Back to Journals » Infection and Drug Resistance » Volume 13
Penicillium janthinellum Pneumonia in an SLE Patient: A Case Study
Authors Li X , Zong L, Zhu Y, Li Y , Zhou Y, Zhou H
Received 30 April 2020
Accepted for publication 17 July 2020
Published 7 August 2020 Volume 2020:13 Pages 2745—2749
DOI https://doi.org/10.2147/IDR.S255968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Xi Li, 1,* Laibin Zong, 1,* Yongze Zhu, 1 Yali Li, 2 Yonglie Zhou, 1 Hua Zhou 3
1Centre of Laboratory Medicine, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, Zhejiang 310014, People’s Republic of China; 2Department of Dermatology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yonglie Zhou; Hua Zhou Tel +86-571-8589-3267
; Fax +86-571-8623-6873
Email [email protected]; [email protected]
Abstract: The risk of opportunistic fungal infections is high in immunocompromised patients. The Penicillium genus is common and diverse in nature. However, it rarely causes infection in humans. Here, we reported a case of Penicillium janthinellum pneumonia in a systemic lupus erythematosus (SLE) patient, and the morphological characteristics of P. janthinellum were also described. The patient was a 64-year-old female. She had been diagnosed with SLE and membranous lupus nephritis 10 months previously. Her medications included methylprednisolone, cyclosporine, and hydroxychloroquine. She was admitted because of fever and diagnosed with pneumonia. P. janthinellum was isolated from sputum and bronchoalveolar lavage (BAL) samples. BAL fluid stained with multiple stains showed the presence of somewhat dichotomously branching septate fungal hyphae. P. janthinellum was identified, and its morphological features were described. Antibiotic susceptibility profiles showed that this strain had higher minimum inhibitory concentration (MIC) values in response to multiple antifungal drugs. The patient died 10 days after diagnosis. To the best of our knowledge, this report is the second to demonstrate that P. janthinellum causes infection and is the first to present an infection (pneumonia) caused by P. janthinellumi in an SLE patient. Clinical and laboratory personnel should be aware that the Penicillium genus also contains pathogenic bacteria that cannot simply be treated as contaminants, especially in immunosuppressed patients.
Keywords: BAL fluid, Penicillium janthinellum, SLE
Introduction
Systemic lupus erythematosus (SLE) is an archetype of systemic autoimmune disease with no available cure and at only remission can be achieved by medication.1 Adrenocortical hormone or immunosuppression is still the main treatment regimen for SLE. However, long-term use of glucocorticoids and immunosuppressants in SLE patients can lead to immune impairment, resulting in is a high risk of opportunistic infections, such as opportunistic fungal infections, which are often severe and can cause patient death if not treated promptly.
The Penicillium genus is common and diverse in nature, and contains approximately 350 species, which may cause opportunistic infection in humans.2 Notably, these species are often contaminants in clinical specimens, and isolated from the lungs as a result of colonization after inhalation of conidia. Here, we reported a case of severe pneumonia in an SLE patient from whom Penicillium janthinellum was isolated from sputum and bronchoalveolar lavage (BAL) samples. The patient died 10 days after diagnosis. We believe that P. janthinellum was the cause of the severe bilateral pneumonia with consolidation in this patient according to results of chest computed tomography (CT), the aspergillus galactomannan (GM) test and culture.
Case Presentation
The patient was a 64-year-old female. She was admitted because of fever and cough for 1 week. She had suffered from hypertension for more than ten years. She had been diagnosed with SLE 10 months previously because after developing a rash, which was mainly distributed in the skin, neck and both upper limbs. Meanwhile, laboratory tests showed positive results for anti-DS-DNA and anti-SSA antibody spectra. She was further diagnosed with membranous lupus nephritis based on pathological findings of renal puncture 7 months ago prior to admission. Her medications included methylprednisolone 24 mg once daily, cyclosporine 50 mg twice daily and hydroxychloroquine 0.2g twice daily, amlodipine 5mg once daily, febuxostat 40 mg once daily combined with a sodium bicarbonate tablet 0.5g three times a day, warfarin tablets 2.5mg once daily, torasemide 10mg once daily, potassium chloride 0.5 g once-daily. After treatment at a local hospital, the rash subsided. However, the urine protein of the patient remained positive, with an elevated erythrocyte sedimentation rate and hypoproteinaemia, and the patient therefore remained on immunosuppressive therapy.
A physical examination revealed a weak state. Her temperature was 36.4°C on admission (maximum temperature 38.5°C), her heart rate was 85 beats/min, her respiratory rate was 18/min, and her blood pressure was 140/76 mmHg. No obvious dry or wet rales were noted in either lung by auscultation. Laboratory data showed a white blood cell count of 6360/mm3, with 92.6% polymorphonuclear cells, 4.9% lymphocytes, and 2.1% monocytes. Platelets (34*109/L) were significantly decreased. Serum complement C3 (0.69g/L) and serum albumin (23.5 g/L) were reduced. Ur Prot/UrCreat (6.51g/g) was significantly elevated. The result of anti-DS-DNA result was negative, but anti-SSA antibody spectra were weakly positive (1:32). CD3+ (348*106/L), CD4+ (168*106/L) and CD8+ (180*106/L) lymphocyte subsets had a significantly decreased. In addition, the patient tested negative for human immunodeficiency virus (HIV).
The chest CT showed pneumonia in both lungs (Figure 1A). The patient was diagnosed with pneumonia, and received treatment with sulfamethoxazole and cefuroxime. The patient’s body temperature fluctuated between 36°C and 38°C during hospitalization, and her pulmonary infection did not improve. It is necessary to exclude pulmonary tuberculosis and pneumocystis jiroveci pneumonia (PJP). Sputum for gram stain, culture and acid-fast smear was obtained. Culture of the sputum generated abundant normal oropharyngeal flora and Candida tropicalis. In addition, blood cultures were negative.
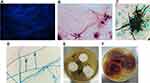
The patient remained febrile, with a temperature as high as 39.1°C. A repeat chest CT also showed further infection in both lungs compared with that at admission (Figure 1B). A bronchoscopy with BAL was performed. Purulent secretions were observed and a sample was taken from the left upper lung. A large amount of mould was also isolated in large amounts from the BAL fluid. BAL fluid stained with Gram, fluorescence and hexamine silver stains showed the presence of somewhat dichotomously branching septate fungal hyphae (Figure 2A–C). The GM results in the BAL and serum were 1.85 μg/L and 0.89 μg/L, respectively. According to the latest guidelines for invasive fungi, the GM results indicated the presence of fungal infection.3 Sulfamethoxazole and cefuroxime were discontinued and a new regimen of micafungin (150mg/d) and ganciclovir was initiated. The patient began to defervesce but the repeat chest CT also showed further infection in both lungs (Figure 1C). The patient died 10 days later. No autopsy was performed.
The purulent secretions were cultured on Sabouraud dextrose agar (SDA) (Emmon’s modification) and on SDA with chloramphenicol at 28°C.The fungus grew at 28°C on SDA and on SDA with chloramphenicol. No growth was observed on Mycosel agar. Fungal morphology stained with Medan lactate showed a branching mycelium. Conidial terrier grew from the side ends of hyphae and formed a short broom at the top. The stem of the bottle was in the shape of a bottle. The conidia were spherical or elliptic (3–6 μm in diameter) (Figure 2D). The organism had white-coloured villiform and produced a slight yellow pigment on the reverse side, showing that had the most pronounced growth at 5 days on potato dextrose agar (Figure 2E). Notably, the organism developed a purple pigment on the reverse side after 14 days of growth on potato dextrose agar (Figure 2F). Attempts to identify the fungus were unsuccessful by morphology and the MALDI-TOF-MS system. The isolate was identified by sequencing using primers (ITS1: 5ʹ-TCCGTAGGTGAACCTGCGG-3ʹ; ITS4: 5ʹ-TCCTCCGCTTATTGATATGC-3ʹ). The mould was later identified as P. janthinellum by its DNA sequence. Antibiotic susceptibility testing was performed using a microdilution technique (CLSI M38-A2) (Clinical and Laboratory Standards Institute, 2008).4 Antibiotic susceptibility profiles showed that this strain had higher minimum inhibitory concentrations (MIC) values in response to many antifungal drugs (Table 1).
 |
Table 1 Fungal Susceptibility |
Discussion and Conclusions
The genus Penicillium includes approximately 350 species, including Paecilomyces, Fusarium, Scopulariopsis, Acremonium and Beauveria. Currently, these species play significant and varied roles in food manufacturing.2,5 P. janthinellum is a very common organism, which that mainly exists in a variety of habitats, such as soil, vegetation, air and food products. It has the characteristics of fast growth and reproduction and strong adaptability, and can grow and reproduce in the absence of oxygen and a certain concentration of carbon dioxide. In addition, this organism can produce a variety of toxins, such as verrucosporin, citrinin, and janthitrems curnlic acid.6 Because Penicillium species are common in nature, these species are usually considered contaminants when they were isolated from clinical specimens, but they have also been demonstrated to cause human disease.7 Systemic infections caused by Penicillium marneffei have been reported in immunocompromised patients.8,9 These mycelial fungi can cause various nosocomial infections in immunocompromised hosts. P. janthinellum had been shown to cause a neurologic disease in sheep and cattle (known as rye grass staggers) as a result of its ability to produce tremorgenic toxins.6,10 In addition, P janthinellum can cause soft drink food poisoning.7 To date, only one study has reported an infection (pneumonia) caused by P. janthinellum in an AIDS patient.11 To the best of our knowledge, this report is the second to demonstrate that P. janthinellum causes infection and is the first to present an infection (pneumonia) caused by P. janthinellum in an SLE patient. Immunocompromised patients are at a high risk for developing severe life-threatening illnesses caused by opportunistic fungal pathogens. This species of Penicillium was detected from the patient’s sputum and bronchoalveolar samples, and this is the second report of pneumonia caused by this organism.
BAL is an effective method to collect bronchial and alveolar secretions from patients with pneumonia to facilitate diagnosis and treatment. In our case, P. janthinellum was isolated from sputum and bronchoalveolar washings samples, which we therefore believe may be the cause of the severe bilateral pneumonia. Although she was not neutropenic during her illness, the patient was severely immunodeficient. Notably, the infection was neglected by the clinics and laboratories due to a lack of understanding of P. janthinellum, which may be an important cause of the patients’ death. Azole drugs may be effective for treating this fungus according to the result of the drug sensitivity analysis. In addition, we are aware of one limitation of this study. Because no autopsy was performed, the nature of the infection was not confirmed histopathologically or by tissue culture.
Our case is notable for several reasons. First, this report is the first to present a P. janthinellum caused infection (pneumonia) in an SLE patient. Second, this case further highlights the need for clinicians to be cognizant of the immunosuppressors capable of inciting disease in patients with compromised immunity. Third, clinical and laboratory personnel should be aware that the Penicillium genus also contains pathogenic bacteria that cannot simply be treated as contaminants, especially in immunosuppressed patients. Fungal identification and antifungal drug sensitivity testing should also be carried out as soon as possible to provide direction for clinical drug selection.
Abbreviations
SLE, systemic lupus erythematosus; BAL, bronchoalveolar; CT, computed tomography; PJP, Pneumocystis jiroveci pneumonia; GM, Aspergillus galactomannan; SDA, Sabouraud dextrose agar; MIC, minimum inhibitory concentration.
Ethics Approval and Consent for Publication
This study has been reviewed and approved by the Research Ethics Committee of the First Affiliated Hospital of Zhejiang University (ref#2019-1488). The husband of this patient provided consent for publication of the clinical details, and written informed consent was obtained.
Acknowledgments
The authors give special thanks to Professor Yunsong Yu (Zhejiang University) for his help with revising the manuscript. Xi Li and Laibin Zong are co-first authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Tsai CY, Shen CY, Liao HT, et al. Molecular and cellular bases of immunosenescence, inflammation, and cardiovascular complications mimicking “Inflammaging” in patients with systemic lupus erythematosus. Int J Mol Sci. 2019;20(16):3878. doi:10.3390/ijms20163878
2. Perrone G, Susca A. Penicillium species and their associated mycotoxins. Methods Mol Biol. 2017;15(42):107–119.
3. Donnelly JP, Chen SC, Kauffman CA. et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2019:
4. Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi.
5. Lopez-Diaz TM, Santos JA, Garcia-Lopez ML, et al. Surface mycoflora of a Spanish fermented meat sausage and toxigenicity of penicillium isolates. Int J Food Microbiol. 2001;68:69–74. doi:10.1016/S0168-1605(01)00472-X
6. Lanigan GW, Payne AL, Cockrum PA. Production of tremorgenic toxins by penicillium janthinellum biourge: a possible aetiological factor in ryegrass staggers. Aust J Exp Biol Med Sci. 1979;57(1):31–37. doi:10.1038/icb.1979.3
7. Wang ZG. Identification of toxigenic mould in soft drink causing food poisoning. Chin J Prev Med. 1992;26:8–10.
8. Supparatpinyo K, Perriens J, Nelson KE, et al. A controlled trial of itraconazole to prevent relapse of Penicillium marneffei infection in patients infected with the human immunodeficiency virus. N Engl J Med. 1998;339(24):1739–1743. doi:10.1056/NEJM199812103392403
9. Sirisanthana T, Supparatpinyo K, Perriens J, et al. Amphotericin B and itraconazole for treatment of disseminated Penicillium marneffei infection in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;26(5):1107–1110. doi:10.1086/520280
10. Gallagher RT, Latch GC, Keogh RG. The janthitrems: fluorescent tremorgenic toxins produced by Penicillium janthinellum isolates from ryegrass pastures. Appl Environ Microbiol. 1980;39(1):272–273. doi:10.1128/AEM.39.1.272-273.1980
11. Gill MV, Schoch PE, Rinaldi MG, et al. Penicillium janthinellum in sputum and bronchoalveolar lavage in an AIDS patient with pneumonia. Clin Microbiol Infect. 1997;3(2):261–264. doi:10.1111/j.1469-0691.1997.tb00607.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.