs within four weeks of surgery. The exclusion criteria were as follows: 1) malignant tumor; 2) severe cardiovascular or cerebrovascular disease (CCVD); 3) hepatic or renal insufficiency; and 4) reoperation at the responsible segment.
Surgical Procedure
In most patients, the L5/S1 interlaminar window was wide enough to accommodate the working cannula; patients in whom it was not were kept in the prone position with flexion of the knees and hips to increase the interlaminar space. Furthermore, a Karrison rongeur was used to enlarge the space.
In all three groups, PEID was conducted using an endoscopic spine system (Joinmax, Karlsruhe, Germany). A posterior transverse 7-mm incision was made approximately 5 mm lateral to the spinous process. The procedural technique varied slightly. In the LA group, the skin, the lumbodorsal fascia and the attachment between the LF and the lamina were anesthetized with 10 mL of a 1:1 mixture of 1% lidocaine and 0.5% ropivacaine, and the extradural intraspinal canal or the surrounding zone of nerve roots was anesthetized with 5–10 mL of a 1:1 mixture of 0.5% lidocaine and 0.25% ropivacaine. Additional analgesic doses were administered intraoperatively if necessary. With the surgeon in direct communication with the patient to achieve adequate pain management, the puncture needle and the working cannula were introduced at the dorsal part of the LF under fluoroscopic guidance, followed by cutting or splitting of the LF, introduction of a cannula into the epidural space, identification of the epidural fat and the dural sac, and removal of the herniated disc under endoscopic view according to the preference of Professor Choi.6 In the MA group, the procedure was conducted using a standard EA technique with a puncture at the L2/3 segment and insertion of a catheter into the epidural space to a depth of approximately 4 cm. With the patient in the prone position, the anesthesiologist injected a mixture of 5 mL of 0.25% or 0.2% ropivacaine and 5 mL of sufentanil (250 μg) according to the height, weight and pain threshold of the patient. A total of 3 mL of the mixture was applied initially, and movement of the leg 5 minutes later indicated that the anesthetic was not in the subarachnoid space. When necessary, the mixture was administered a second time to adjust the sensory level and achieve the aim of sensory-motor separation. In the optimal state of anesthesia, the patient could still experience minimal sustained pain, but the motor nerves were not blocked. After successful EA, the puncture needle and the working channel were placed at the level of the herniated disc in sequence, just dorsal to the LF. The remainder of the procedure was similar; it involved separating or dissecting the LF, relocating the working channel, identifying the nerve root and the herniated disc, and completing the discectomy under endoscopy according to the procedure described above.7,8 In the GA group, propofol (2–3 mg/kg), sufentanil (0.2 μg/kg), and cisatracurium (0.2 mg/kg) were administered, and ventilation was controlled to maintain end-tidal carbon dioxide (CO2) between 32 and 38 mmHg. To maintain anesthesia, 0.05 mg/kg of the muscle relaxant cisatracurium was added at 40-minute intervals according to the conditions of the operation. Since the patient was completely unconscious, the puncture needle and working channel were placed using a technique similar to that used in the MA group.
Follow-Up Evaluations
The clinical outcomes were compared among the three groups using a self-reported visual analog scale (VAS), the Oswestry Disability Index (ODI), and the Short-Form Health Survey 36 (SF-36);15 the scores on these instruments were recorded preoperatively and 1 and 3 months postoperatively. Peri- and postoperative data, including the preparation and anesthesia time, operative duration (from skin incision to closure), recovery time (from incision closure to leaving the operating room), incidence of complications, length of hospital stay, ambulation time, and reoperation rate, were collected. Spinal magnetic resonance imaging (MRI) was performed to evaluate the extent of decompression and removal of the nucleus pulposus on postoperative day 1 and at the 6-month follow-up. Recurrence of disc herniation was defined as disc herniation at the primary operative site after successful initial removal of the protruding disc and a pain-free interval that lasted for at least 2 weeks as revealed on the subsequent MRI.
Statistical Analysis
Statistical analysis was performed using SPSS 17.0 software. For demographic information and clinical parameters before and after the operation, group differences were examined by the χ2 test, one-way ANOVA, the least significant difference t-test (LSD-t) and Fisher’s test, as applicable. Measurement data are expressed as the mean and standard deviation (SD). Statistical significance was defined as P < 0.05.
Results
All patients in the three groups who suffered from L5/S1 disc herniation underwent PEID. The demographic characteristics of the patients are summarized in Table 1. The LA group included 17 males and 9 females, with a mean age of 34.2 years (range, 16–71 years) and a mean symptom duration of 11.7 months (1.5–102 months). Eight of these patients suffered from disc herniation with epiphyseal annulus separation or calcification. In the GA group, there were 18 men and 11 women with a mean age of 38.8 years (range, 22–68 years) and a mean symptom duration of 12.5 months (1–108 months). Seven of the patients in this group suffered from disc herniation with epiphyseal annulus separation or calcification. In the MA group, there were 19 males and 10 females, with a mean age of 37.4 years (range, 17–65 years) and a mean symptom duration of 11.5 months (1–96 months). Eight patients in the MA group suffered from disc herniation with epiphyseal annulus separation or calcification. The mean follow-up periods in the LA, GA and MA groups were 16.4 months (11–26 months), 15.6 months (10–24 months) and 15.1 months (11–23 months), respectively. No differences in the above parameters or in the composition of herniated disc type were found among the three groups.
 |
Table 1 Preoperative Demographic and Clinical Characteristics |
The perioperative characteristics are summarized in Table 2. The mean preparation and anesthesia time was 5.5 minutes (P<0.001 vs MA) in the LA group, 21.93 minutes (P<0.001 vs MA) in the GA group and 12.59 minutes in the MA group. Conducting the EA procedure in MA often requires a certain amount of time. However, patients in the MA group (8.90 minutes) had almost the same recovery time before leaving the operating room as patients in the LA group (8.69 minutes, P>0.05), and in both groups the recovery time was shorter than that in the GA group (32.72 minutes, P<0.001). The average operative duration after skin incision was 54.23 minutes (P<0.001 vs MA) in the LA group, 58.75 minutes (P<0.001 vs MA) in the GA group and 42.51 minutes in the MA group. With regard to nerve root or dural damage, three patients in the GA group (3/29, 10.34%) showed obvious sciatica after recovery from anesthesia; this was attributed to intraoperative working tube adjustments that were made without patient feedback. Moderate postoperative sensory paralysis due to an overdose of anesthetic was observed in one patient in the MA group (1/29, 3.45%), but no significant differences in this parameter were found among the three groups. Patients who received an overdose of anesthetics usually recovered within 6 hours after returning to the ward, and recovery within this period could be used to differentiate between the effect of ropivacaine and iatrogenic nerve damage. Additionally, the ambulation time data indicated that faster rehabilitation was achieved in the LA (5.5 hours) and MA (5.79 hours) groups than in the GA group (11.41 hours, P<0.001 vs MA/LA). Moreover, the mean length of hospital stay was 2.96 days in the LA group (P>0.05 vs MA) and 3.10 days in the MA group; in both of these groups, the rehabilitation time was shorter than the 4.76 days in the GA group (P<0.001 vs MA/LA). During the follow-up period, disc herniation recurrence requiring subsequent reoperation was observed in one patient in the GA group (1/29) and in one patient in the LA group (1/26), yielding a total recurrence rate of 2.4% (2/84).
 |
Table 2 Summary of Perioperative Outcomes, Complication, and Reoperation |
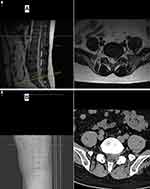
The preoperative and postoperative scoring parameters are shown in Table 3. The mean postoperative VAS, ODI and SF-36 scores were obviously improved compared with the preoperative scores in all three groups (P<0.05), and no significant differences in these scores were found among the three groups (P>0.05), indicating that similar clinical efficacy was obtained in all three groups. Nevertheless, as mentioned above, subjects who received LA were more sensitive to nerve stimulation and exhibited higher intraoperative VAS scores (6.35) than those in the MA group (4.51) (P<0.05). During the operation, most of the patients complained of frequent pain around the nerve tissue, and this influenced the mental state of the surgeon and required proper management by the surgeon. This was considered the main cause of the prolonged operative duration. When the nerve root was exposed and the herniated disc was removed, the intraoperative VAS scores of all patients in the LA and MA groups, especially those in the former group, increased suddenly. In all three groups, the patients had good MRI findings at the last follow-up (Figures 1 and 2). In summary, MA demonstrated obvious advantages over LA with respect to operative duration and intraoperative experience and resulted in a reduced perioperative period compared with GA.
 |
Table 3 Clinical Improvement According to the Parameters |
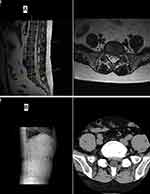 |
Figure 2 One-year postoperative MRI (A) and CT (B) of the patient whose MRI and CT findings are shown in Figure 1. |
Discussion
PEID is an effective and alternative surgery for the treatment of L5/S1 disc herniation and even for the treatment of special cases of L4/5 disc herniation, as this procedure can be easily performed by spinal surgeons who have extensive experience in open surgery and with the local anatomy. Compared with percutaneous endoscopic transforaminal discectomy (PETD), the wide interlaminar space between L5 and S1 makes it easy to enter the spinal canal, decompress the nerve root and remove the protruding disc.16,17 However, in PEID, the operating procedure involves entry into the spinal canal and direct retraction of the nerve root, and this may cause postoperative complications. In general, most spinal surgeons prefer to perform PEID to treat L5/S1 disc herniation, while other physicians, such as pain specialists and interventional radiologists, always select PETD for all types of LDH, even in cases of high iliac crests. In our department, PETD is mostly applied to treat far lateral disc herniation at the L5/S1 level. In regard to long-term clinical efficacy, PEID and PETD do not seem to always differ significantly.18
Endoscopic discectomy is a complicated procedure that relies heavily on patient feedback during surgery. This applies to both the transforaminal approach and the interlaminar approach. GA has been widely adopted in almost all spinal surgeries due to the ease it allows of controlling vital signs. Nonetheless, GA also has obvious drawbacks, such as the lack of timely feedback while the patient is unconscious, and it requires greater surgical skill and experience.19 Relevant studies have shown that GA may be associated with a greater risk of neurological complications, rendering patients unable to cooperate with the surgeon.19 Compared with those of PETD, the complications of PEID are much more troublesome and serious.20 Since the spinal canal is opened and the dura mater or nerve root must be retracted during the surgical procedure, complications such as dural avulsion, nerve root damage, and epidural hematoma are more likely to occur.21 In this study, 3 of the 29 patients in the GA group experienced neurological deficits after the operation, a higher proportion than was observed in the other two groups. The lack of a significant difference in this parameter could be attributed to the small sample size. However, the occurrence of nerve injury in patients in the GA group highlights the potential importance of real-time intraoperative patient feedback during these procedures. In the early stage of learning PEID under GA, junior surgeons may have difficulty accurately distinguishing the nerve root from the surrounding tissue. Furthermore, under endoscopic view, the process of inserting a working cannula with an external diameter of 6.9 or 6.3 mm through the LF and relocating the instrument in the spinal canal may tear the dura directly. Sometimes, when far-migrated discs are being dissected, the nerve root or dura are roughly handled and are subjected to prolonged or strenuous retraction without any reaction from the patient. Fortunately, all 3 of the patients who experienced neurological deficits in this study recovered well after 3 months. Based on our experience, any movement toward the nerve roots should be performed carefully and sufficiently slowly to establish a “controlled” environment and avoid damage, because no subjective responses are obtained from unconscious patients during the operation. Consequently, the operative duration increased with the use of GA (58.75±5.98 minutes, Table 2); thus, anesthetic accidents or postoperative nausea and emesis are more likely to occur in patients in poor physical condition.22 In general, intraoperative neurophysiological monitoring should be considered if GA is applied, and this may increase the cost of treatment.
LA has always been employed by pain specialists and interventional practitioners for the purpose of reducing the risk of nerve injury.23 During this procedure, patients can provide instant feedback on their feelings and communicate information regarding their physical condition to the doctor, reporting any sensations of temporary or sharp pain.24 However, sharp pain and agitation can sometimes disturb the progress of the surgery (this may have contributed to the average operative duration of 54.23±7.32 minutes in the LA group, Table 2) and increase the mental burden on the surgeon, especially if the surgeon is inexperienced, because the surgeon will be concerned about nerve damage while performing the procedure, and this may require the procedure to be stopped.25 Thus, not only did the patients usually have a poor surgical experience when LA was used, but the surgeon also usually experienced unpleasant challenges, and this was supported by the low willingness of LA patients to undergo the same procedure again.
To assist spine surgeons and beginners in the early period of learning PEID, we attempted a modified anesthesia method that used the sensation-motion separation effect of ropivacaine and the synergistic effect of sufentanil to maintain a condition of consciousness, painlessness and freedom of movement during the operation. Our goal was to minimize discomfort and pain and allow real-time communication through continuous feedback from patients to prevent neural damage and help monitor clinical improvements during the operation. The patients who were anesthetized using this method could also move their lower limbs when needed to help the surgeons identify nerve injury. However, studies in the literature have suggested that the concentration of ropivacaine used plays an important role in the alleviation of pain.26 Kathuria27 and Zhu28 et al separately reported that low concentrations of ropivacaine (0.25% or 0.375%) blocked the sensory nerves without completely blocking the motor nerves, resulting in better efficacy in pain management when used in EA. Previously, with the help of anesthesiologists, we tested concentrations of ropivacaine ranging from 0.1% to 0.375% according to the physical condition of the patients. For most patients, 0.2% or 0.25% ropivacaine was the ideal concentration, and the administration of a total of 10 mL of the anesthetic was satisfactory. Another advantage of MA is the ease of controlling the dosage. Nevertheless, attention should also be paid to individual variation in pain and drug responses as well as to nerve root anomalies.
It is not surprising that patients who received spinal anesthesia had better intraoperative experiences than patients who received LA. All of the 29 patients who received MA achieved free movement of the lower limbs, and none complained of severe pain; thus, the result was satisfactory (intraoperative VAS score 4.51±1.13, Table 3). Although the amount of pain experienced by the patient was not easy to measure accurately, it reflected patient comfort to a certain extent. In addition, it is usually time consuming and painful to treat disc herniations with epiphyseal annulus separation or calcification in PEID. In the current research, since no differences in the type or location of protrusions were identified (Table 1), the difference in the intraoperative pain intensity and the operative duration between the LA and MA groups was not associated with operational discrepancies between individuals. Only one patient had lower limb paresthesia; this was found to be caused by an excessive concentration and dosage of anesthetic that was injected in an attempt to obtain a satisfactory painless condition in the early stage, and the disorder subsided 6 hours later. Due to the indispensable feedback and the absence of unnecessary distractions from the patients, the surgeon was able to complete the operation in a leisurely manner; this could explain why the operative duration was significantly shortened in the MA group (P<0.001 vs GA and LA groups).
Another noteworthy matter was the similar recovery rates of the MA and LA groups after surgery. Because of the efficacy of sensation-motion separation and the more rapid metabolism of the anesthetics, patients who received MA could be resuscitated from anesthesia more quickly than patients who received GA and, upon leaving the operating table, could immediately cooperate in the routine examination of lower limb activity; thus, they could return to the ward faster (Table 2). Ye et al29 also demonstrated a higher score of postoperative cognitive function in patients treated with PELD under EA than in patients treated with PELD under GA, suggesting that EA had a positive effect on the improvement in cognitive function. In the current study, the ambulation time in the MA group, as well as that in the LA group, was obviously shorter than that in the GA group (P<0.001). In addition, before performing GA, comprehensive preoperative physical examinations, especially examinations to detect cardiopathy and pneumopathy, are necessary to avoid the risk of perioperative accidents. Vital signs are usually monitored and evaluated for 24 hours before discharge in patients who receive GA. Hence, the length of hospital stay in the GA group was prolonged (P<0.001 vs LA and MA groups).
With regard to the reoperation rate in this study, only one patient in the GA group underwent revision surgery at 2 months postoperatively due to a fall while intoxicated; this made it difficult to determine whether there were statistically significant correlations between the reoperation rate and the anesthesia method used in PEID. Previous studies have suggested that the incidence of recurrence was 5.5% after PEID with annular sealing and 13.5% after PEID without annular sealing, and patient age was correlated with overall recurrence and late recurrence, whereas the operative technique was correlated only with early recurrence.19 Of note, we found no significant differences in the recurrence rate among the three groups, and the low reoperation rate in this study may be due to the relatively small sample size and the short follow-up period.
In addition, although it did not occur in this study and has been reported to normally disappear within hours, postoperative dysuria is a side effect of excessive EA19,30 that needs to be considered. In the case of MA, the use of an experienced anesthesiologist is recommended to avoid damage to the spinal cord/cauda equina and to control sensory-motor separation.
In conclusion, the best anesthetic approach for endoscopic surgery allows the real-time monitoring of the nerve root status and avoids pain. The current findings show that for spinal surgeons who are inexperienced in the use of PEID to treat L5/S1 disc herniation, MA would be a superior choice because of its efficacy in allowing the surgeon to avoid nerve root injury, increasing pain tolerance, easing the anxiety of beginner surgeons, and accelerating patient recovery. Our practical experience suggests that LA be used when the surgeon has gained sufficient experience with various endoscopic surgeries in more than 100 cases and has less fear of distractions from patients. After the surgeon attains sufficient endoscopic operational experience in more than 200 cases and can perform endoscopic surgeries perfectly and address complex situations, the use of GA in PEID would be more appropriate.
Limitations
The retrospective design and the limited number of cases are the main inherent limitations of this study, and they may have led to biases. Since analgesic drugs are not routinely used after surgery in our department, no relevant data were included in this research. Thus, a comprehensive analysis of the effect of postoperative analgesia on the results of the experiment was not possible. A larger sample size is needed to corroborate the favorable effect of MA in PEID for treating L5/S1 disc herniation.
Conclusion
In this study, we aimed to share our experience during the learning curve. Specifically, MA demonstrated obvious advantages over LA with respect to the operative duration and intraoperative experience and led to a shortened perioperative period compared with GA, suggesting that modified anesthesia with sensation-motion separation is a suitable method for use by spinal surgeons who are inexperienced with PEID in the treatment of L5/S1 disc herniation.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Affiliated Hospital of Qingdao University (QYFYWZLL 26036) and conducted in accordance with the principles set forth in the Declaration of Helsinki. All patients involved provided verbal informed consent to a review of their medical records, and that was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University.
Acknowledgments
The authors acknowledge Chao Wang, Shuo Han, Xing Han and Xiaoliang Chen (Department of Spinal Surgery, Affiliated Hospital of Qingdao University) for their contributions to this work.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81672200, 81871804) and the National Key Research and Development Project (CN) (2019YFC0121400).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Choi K-C, Lee J-H, Kim J-S, et al. Unsuccessful percutaneous endoscopic lumbar discectomy: a single-center experience of 10,228 cases. Neurosurgery. 2015;76(4):
2. Hirano Y, Mizuno J, Takeda M, Itoh Y, Matsuoka H, Watanabe K. Percutaneous endoscopic lumbar discectomy - early clinical experience. 2012;52(9):625.
3. Choi G, Lee SH, Bhanot A, Raiturker PP, Chae YS. Percutaneous endoscopic discectomy for extraforaminal lumbar disc herniations: extraforaminal targeted fragmentectomy technique using working channel endoscope. Spine. 2007;32(2):E93–99.
4. Ruetten S, Komp M, Merk H, Godolias G. Recurrent lumbar disc herniation after conventional discectomy: a prospective, randomized study comparing full-endoscopic interlaminar and transforaminal versus microsurgical revision. J Spinal Disord Tech. 2009;22(2):122–129.
5. Mirkovic SR, Schwartz DG, Glazier KD. Anatomic considerations in lumbar posterolateral percutaneous procedures. Spine. 1995;20(18):1965–1971.
6. Choi G, Lee SH, Raiturker PP, Lee S, Chae YS. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5-S1 using a rigid working channel endoscope. Neurosurgery. 2006;58(1Suppl):
7. Ruetten S, Komp M, Merk H, Godolias G. Use of newly developed instruments and endoscopes: full-endoscopic resection of lumbar disc herniations via the interlaminar and lateral transforaminal approach. J Neurosurg Spine. 2007;6(6):521–530.
8. Ruetten S, Komp M, Godolias GA. New full-endoscopic technique for the interlaminar operation of lumbar disc herniations using 6-mm endoscopes: prospective 2-year results of 331 patients. Minimally Invasive Neurosurg. 2006;49(2):80–87.
9. Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine. 2008;33(9):931–939.
10. Mason SE, Noel-Storr A, Ritchie CW. The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: a systematic review with meta-analysis. J Alzheimer’s Dis. 2010;22(Suppl 3):67–79.
11. Fang G, Ding Z, Song Z. Comparison of the effects of epidural anesthesia and local anesthesia in lumbar transforaminal endoscopic surgery. Pain Phys. 2016;19(7):E1001–1004.
12. Ren Z, He S, Li J, et al. Comparison of the Safety and Effectiveness of Percutaneous Endoscopic Lumbar Discectomy for Treating Lumbar Disc Herniation Under Epidural Anesthesia and General Anesthesia. Neurospine. 2020;17(1):254–259.
13. Cherng CH, Yang CP, Wong CS. Epidural fentanyl speeds the onset of sensory and motor blocks during epidural ropivacaine anesthesia. Anesth Analg. 2005;101(6):1834–1837.
14. Leighton BL, Arkoosh VA, Huffnagle S, Huffnagle HJ, Kinsella SM, Norris MC. The dermatomal spread of epidural bupivacaine with and without prior intrathecal sufentanil. Anesth Analg. 1996;83(3):526–529.
15. Ware JE
16. Choi KC, Kim JS, Ryu KS, Kang BU, Ahn Y, Lee SH. Percutaneous endoscopic lumbar discectomy for L5-S1 disc herniation: transforaminal versus interlaminar approach. Pain Phys. 2013;16(6):547–556.
17. Choi KC, Park CK. Percutaneous Endoscopic Lumbar Discectomy for L5-S1 disc herniation: consideration of the relation between the iliac crest and L5-S1 disc. Pain Phys. 2016;19(2):E301–308.
18. Choi KC, Kim JS, Park CK. Percutaneous endoscopic lumbar discectomy as an alternative to open lumbar microdiscectomy for large lumbar disc herniation. Pain Phys. 2016;19(2):E291–300.
19. Zhu Y, Zhao Y, Fan G, et al. Comparison of 3 anesthetic methods for percutaneous transforaminal endoscopic discectomy: a prospective study. Pain Phys. 2018;21(4):E347–e353.
20. Sairyo K, Matsuura T, Higashino K, et al. Surgery related complications in percutaneous endoscopic lumbar discectomy under local anesthesia. J Med Investigation. 2014;61(3–4):264–269.
21. Cong L, Zhu Y, Tu G. A meta-analysis of endoscopic discectomy versus open discectomy for symptomatic lumbar disk herniation. Eur Spine J. 2016;25(1):134–143.
22. Albayrak S, Erol FS, Demirel I, Ayden O, Ucler N. Lumbar disc surgery with epidural anesthesia: review of 700 cases. Turk Neurosurg. 2016;26(3):399–403.
23. Sairyo K, Egawa H, Matsuura T, et al. State of the art: transforaminal approach for percutaneous endoscopic lumbar discectomy under local anesthesia. J Med Investigation. 2014;61(3–4):217–225.
24. Li ZZ, Hou SX, Shang WL, Song KR, Zhao HL. The strategy and early clinical outcome of full-endoscopic L5/S1 discectomy through interlaminar approach. Clin Neurol Neurosurg. 2015;133:40–45.
25. Ahn Y. Transforaminal percutaneous endoscopic lumbar discectomy: technical tips to prevent complications. Expert Rev Med Devices. 2012;9(4):361–366.
26. Akakin A, Yilmaz B, Akay A, Sahin S, Eksi MS, Konya D. Epidural anesthesia in elective lumbar microdiscectomy surgery: is it safe and effective? Turk Neurosurg. 2015;25(1):117–120.
27. Kathuria S, Gupta S, Dhawan I. Dexmedetomidine as an adjuvant to ropivacaine in supraclavicular brachial plexus block. Saudi J Anaesth. 2015;9(2):148–154.
28. Zhu Y, Zhao Y, Fan G, et al. Comparison of the effects of local anesthesia and epidural anesthesia for percutaneous transforaminal endoscopic discectomy in elderly patients over 65 years old. Int J Surg. 2017;48:260–263.
29. Ye H, et al. Therapeutic effect of percutaneous transforaminal nucleus pulposus extraction on lumbar disc herniation under different anesthesia. China J Endoscopy. 2019;25(10):74–78.
30. Dagistan Y, Okmen K, Dagistan E, Guler A, Ozkan N. Lumbar microdiscectomy under spinal and general anesthesia: a comparative study. Turk Neurosurg. 2015;25(5):685–689.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.