Back to Journals » Journal of Asthma and Allergy » Volume 16
Percent Recovery Index Predicts Poor Asthma Control and Exacerbation in Adults
Authors Kuang L , Ren C, Liao X, Zhang X, Zhou X
Received 25 March 2023
Accepted for publication 3 July 2023
Published 13 July 2023 Volume 2023:16 Pages 711—722
DOI https://doi.org/10.2147/JAA.S414164
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor David Price
Lisha Kuang,1,* Cheng Ren,2,* Xiuqing Liao,2 Xiaobin Zhang,2 Xuegang Zhou2
1Department of Health Management Center, Chongqing University Fuling Hospital, Chongqing, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Chongqing University Fuling Hospital, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiuqing Liao, Department of Respiratory and Critical Care Medicine, Chongqing University Fuling Hospital, Chongqing, People’s Republic of China, Tel +86 23-72250973, Email [email protected]
Background: Previous studies indicate that the percent recovery index (PRI: the percentage increase from the maximally reduced FEV1 after bronchodilator inhalation), one of the indexes of methacholine bronchial provocation, may predict acute asthma exacerbations in childhood and elderly asthmatics. It is known that childhood (< 12) and elder (> 60) asthmatics may be different to adult patients in many aspect including prognosis. However, in adults, a research for predicting value of PRI to exacerbation is still absence. Besides exacerbation, predicting value of PRI to poor asthma control is also unknown. We try to detect whether PRI can predict poor asthma control and exacerbation in adults in this research. Meanwhile, we try to detect whether treatment can influence PRI.
Methods: In 61 adults with asthma, baseline PRI was measured during enrollment. And then baseline PRI was evaluated as a predictor of exacerbation or poor asthma control at an upcoming 3-month follow-up. The covariates included age, sex, BMI, previous exacerbation, smoking status and baseline lung function. After treatment for 3 months, PRI was measured again and compared with baseline PRI.
Results: After the 3-month follow-up, we found that baseline PRI was significantly related to asthma exacerbation (P = 0.023), poor asthma control (ACT at 3 months, P = 0.014), decreased quality of life (decrease of MiniAQLQ, P = 0.010) and cumulative number of EDHO at 3 months (P = 0.039). Meanwhile, no significant correlation was observed between baseline PRI and inflammation factors (FENO, CaNO, and EOS). Finally, PRI was dramatically reduced after standard treatment for 3 months.
Conclusion: PRI is efficient in the prediction of poor asthma control and exacerbation in adults. The predictive value of PRI may rely on the inherent property of asthmatic airway smooth muscle (ASM) independent of inflammation factors. Effective treatment can alleviate PRI dramatically and that indicate PRI may also be valuable in evaluation of curative effect.
Keywords: asthma, exacerbation, poor control, percent recovery index, bronchodilator reversibility
Introduction
Poor asthma control and exacerbation are the main problems for the prognosis of asthma, and the efficient prediction is very valuable for the effective treatment of asthma. It is known that the following factors may participate in poor asthma control and exacerbation: older age, female sex, smoking, obesity, comorbidities (COPD, gastroesophageal reflux, sleep apnoea, etc.), incorrect inhalation techniques, previous history of exacerbations, improper drug use, low economic status, worsening lung function, and so on.1–8 In addition to these respects, the predictive value of the percent recovery index (PRI) has been noticed recently.
PRI is one of the metrics generated from the methacholine bronchial provocation test (MBPT), and it is the percent increase from the maximally reduced FEV1 after bronchodilator inhalation. MBPT can generate other metrics: PD20 (the cumulative amount causing a 20% decrease in forced expiratory volume in 1 s (FEV1) from baseline), PC20 (the threshold causing a 20% decrease in FEV1 from baseline), and CIR (the continuous index of responsiveness: the percent decline from baseline FEV1 after the last dose of methacholine). In all these indexes, PC20 and PD20 are most widely used in the clinic as indicators for airway hyperresponsiveness (AHR). However, only PRI is effective in predicting acute exacerbation in both elderly and childhood patients with asthma.9
Research on the predictive value of PRI to asthma is limited. First, there is still a lack of detection to the relationship between poor asthma control and PRI at any age group. Second, previous research was carried out in children (aged 5–12 years) and elderly (aged ≥65 years) asthmatics. It is known that childhood (<12) and elder (>60) asthmatics are different to adult patients in many aspects including pathogenesis, epidemiology, comorbidities, treatment and prognosis.10–14 For example, in exacerbations, old patients had more exacerbation and atypical manifestation,15,16 and children are prone to different environmental or biological factors which may lead to more exacerbation.17 Meanwhile, adult patients are major part of the whole asthmatic population.18 Therefore, research on adult asthma patients is indispensable. To our knowledge, this is the first study of PRI predicting poor asthma control and exacerbation in adults.
In addition, whether PRI can be changed by treatment is still unknown. We will detect the dynamics change of PRI during procession of follow-up.
Methods
Each study was approved by the Institutional Review Board of the corresponding institution (Chongqing University Fuling Hospital, Chongqing city, China (number: 2019CQSFLZXYYEC-013)), and informed consent was obtained from all study participants. Our study complies with the Declaration of Helsinki. The clinical research was registered at http://www.chictr.org.cn (Registration number: ChiCTR1900026436).
Study Populations
Patients diagnosed with asthma and meet the following criteria were enrolled. Inclusion criteria: (1) Age 18–60; (2) Participants have the ability to follow instructions and meet the quality assurance standards of the NIH/NHLBI’s Severe Asthma Research Program (SARP)19 to perform accurate and reproducible spirometry. Exclusion criteria: (1) Patients with chronic cardiopulmonary disorders other than asthma, such as congenital heart disease, bronchiectasis, cystic fibrosis, interstitial lung disease, major systemic disorders, bronchopulmonary dysplasia, and so on. (2) Patients who have communication disorders. (3) Patients who were critically ill. Finally, sixty-one adult patients with current asthma participated in the study.
After enrolment, participants were treated with conventional medications based on the Global Initiative for Asthma guidelines.20 Follow-up visits were scheduled every 3 months. Besides the general data, the following metrics were recorded at enrollment (baseline) and every 3-month visit: MBPT: include PRI, CIR and PD20; lung function; asthma exacerbation; ACT; MiniAQLQ; inflammation factors: fractional exhaled nitric oxide (FENO), blood eosinophils (EOS) and alveolar NO (CaNO). In this article, we reported the data of the first follow-up visit for 3 months.
Diagnosis of Asthma
The diagnosis of asthma was made by a specialist in respiratory medicine based on a typical history (wheezing or attacks of breathlessness; chest tightness; cough triggered by exercise, exposure to allergens or irritants or respiratory infections) and at least one of the following: (1) FEV1 reversibility >12% (and an absolute increase of >200 mL) after a standard dose of short-acting β2-agonist (SABA). (2) Positive bronchial challenge test. (3) Average daily diurnal PEF variability >10%, or PEF weekly variation rate (within 2 weeks) >20%.18
Asthma Exacerbation
An asthma exacerbation was defined when one of the following criteria was satisfied: use of systemic corticosteroids for at least three successive days, unscheduled asthma-specific emergency department visits or hospitalizations (EDHO).9 Besides the exacerbations in follow-up of 3 months, a history of a previous exacerbation (no versus yes) during one year before enrolment was also recorded.
Methacholine Bronchial Provocation Test (MBPT) and Index Analysis
For all participants, MBPT was carried out using the modified method described by Park et al.9 Methacholine was diluted to concentrations of 1.25, 2.5, 6.25, and 12.5 mg·mL−1 with buffered saline, and the aerosol was generated and delivered in general method.21 FEV1 after inhaling aerosol generated only from buffered saline (Baseline FEV1) was evaluated. Participants inhaled increasing concentrations of methacholine stepwisely until fall of FEV1 from baseline ≧ 20% (FEV1 at the last dose of methacholine). Then, a bronchodilator (two puffs of salbutamol) was administered, and FEV1 (post-bronchodilator FEV1) was measured 15 mins after bronchodilator inhalation. The calculation method of PRI and CIR are listed in Figure 1.
 |
Figure 1 The calculation process of indices from the MBPT. |
Mini Asthma Quality of Life Questionnaire (Mini-AQLQ)
Mini-Asthma Quality of Life Questionnaire (Mini-AQLQ) includes 15 questions in 4 domains (symptoms, activity, emotions, and environment). Scores range from 1 to 7, with higher scores indicating a better quality of life.22 According to change of Mini-AQLQ score during 3-month follow-up, we divided participants into an elevated life quality group and a decreased life quality group. For elevated life quality group, (Mini-AQLQ score at 3 months-Mini-AQLQ score at baseline) > 0. For decreased life quality group, (Mini-AQLQ score at 3 months-Mini-AQLQ score at baseline) < 0. Mini-AQLQ score included not only total score but also score of four domains separately.
The Asthma Control Test (ACT)
The Asthma Control Test (ACT) questionnaire23 was used to classify patients as having controlled or uncontrolled asthma. The ACT questionnaire is a validated, five-item, patient-completed assessment of their asthma control in the prior 4 weeks. Poor asthma control is defined as a score of <19.
Statistical Analysis
All data were collected at two timepoints seperately: enrollment (baseline) and visit at the 3-month follow-up (3 months). The assumptions of the normal distribution for demographic statistics were evaluated using the Kolmogorov–Smirnov test. A normal distribution is presented as the mean and standard deviation (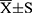 ), and an abnormal distribution is presented as the median (inter-quartile range, IQR). For impact of treatment, the difference was detected between baseline and 3 months: Statistics with normal distribution were compared using paired sample t-test, and statistics with abnormal distribution were compared using the non-parametric Wilcoxon test.
), and an abnormal distribution is presented as the median (inter-quartile range, IQR). For impact of treatment, the difference was detected between baseline and 3 months: Statistics with normal distribution were compared using paired sample t-test, and statistics with abnormal distribution were compared using the non-parametric Wilcoxon test.
For predicting ability of PRI (baseline) to poor asthma control and exacerbation, a logistic regression was used for the categorical variable, and a linear regression was used for the continuous variable. The primary outcomes were acute exacerbation (no versus yes, during the follow-up of 3 months), poor asthma control (ACT at 3 months) and decrease of life quality (comparison of MiniAQLQ between baseline and 3 months). Secondary outcome was the cumulative number of EDHOs during the follow-up of 3 months (Figure 2). Covariates included age, sex (female versus male), BMI, previous history of exacerbation (no versus yes, 1 year before enrollment), smoking status (no versus yes), and spirometry of baseline (include FEV1% predicted, FEV1/FVC, FEF25, FEF50, and FEF75). For the difference of PRI (baseline) between the patients with poor asthma control or exacerbation and the corresponding normal patients: Comparison was made separately between population with or without exacerbation (exacerbations), ACT (3 months) ≧19 and <19 (poor asthma control), increased and decreased scores of Mini-AQLQ (decrease of life quality). And the difference was calculated using the non-parametric Mann–Whitney-Wilcoxon U-test.
For correlation between PRI and other indexes at baseline, a Spearman correlation analysis was used due to abnormal distribution of PRI. The other indexes include MBPT (PD20 and CIR), inflammation factors (FENO, CaNO and EOS) and spirometry (include FEV1% predicted, FEV1/FVC, FEF25, FEF50, and FEF75). SPSS 24.0 was used for statistical analyses. P values ≤0.05 indicated statistical significance.
Results
Baseline characteristics of the participants are summarized in Table 1. In our research, the proportion of males (31.15%) and smokers (14.76%) is relatively small. All patients got ICS contained therapy, and ICS-LABA (93.44%) is the main medication.
 |
Table 1 Baseline Characteristics |
Difference of metrics was compared between the baseline and 3 months (Table 2). After treatment for 3 months, score of ACT and MiniAQLQ, PD20 and spirometry (FEV1, FVC and FEF) were increased significantly. Meanwhile, PRI was decreased significantly. The results indicate that the effective treatment can reduce PRI (Table 2). To our knowledge, this is the first study to detect the influence of treatment to PRI.
 |
Table 2 Comparison Between the Baseline and the 3-Month Follow-Up Visit |
Predicting value of PRI to poor asthma control and exacerbation was detected by regression analysis. Using the variance inflation factor, we evaluated the multicollinearity in our models. By removing FVC, FEF50 and FEF75 from final models, we obtained acceptable variance inflation factor values (<5) (Table 3). Results of regression analysis are shown in Table 4. PRI at baseline was significantly associated with acute exacerbations during follow-up of 3 months, cumulative number of EDHO during follow-up of 3 months, ACT at 3 month and changes of MiniAQLQ during follow-up of 3 months. In the four domains of the MiniAQLQ, a significant correlation with PRI was observed in the symptoms, environmental and emotion domains (Table 5). To our knowledge, this is the first research for predicting value of PRI to exacerbation and poor asthma control in adults, and this is also the first research for predicting value of PRI to decreased life quality.
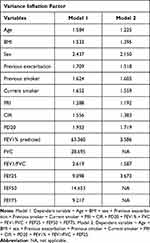 |
Table 3 Collinearity Diagnostics for Logistic Regression Models |
 |
Table 4 Relationships Between PRI and Clinical Outcomes |
 |
Table 5 Relationships Between PRI and the Four Domains of MiniAQLQ |
Difference of PRI at baseline between poor asthma control or exacerbation group and corresponding control group were detected. PRI was significantly higher in patients with acute exacerbations (yes), poor asthma control (ACT < 19) or decreased life quality (MiniAQLQ at 3 months - MiniAQLQ at baseline < 0) (Figure 3).
The correlation between PRI and other metrics was measured using spearman’s correlation coefficients, all the data in this section were generated from the baseline. High correlations were noted between PRI and PD20 (Table S1). No significant correlation was found between PRI and inflammation factors (Table S2). No significant correlations were found between PRI and airway obstruction indexes (FEV1, FVC, FEV1/FVC and FEF) (Table S3).
Discussion
Results showed that PRI is efficient in the prediction of poor asthma control and exacerbation in adult. That is support by previous study: In children and elder asthmatics, PRI also showed homologous ability in predicting future exacerbation of asthma.9 To our knowledge, this is the first report that PRI is valuable for the prediction of poor asthma control and exacerbation in adults.
The predicting ability of PRI may be based on the inherent property of asthmatic airway smooth muscle (ASM). In our research, we found a significant positive correlation between PRI and PD20, which is consistent with a previous study. Heung-Woo Park also found a significant positive correlation between PRI and PC20.9 The correlation indicates that a higher PRI is related to more AHR.
The inherent property of ASM is one of the main mechanisms of AHR, and it plays important roles in acute exacerbation of asthma.24,25 In asthmatic ASM, these inherent properties include increased mass and hypercontractility, which present as an increase in the maximum capacity and velocity of shortening.24 In addition to inherent properties, ASM is also affected by inflammation or neuroendocrine factors in the asthmatic state. Affected by these internal or external factors, asthmatic ASM is usually in a shortened and hypercontractile state, and this abnormal state may be the foundation of a high rate of reversion to bronchodilators.24
Compared to traditional bronchodilator reversibility (BR), PRI has a different basic status of the airway. The use of a short-β2-agonist (salbutamol) in the measurement of PRI is based on methacholine-induced contraction of the trachea, not the natural state of the airway in BR. We hypothesized that this contraction induced by methacholine may cover the basic shortening state of asthmatic ASM, and bronchodilation based on this contraction (PRI) may mainly depend on the inherent properties of ASM independent of external factors such as inflammation. Surprisingly, this hypothesis got a support in our study: PRI indeed has no correlation with some inflammation factors that may correlate with BR. Besides the positive correlation between PRI and AHR,9 the results indicate that PRI may be determined by the inherent properties of ASM independent of external factors, and that may be the basis of the predictive value of PRI.
The comparison between PRI and BR is also instructive. BR, including the bronchial dilation test (BDT) and bronchodilator-dose responsiveness (BDR),26 can also predict future exacerbation of asthma in children or adolescents.26–28 The basis of BR is the natural state of ASM, which is determined by both inherent properties of ASM and external factors as mentioned before, and that may also lead to some difference compared to PRI: First, BR and inflammation factors present a certain degree of positive correlation. The related inflammation factors include sensitization to aeroallergen, serum IgE, reactivity on a skin test, EOS27 and FENO (present large airway inflammation).29 Secondly, BR is closely correlated with airway obstruction (FEV1/FVC, FEV1% predicted and PEF),30,31 meanwhile PRI had no correlation with these metrics (Tables S2 and S3). Finally, no significant correlation was detected between PC20 and BR.31 These evidence indicate that BR may reflect a comprehensive effect caused by internal and external factors that affect asthmatic ASM.
Whether the difference between PRI and BR can lead to different efficiencies of prediction is unknown. In previous studies, children and adolescents with poor BDR did not influence current symptoms or quality of life.26 In our research, a higher PRI was not only related to poor asthma control and exacerbation but also to a decrease in quality of life. However, due to the lack of direct comparison, it cannot be deduced that PRI is more efficient than BR in predicting prognosis of asthma.
As an efficient tool for the reflection of life quality, it has been accepted that the MiniAQLQ is valuable guidance for treatment of asthma.22 PRI has a significant positive correlation with a decrease in MiniAQLQ score in the course of treatment, indicating that higher PRI is a hazard factor for a decreased life quality. Further analysis observed same positive correlation of PRI with the symptoms, environmental and emotion domains in MiniAQLQ. The result indicate PRI may be valuable to evaluation of curative effect in asthma. To our knowledge, this is also the first report showing that PRI is valuable for predicting a decreased life quality in asthma patients.
Besides evidence of MiniAQLQ, we also found that effective treatment for asthma can reduce PRI and that indicate PRI is valuable to evaluation of curative effect in asthma more directly. To our knowledge, this is also the first research for the influence of treatment to PRI. Inherent property of ASM may be determined by abnormalities of calcium Homeostasis or contractile proteins in asthmatic state and that can be alleviated dramatically by ICS or LABA.24 Considering that ICS (100.0%) and LABA (95.1%) were the main medications in our research, we proposed that effective medicine may reduce PRI through the alleviation of inherent property of ASM and that also provide another evidence to indicate that PRI may be related to inherent property of ASM.
Limitation
As mentioned before, the comparison between PRI and BR is instructive. However, due to the initial design of the project, the direct contrast between PRI and BR is absent in our research. This leaves a gap that needs to be filled in the future.
We have more female patients in this study, and that is in accord with the natural character of asthma. The research in recent years has also shown that females had poorer asthma control and more exacerbations.32 The role of sex difference in prediction of asthma is deserve to detect. However, our data indicate no significant difference in PRI between male and female. That may be due to the limited number of participants, and larger related research is needed in the future.
Our main proposal, PRI may reflect inherent property of ASM, is based on deduction, and lack direct evidence. That may also provide a new direction for future research.
In conclusion, our research found that PRI is valuable for the prediction of poor asthma control, exacerbation and decreased life quality in adults. Meanwhile, PRI may be also valuable to the evaluation of curative effect in asthma. Finally, we proposed that predicting ability of PRI may be based on the inherent property of ASM.
Data Sharing Statement
The authors are willing to share the individual deidentified data of this article, including sex, age, BMI, main spirometry, MBPT data (PRI, CIR and PD20), and Questionnaire score (ACT and MiniAQLQ). Except these data, no additional study documents will be available. The data will be sharing in clinical research website http://www.chictr.org.cn once the article is accepted and published.
Disclosure
The authors report grants from Chongqing Science and health joint medical research project (2019ZDXM053), during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Patel M, Pilcher J, Reddel HK, et al. Predictors of severe exacerbations, poor asthma control, and β-agonist overuse for patients with asthma. J Allergy Clin Immunol Pract. 2014;2(6):751–758. doi:10.1016/j.jaip.2014.06.001
2. Ban GY, Trinh TH, Ye YM, Park HS. Predictors of asthma control in elderly patients. Curr Opin Allergy Clin Immunol. 2016;16(3):237–243.
3. Kuti BP, Omole KO, Kuti DK. Factors associated with childhood asthma control in a resource-poor center. J Family Med Prim Care. 2017;6(2):222–230. doi:10.4103/jfmpc.jfmpc_271_16
4. Sorkness RL, Zoratti EM, Kattan M, et al. Obstruction phenotype as a predictor of asthma severity and instability in children. J Allergy Clin Immunol. 2018;142(4):1090–1099.e4. doi:10.1016/j.jaci.2017.09.047
5. Smith CJ, Spaeder MC, Sorkness RL, Teague WG. Disparate diagnostic accuracy of lung function tests as predictors of poor asthma control in children. J Asthma. 2020;57(3):327–334. doi:10.1080/02770903.2019.1566471
6. Almomani BA, Al-Qawasmeh BS, Al-Shatnawi SF, Awad S, Alzoubi SA. Predictors of proper inhaler technique and asthma control in pediatric patients with asthma. Pediatr Pulmonol. 2021;56(5):866–874. doi:10.1002/ppul.25263
7. Tupper OD, Ulrik CS. Long-term predictors of severe exacerbations and mortality in a cohort of well-characterised adults with asthma. Respir Res. 2021;22(1):269. doi:10.1186/s12931-021-01864-z
8. Mulugeta T, Ayele T, Zeleke G, Tesfay G, Plavec D. Asthma control and its predictors in Ethiopia: systematic review and meta-analysis. PLoS One. 2022;17(1):e0262566. doi:10.1371/journal.pone.0262566
9. Park HW, Song WJ, Chang YS, et al. Bronchodilator response following methacholine-induced bronchoconstriction predicts acute asthma exacerbations. Eur Respir J. 2016;48(1):104–114. doi:10.1183/13993003.00182-2016
10. Skloot GS, Busse PJ, Braman SS, et al. An official American thoracic society workshop report: evaluation and management of asthma in the elderly. Ann Am Thorac Soc. 2016;13(11):2064–2077. doi:10.1513/AnnalsATS.201608-658ST
11. Xiang L, Zhao J, Zheng Y, et al. Uncontrolled asthma and its risk factors in Chinese children: a cross-sectional observational study. J Asthma. 2016;53(7):699–706. doi:10.3109/02770903.2016.1144199
12. Kercsmar CM, Shipp C. Management/comorbidities of school-aged children with asthma. Immunol Allergy Clin North Am. 2019;39(2):191–204. doi:10.1016/j.iac.2018.12.004
13. Liu L, Liu C, Chen R, et al. Associations of short-term exposure to air pollution and emergency department visits for pediatric asthma in Shanghai, China. Chemosphere. 2021;263:127856. doi:10.1016/j.chemosphere.2020.127856
14. Association PPCoCME, Asthma Collaborative Group RG, Association PBoCM. Expert consensus on diagnosis and management of bronchial asthma comorbidities in children. Chin J Appl Clin Pediatr. 2023;38(4):245–259.
15. Gonzalez-Garcia M, Caballero A, Jaramillo C, Maldonado D, Torres-Duque CA. Prevalence, risk factors and underdiagnosis of asthma and wheezing in adults 40 years and older: a population-based study. J Asthma. 2015;52(8):823–830. doi:10.3109/02770903.2015.1010733
16. Lin J, Wang W, Chen P, et al. Prevalence and risk factors of asthma in mainland China: the CARE study. Respir Med. 2018;137:48–54. doi:10.1016/j.rmed.2018.02.010
17. Murray CS, Jackson DJ, Teague WG. Prevention and outpatient treatment of asthma exacerbations in children. J Allergy Clin Immunol Pract. 2021;9(7):2567–2576. doi:10.1016/j.jaip.2021.03.035
18. Asthma group of Chinese Thoracic Society. 支气管哮喘防治指南(2020年版)[Guidelines for bronchial asthma prevent and management(2020 edition). Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(12):1023–1048. Chinese. doi:10.3760/cma.j.cn112147-20200618-00721
19. Gaffin JM, Petty CR, Sorkness RL, et al. Determinants of lung function across childhood in the Severe Asthma Research Program (SARP) 3. J Allergy Clin Immunol. 2023;151(1):138–146.e9. doi:10.1016/j.jaci.2022.08.014
20. Reddel HK, Bacharier LB, Bateman ED, et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. Eur Respir J. 2022;59(1):2102730. doi:10.1183/13993003.02730-2021
21. Cockcroft DW, Berscheid BA. Slope of the dose-response curve: usefulness in assessing bronchial responses to inhaled histamine. Thorax. 1983;38(1):55–61. doi:10.1136/thx.38.1.55
22. Schatz M, Zeiger RS, Mosen D, Vollmer WM. Asthma-specific quality of life and subsequent asthma emergency hospital care. Am J Manag Care. 2008;14(4):206–211.
23. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008
24. Berair R, Hollins F, Brightling C. Airway smooth muscle hypercontractility in asthma. J Allergy. 2013;2013:185971. doi:10.1155/2013/185971
25. Xiong D, Martin JG, Lauzon AM. Airway smooth muscle function in asthma. Front Physiol. 2022;13:993406. doi:10.3389/fphys.2022.993406
26. Grunwell JR, Nguyen KM, Bruce AC, Fitzpatrick AM. Bronchodilator dose responsiveness in children and adolescents: clinical features and association with future asthma exacerbations. J Allergy Clin Immunol Pract. 2020;8(3):953–964. doi:10.1016/j.jaip.2019.09.033
27. Howrylak JA, Fuhlbrigge AL, Strunk RC, Zeiger RS, Weiss ST, Raby BA. Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications. J Allergy Clin Immunol. 2014;133(5):1289–300, 1300.e1–12. doi:10.1016/j.jaci.2014.02.006
28. Heffler E, Crimi C, Campisi R, et al. Bronchodilator response as a marker of poor asthma control. Respir Med. 2016;112:45–50. doi:10.1016/j.rmed.2016.01.012
29. Puckett JL, Taylor RW, Leu SY, et al. An elevated bronchodilator response predicts large airway inflammation in mild asthma. Pediatr Pulmonol. 2010;45(2):174–181. doi:10.1002/ppul.21172
30. Ryan G, Latimer KM, Dolovich J, Hargreave FE. Bronchial responsiveness to histamine: relationship to diurnal variation of peak flow rate, improvement after bronchodilator, and airway calibre. Thorax. 1982;37(6):423–429. doi:10.1136/thx.37.6.423
31. Louis R, Bougard N, Guissard F, Paulus V, Henket M, Schleich F. Bronchodilation test with inhaled salbutamol versus bronchial methacholine challenge to make an asthma diagnosis: do they provide the same information. J Allergy Clin Immunol Pract. 2020;8(2):618–625.e8. doi:10.1016/j.jaip.2019.09.007
32. Senna G, Latorre M, Bugiani M, et al. Sex differences in severe asthma: results from severe asthma network in Italy-SANI. Allergy Asthma Immunol Res. 2021;13(2):219–228. doi:10.4168/aair.2021.13.2.219
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


