Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Pencil Beam Scanning Carbon Ion Radiotherapy for Hepatocellular Carcinoma
Authors Zhang W, Cai X, Sun J, Wang W, Zhao J, Zhang Q, Jiang G, Wang Z
Received 5 July 2023
Accepted for publication 16 December 2023
Published 29 December 2023 Volume 2023:10 Pages 2397—2409
DOI https://doi.org/10.2147/JHC.S429186
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Wenna Zhang,1– 3 Xin Cai,1– 3 Jiayao Sun,2– 4 Weiwei Wang,2– 4 Jingfang Zhao,2– 4 Qing Zhang,1– 3 Guoliang Jiang,1– 3,5 Zheng Wang1– 3,5
1Department of Radiation Oncology, Shanghai Proton and Heavy Ion Center, Shanghai, People’s Republic of China; 2Shanghai Key Laboratory of Radiation Oncology (20dz2261000), Shanghai, People’s Republic of China; 3Shanghai Engineering Research Center of Proton and Heavy Ion Radiation Therapy, Shanghai, People’s Republic of China; 4Department of Medical Physics, Shanghai Proton and Heavy Ion Center, Shanghai, People’s Republic of China; 5Department of Radiation Oncology, Shanghai Proton and Heavy Ion Center, Fudan University Cancer Hospital, Shanghai, People’s Republic of China
Correspondence: Guoliang Jiang; Zheng Wang, Department of Radiation Oncology, Shanghai Proton and Heavy Ion Center, Fudan University Cancer Hospital, No. 4365 Kangxin Road, Pudong, Shanghai, 201315, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Carbon ion radiotherapy (CIRT) has emerged as a promising treatment modality for hepatocellular carcinoma (HCC). However, evidence of using the pencil beam scanning (PBS) technique to treat moving liver tumors remains lacking. The present study investigated the efficacy and toxicity of PBS CIRT in patients with HCC.
Methods: Between January 2016 and October 2021, 90 consecutive HCC patients treated with definitive CIRT in our center were retrospectively analyzed. Fifty-eight patients received relative biological effectiveness-weighted doses of 50– 70 Gy in 10 fractions, and 32 received 60– 67.5 Gy in 15 fractions, which were determined by the tumor location and normal tissue constraints. Active motion-management techniques and necessary strategies were adopted to mitigate interplay effects efficiently. Oncologic outcomes and toxicities were evaluated.
Results: The median follow-up time was 28.6 months (range 5.7– 74.6 months). The objective response rate was 75.0% for all 90 patients with 100 treated lesions. The overall survival rates at 1-, 2- and 3-years were 97.8%, 83.3% and 75.4%, respectively. The local control rates at 1-, 2- and 3-years were 96.4%, 96.4% and 93.1%, respectively. Radiation-induced liver disease was not documented, and 4 patients (4.4%) had their Child–Pugh score elevated by 1 point after CIRT. No grade 3 or higher acute non-hematological toxicities were observed. Six patients (6.7%) experienced grade 3 or higher late toxicities.
Conclusion: The active scanning technique was clinically feasible to treat HCC by applying necessary mitigation measures for interplay effects. The desirable oncologic outcomes as well as favorable toxicity profiles presented in this study will be a valuable reference for other carbon-ion centers using the PBS technique and local effect model-based system, and add to a growing body of evidence about the role of CIRT in the management of HCC.
Keywords: hepatocellular carcinoma, carbon ion radiotherapy, pencil beam scanning, local effect model
Introduction
Historically, use of external beam radiotherapy in the management of hepatocellular carcinoma (HCC) has been limited by the relative radiosensitivity of normal liver tissue.1 With advances in modern radiation and imaging techniques, such as intensity modulated radiotherapy (IMRT) and stereotactic body radiotherapy (SBRT) with image guidance, the indications for radiation treatment of HCC have been greatly extended.2,3 In addition, for the last 2 decades, charged particle radiation therapy such as proton radiotherapy (PRT) and carbon ion radiotherapy (CIRT) has been applied in clinical practice and demonstrated encouraging results.4–7 Unlike photon modalities, PRT and CIRT have a dose-focusing Bragg peak and much more conformal dose distribution, which could spare the adjacent organs at risk (OARs) more effectively.8 Besides, carbon ion, as a high linear energy transfer (LET) beam, produces more dense ionizations, causing higher relative biological effectiveness (RBE), along with reduced oxygen enhancement ratio, thus potentially leads to greater cell-killing effects, especially for radioresistant hypoxic tumor cells, which is common in large-size HCC.9
Since 1995, CIRT had been used to treat HCC in Japan, and yielded excellent clinical outcomes.10,11 However, the traditional beam delivery technique, passive scattering (PS), was adopted in all Japanese studies of CIRT for HCC so far, whereas the report by the Heidelberg Ion Beam Therapy Center in Germany was the only one to use a more advanced technique, pencil beam scanning (PBS), although with a very small number of patients.12 PBS has been rapidly developed in recent years and is gradually becoming the new standard technique in particle therapy. It not only performs modulation in the lateral direction like photon IMRT, but also provides modulations in depth by varying energies, thus achieving a true three-dimensional dose painting.13 As such, it is often the only treatment modality for newly built centers. Although with significant advantages in conformity of target coverage over PS, especially the lower normal tissue doses at the proximal side of the target, it also brings the great challenges when treating a moving target due to the interplay effects between the scanning beam and respiratory motion, which may exacerbate dose delivery uncertainties with poor dose homogeneity.14 Apart from this, another major issue that has to be considered is the clinical application of different RBE models in different centers, thus the microdosimetric kinetic model (MKM) based dose prescription and relevant outcomes in Japanese centers may not be the same story in our center where the local effect model (LEM) is used.15 Hence, the need to verify the reproducibility of clinical findings and explore the potential benefits in the context of the newer PBS technique and another RBE system is increasingly desirable.
In our center we have used CIRT with PBS technique to treat HCC since 2015. Here we reported the efficacy and toxicity of moderate hypofractionated CIRT for HCC, and simultaneously showed the feasibility of PBS to treat moving targets, which would be a valuable reference for other LEM-based facilities and provide more evidence to support CIRT as an alternative curative modality in HCC management.
Patients and Methods
Patient Selection Criteria
The patient selection criteria were: (1) HCC confirmed by histology or cytology, or clinically diagnosed by the criteria proposed by the Chinese Society of Clinical Oncology or the American Association for the study of Liver Diseases;16,17 (2) surgically unresectable, medically inoperable, refusal of surgery, or recurrences after surgery or ablation therapy; (3) Child–Pugh (CP) scores of 5–7 points; (4) Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; (5) no evidence of distant metastases; (6) gastrointestinal (GI) tract not invaded; and (7) absence of uncontrolled ascites. Patients who had previous radiation history of liver, or had another primary malignancy, were excluded. This study was reviewed and approved by the ethical committee of our center (approval number: 221122EXP-01), and all patients signed an informed consent form before treatment initiation.
CIRT Details
Briefly, patients were immobilized in supine or prone position and underwent CT simulation. Alternative techniques for motion mitigation included: respiratory gating (AZ-733V, Anzai Medical, Tokyo, Japan), active breath coordinator (ABC, Elekta, Stockholm, Sweden), or abdominal compression. For respiratory gating, patients should be trained well to make his/her breathing smooth and regular. An Anzai gating pressure sensor, which took the respiratory signals from patients, should be placed on an appropriate site of patient’s body surface, and pressure on sensor adjusted to make it sensitive enough to detect the respiration. Finally, the synchronization between respiratory signal taken and internal target motion should be confirmed by X-ray fluoroscope using the conventional simulator. For ABC, patients should be trained to cooperate well with the ABC device, and the reproducibility of target position after each breath hold had to be assessed and recorded. The gross tumor volume (GTV) was lesion shown on contrast-enhanced CT/MRI, and PET-CT. Internal gross tumor volume (IGTV) was a fused GTV from all GTVs contoured on each breath phase within the gating window for gating patients, or formed by adding the reproducibility deviation among each breath holding to GTV for ABC patients, or by combining the GTVs in all phases for compression patients. The clinical target volume (CTV) was formed by adding a margin of 5 mm around IGTV. The planning target volume (PTV) was produced by adding margins of 5–10 mm around CTV for the set-up error and beam range uncertainty with more margins along the beam direction. For those cases with overlap of the PTV and the planning risk volume (PRV) created by a 3mm expansion of GI tract, a modified planning target volume (mPTV) was introduced, which was the PTV subtracting the overlap area with this PRV.
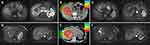
In general, 2–3 horizontal or 45-degree oblique beams with different couch positions were arranged. Treatment plans were generated and evaluated by Siemens Syngo® treatment planning system VC13 (Siemens AG, Manheim, Germany) and doses were delivered by 88–430 MeV/u carbon ion beam using the PBS technique. The biological dose was calculated by the LEM version I. CIRT were delivered by 5 fractions a week. For tumors adjacent to the GI tract, main bile ducts, or chest wall within 1 cm, 15-fraction regimens were administrated, and for other situations, 10-fraction ones were used. To compare the biological effect among the different total doses and fractionations, biological equivalent dose (BED) was estimated by linear-quadratic (LQ) model with an alpha/beta ratio of 10 Gy. For both fractionation regimens, at least 95% of the PTV or mPTV had to be covered by 95% of the prescribed dose, and the minimum dose of GTV should be maximized as much as possible. The respective dose constraints of OARs for the two regimens are detailed in Table 1. Figure 1 shows one typical case for each regimen of the dose distribution and MRI images before and after CIRT.
 |
Table 1 OAR Constraints for 10- and 15-Fraction Regimens |
For the dosimetric patient-specific quality assurance, the dose distribution of each beam was measured by an ionization chamber array set in a commercial 3-dimensional water phantom and compared with that recalculated by treatment planning system using a homogeneous medium prior to therapeutic irradiation. For patient position verification, a pair of orthogonal kilovoltage X-ray films were taken immediately before each treatment fraction to measure and correct the set-up errors online. For in vivo beam range verification, offline PET/CT images were obtained shortly after the first fraction of CIRT. For treatment adaptation, weekly repeated CT and potential replanning were performed to minimize the possibility of unacceptable geometric misses.
Follow-Up and Evaluation
After completion of CIRT, patients were assessed at one month, then every 3 months during the first 2 years, every 6 months for another 3 years, and annually thereafter. Physical examination, laboratory tests, and dynamic-enhanced MRI or CT were performed on each follow-up visit.
Local control (LC) was defined as no evidence of radiographic tumor progression within the radiation treatment field. The recurrences that occurred outside the irradiated volume but inside the liver were regarded as elsewhere liver recurrences (ELRs), and those outside the liver were considered as distant metastases. The tumor response after CIRT was determined by the modified Response Evaluation Criteria in Solid Tumors (mRECIST).18 The efficacy endpoints of LC, elsewhere liver recurrences-free survival (ELRFS), overall survival (OS), distant metastasis-free survival (DMFS) and progression-free survival (PFS) were calculated from the date of CIRT start until the event or last follow-up visit. Adverse events that occurred within 90 days from CIRT initiation were defined as acute toxicities, and those beyond 90 days, as late toxicities, evaluated by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 5.0). Radiation-induced liver disease (RILD) was defined as anicteric hepatomegaly and ascites with elevation of alkaline-phosphatase to more than twice the upper limit of normal (classic form), and evaluation of serum transaminases to more than five times the upper limit of normal or a worsening of CP score by ≥2 points (non-classic form), in the absence of intrahepatic tumor progression within 3 months after CIRT.
Statistics
LC and ELR rates were estimated using the cumulative incidence function, and actuarial survivals were calculated by Kaplan–Meier method. Univariate analysis was conducted by the Log rank test. Multivariate analysis was performed with the Cox proportional hazards model while adjusting for covariates. All statistical analyses were carried out using SPSS software version 26.0 (IBM Corp., Armonk, NY, USA) and p values of < 0.05 were considered statistically significant.
Results
Patient and Treatment Characteristics
From January 2016 to October 2021, a total of 90 HCC patients who underwent definitive CIRT were included in this study. Among the patients, 23 were from a Phase I dose escalation study reported elsewhere,19 and the other 67 were consecutively treated. The patient and treatment characteristics are detailed in Table 2. The median tumor size was 4.6 cm (range 1.6–15.5 cm). Twenty-two patients (24.4%) were recurrences after hepatectomy or radiofrequency ablation, and 78 (86.7%) were assessed as unresectable or inoperable. Twenty-three (25.6%) patients had multiple tumors, and 30 (33.3%) presented with vascular tumor thrombosis. Prior to CIRT, 65 patients (72.2%) had received 1–3 cycles of transcatheter arterial chemoembolization (TACE). Respiratory gating was the most applied technique for motion mitigation, accounting for 80% of all cases. The dose and fractionation schedules used were determined by the tumor location and normal tissue constraints, and consequently 58 patients (64.4%) received RBE-weighted doses (DRBE) of 50–70 Gy in 10 fractions, while 32 (35.6%), 60–67.5 Gy in 15 fractions.
 |
Table 2 Patient and Treatment Characteristics |
Treatment Outcomes
The median follow-up time was 28.6 months (range 5.7–74.6 months). At the last follow-up visit, 65 patients were alive with no evidence of disease in 49 cases; 20 patients died of local recurrence (1 case), ELR (8 cases), distant metastases (5 cases), both ELR and distant failures (4 cases), and TACE- or radiation-related complications (2 cases); 5 patients were lost to follow-up but without disease progression at the time of the last evaluation. Overall, 27 patients developed intrahepatic recurrences (2, inside of irradiated volume; 23, ELR; and 2, both inside of irradiated volume and ELR). Twenty patients developed distant metastases (14, in lung; 3, in retroperitoneal nodes; 1, in bones; 1, in both lung and bones; and 1, in lung, bones and brain). The objective response rate (combination of complete and partial response) was 75.0% for all 90 patients with 100 treated lesions. The OS rates at 1-, 2- and 3-years were 97.8%, 83.3% and 75.4%, respectively. The LC rates at 1-, 2- and 3-years were 96.4%, 96.4% and 93.1%, respectively. The ELR rates at 1-, 2- and 3-years were 13.7%, 26.1% and 29.3%, respectively. The DMFS rates at 1-, 2- and 3-years were 89.8%, 76.6% and 76.6%, respectively. The PFS rates at 1-, 2- and 3-years were 76.4%, 60.1% and 53.2%, respectively (Figure 2).
By univariate analysis, ECOG score of 0 (p = 0.012), absence of vascular tumor thrombosis (p = 0.010), American Joint Committee on Cancer T1 disease (p = 0.007) and Barcelona Clinic Liver Cancer stage 0-B (p = 0.005) were found to be significantly associated with longer OS, and TACE before or after CIRT was strongly related to higher LC (p = 0.004). Furthermore, patients with solitary lesion had lower ELR rates (p = 0.030), and those with tumor size ≤ 5 cm (p = 0.033) or AFP ≤ 20 ng/mL before CIRT (p = 0.011) or T1 disease (p = 0.048) suffered lower risks of distant metastasis. However, no independent prognostic factors were identified for all endpoints based on multivariate analysis. The results of Log rank tests for the prognostic factors are shown in Table 3.
 |
Table 3 Two-Year Clinical Outcomes of Prognostic Factors |
Toxicities
Acute toxicities of grades 1–2 were observed in 63 patients (70%), and the most common ones were hematological abnormalities, dermatitis radiation and liver function impairment. Within 3 months of CIRT completion, 4 patients (4.4%) had their CP scores elevated by 1 point without evidence of intrahepatic tumor progression, and the other 86 patients had stable CP scores as before. Neither classic RILD, nor non-classic RILD was documented in the entire cohort. For late toxicities, 3 patients experienced grade 3 of stomach bleeding, and 1 died of stomach bleeding 13 months after CIRT, attributed to their cirrhotic portal hypertension. Bile duct stricture occurred in 2 patients (2.2%), whose tumors were located in the porta hepatis. One of the 2 presented the stricture 18 months after CIRT of 65 Gy in 10 fractions and died of repeated jaundice 25 months after CIRT. Another one developed that 5 months after CIRT of 67.5 Gy in 15 fractions and recovered after a stent insertion. One patient (1.1%) with a peripheral lesion in close proximity to the chest wall, irradiated by 65 Gy in 10 fractions, was recorded an asymptomatic rib fracture on CT images 24 months after CIRT. Details of these toxicities are listed in Table 4.
 |
Table 4 Acute and Late Toxicities |
Discussion
Because of a very limited number of CIRT centers in the world, CIRT for HCC was mainly performed in Japan. Kato et al first reported 24 unresectable or recurrent HCC treated with CIRT in 2004, and the 3-year OS and LC rates were 50% and 81%, respectively.20 Following this encouraging outcome more clinical trials have been carried out since then. In all of these trials, the dose schemes were changed from moderate hypofractionation (15, 12 fractions) to ultrahypofractionation (8, 4, 2 fractions), with the most commonly used being 2 or 4 fractions at the present stage. Their outcomes were excellent with 3-year LC of 76.5–91.4% and 3-year OS of 50–76.7%, irrespective of the fraction number.21,22 Although employing different beam delivery systems and RBE models, our data with moderate hypofractionation of 10 or 15 fractions, in which the 3-year LC and OS were 93.1% and 75.4%, were comparable with those in previous Japanese studies.
Tumor dose is important for HCC local tumor control. Kim et al recently published a pooled analysis of Asian HCC treated by photon SBRT, and showed that a prescribed BED10 of ≥ 100 Gy was significantly associated with better local tumor control and OS.23 However, the nominal term of BED was estimated by LQ model, which was from photon biological experiments. Whether LQ model could be applied to CIRT was questionable because of their quite different radiobiology. As no CIRT biological model has been available so far, we used LQ model to evaluate the biological effect for different dose fractionations. In the present study, although there was no significant difference in LC and OS between patients receiving BED10 of ≥ 100 Gy and BED10 of < 100 Gy (p = 0.688 and p = 0.747), the median tumor dose of 63 Gy in local recurrent patients was less than 65 Gy in those without local recurrence (p < 0.0001), which implied that the dose in the setting of CIRT may also be an issue for LC. Another vital point to be considered is that high LET radiations such as carbon ions exhibit lower oxygen effects, which makes them able to eradicate hypoxic cancer cells without using an extremely high dose. This may be the reason why LC of CIRT appeared to be superior to that of PRT for larger size HCCs (> 5.0 cm diameter), as reported by the Hyogo Ion Beam Medical Center.24 Wakatsuki et al have recently shown the clinical outcomes of CIRT for bulky (≥ 4 cm) locally advanced HCC with a LC rate of 86.7% at 2-years,25 which lies within the range of those reported for patients with relatively small tumors. This is consistent with our findings that tumor size had no significant impact on LC rates of CIRT for HCC. In our cohort, the local recurrences inside the irradiation field occurred in 4 (4.4%) patients, but ELRs in 25 (27.8%) patients and distant metastases in 20 (22.2%) patients. ELRs could be either the intrahepatic spreading of primary lesion, or metachronous primary HCC in patients with long-term hepatitis B virus infection. Due to the high LC rate achieved by CIRT, preventing both ELRs and distant metastases after CIRT is very important to further improve the prognosis of HCC patients. Therefore, additional regional and systemic therapies should be given before and/or after CIRT for those patients associated with high probabilities of out-of-field failures. Although to date there are no effective treatment strategies with high categories of evidence and consensus available, the use of more sessions of TACE, as well as targeted agents and immune checkpoint inhibitors were proposed.
With regard to radiation hepatotoxicity, its rates across the various reports for HCC patients treated with CIRT ranged from none to 22%, mainly but not exclusively using CP score progression ≥ 2 points at 3 months post-treatment as the endpoint of RILD, while that ranged from 7–13% in SBRT studies.26,27 However, the high heterogeneity of tumor size and location, underlying liver function, hepatotoxicity definition, and the number of patients makes the direct comparison between these existing data quite challenging. Even so, CIRT is still greatly expected to provide superior sparing of normal liver tissue over photon irradiation due to its Bragg peak effect, and slightly better dose profiles in the entrance and lateral sides of target volume compared with PRT. In the present study, only mild abnormalities of hepatic and biliary biochemical parameters were noted in a minority of patients, which had already pre-existed, but CIRT worsened them slightly. While evaluating the CP score change, an increase of 1 point was found in 4 patients, but that of ≥2 points, in none. The minimal liver toxicities may well be partly contributed by the application of a scanning beam that provides improved dose optimization and proximal conformity minimizing radiation exposure to normal liver in front of the treatment target. In our cohort, the median mean dose to normal liver (MDTNL) was 14.7 Gy (range, 6.7–23.3 Gy), which is currently the most commonly used predictor of hepatotoxicity in photon irradiation. However, attention is needed when exclusively applying this traditional metric in the context of particle therapy planning, which exhibits a totally different depth-dose profile without a low dose bath to uninvolved liver parenchyma. The percentage of unirradiated liver volume (LV) was put forward by Mizumoto et al as a more meaningful parameter to predict RILD for PRT.28 Other than the irradiated dose and volume of normal liver, its baseline function is also a critical determinant of RILD. The study by Hsieh et al further took account of the varying demand of LV in individual patients, and proposed to use the ratio of unirradiated LV/standard LV by correcting for body surface area as a better RILD predictor than MDTNL in PRT, which was copied from the surgery experience.29 Unfortunately, available data on the optimal dose-volumetric parameters to predict RILD in the setting of CIRT are lacking so far.
Besides the hepatotoxicity, we had to take notice of two radiotherapy-related late complications as well. The first one was bile duct stricture, in the case of which the potential secondary biliary infection and hepatic failure could be serious or even fatal for HCC patients with background liver disease. Although seldom reported, its incidence may increase accordingly due to the wider use of dose-escalated radiotherapy for centrally located HCC. Osmundson et al described the utility of SBRT for liver tumors with a median BED10 of 85.5 Gy in 1–5 fractions, showing grade 3 or higher biliary stricture/obstruction requiring intervention in 13 of 96 (13.5%) patients.30 An earlier study by the University of Tsukuba reported that 3 of 162 (1.9%) HCC patients experienced late common bile duct stenosis following PRT (2 patients at a DRBE of 79.2 Gy in 16 fractions, and 1 at 92.4 Gy in 24 fractions).31 Therefore, a lower dose fractionation scheme of 72.6 Gy in 22 fractions was subsequently applied to reduce the risk of late biliary toxicities for those tumors located adjacent to the porta hepatis, and no bile duct stenosis was observed in that study including a total of 53 subjects.32 Recently, the safety of this dose scheme was further validated by a separate Korean cohort.33 Although, as yet, no CIRT-associated biliary stenosis was documented in published literature even with short-course treatment,10,21 our results demonstrated a relatively low incidence of 2.2%, which was consistent with the findings in previous studies with photon and proton beams.31,34 The second one was rib fracture which commonly occurred at least 6 months after the completion of radiotherapy with varying rates, mainly attributed to the heterogeneous tumor locations, dose regimens and irradiation techniques. Kanemoto et al evaluated the incidence and predictor of rib fracture with PRT at a DRBE of 66 Gy in 10 fractions for peripherally located HCC.35 Eleven of 67 (16.4%) patients developed rib fractures, and the volume of rib receiving a BED of more than 60 Gy3 (V60) with cut-off point of 4.48 cm3 was determined as the most significant predictive parameter. Yeung et al reported that no radiographic evidence of rib fracture was observed after PRT using a 15-fraction regimen for 39 liver tumors.36 As for CIRT, only 5 patients experienced rib fracture (grade 2 in 4, grade 3 in 1) among a total of over 250 HCC patients reported in several publications from four carbon-ion centers in Japan,10,22,24 while a similar incidence of 1.1% was found in our cohort. During the early period at our center, all patients with HCC were treated by 10 fractions, except for those whose tumor located within 1 cm of the GI tract. However, with the lessons of the above two toxicities occurring in one case each, we paid special attention to avoid high doses to central biliary tree and ribs, and instead adopt the more moderate regimens of 15 fractions for tumors in close proximity to the main bile ducts or chest wall, as well as the GI tract, so as to achieve an ablative dose while staying within the tolerance of OARs.
Pencil beam raster scanning is the only available delivery mode in our center, and over 1000 patients with moving tumors in the thorax and abdomen have been treated to date. As pointed out by the PTCOG consensus and AAPM report, implementing PBS for moving target had great technical challenges, among which the interplay effect was an issue of major concern.14,37 For liver tumors, their motions in the craniocaudal direction were generally the most dominant with an amplitude of about 1–3 cm during respiration.38,39 To mitigate interplay effect and the resulting dose uncertainty we adopted the following necessary strategies: (1) Active motion-management techniques. Breath hold was believed to be the best way but required the highest level of patient training to achieve the satisfactory results. Unfortunately, a majority of our patients did not cooperate very well with the ABC device probably due to its involuntary manner of breath hold. Abdominal compression was less commonly used because of the discomfort experienced by the elderly or weak patients, as well as sometimes the device itself being in the beam path. Anzai gating was used in 80% of our patients, but the magnitude of residual motion within the gating window should be noted. To evaluate the system-specific window size, using which the target dose homogeneity would not be significantly affected by the residual motion, a moving phantom study was performed in our center and 5 mm was eventually determined as a threshold.40 (2) Robust beam angle selection. Beams were arranged to maintain the shortest distance to the target and go through homogeneous tissues as much as possible. (3) Proper beam spot size and spacing. During the planning process a large spot size of 10 mm with a small spacing of 2 mm was used to improve treatment plan robustness. (4) Appropriate optimization strategy. Single beam optimization (SBO) was used whenever feasible but not intensity modulated technique, which can be highly sensitive to motion. Fortunately, intensity modulation was not normally necessary owing to the regular shape of HCC targets. (5) Multiple beam angles. Multiple fields were employed in each treatment fraction to improve the robustness of SBO-based plans and smooth out the dose heterogeneities induced by interplay effect. (6) Increased fractionations. Because both techniques of robust optimization and rescanning had not been available in our system, which were mandated for short fractionation schemes (e.g., ≤ 5 fractions), increased fractionations (i.e., 10 or 15 fractions) were applied as a compensatory strategy.
It also has to be noted that our aforementioned prescription doses and clinical results were based on the LEM that differs from the DRBE calculation system at Japanese centers. Although the application of conversion factors for both target dose and OAR constraints between the two different models were proposed by several authors to minimize their deviations of RBE dependence on dose level, the discrepancy in LET spectra caused by different clinical irradiation conditions, including target volume characteristics (size, shape and position), beam configuration (number and orientation) and delivery technique, could not be fully avoided, which led to inconsistent physical dose distributions as well as the clinical effects. Therefore, our data can be taken as valuable references for immediate use in other centers like ours using LEM-based system without worrying about the DRBE conversion issue.
There are several limitations of the present study that should be considered. First, the retrospective nature of our cohort is more prone to selection bias, thus including few patients with CP class of B who may benefit more from CIRT over photon-based radiotherapy. Hiroshima et al recently published their experience on the use of CIRT in CP-B patients, showing a high 2-year LC rate of 96.4% with minimal hepatic radiation toxicities.41 Second, conclusions may be limited by the moderate sample size, the relatively short follow-up period, and the single-center design of this study. Third, the peri-CIRT use of TACE was not uniform, which might differently affect the overall oncologic outcomes by reducing intrahepatic tumor dissemination. Lastly, the treatment-related adverse events could be underestimated in non-prospective studies because they may not have been fully documented in the medical records. Nevertheless, our study has yielded promising results in a series of consecutive cases that were homogeneously treated by a consistent CIRT protocol. Prospective trials with more cases and long-term data especially focusing on unfavorable patient subgroups, such as those with impaired liver function, large tumors, or a history of previous hepatic irradiation, are warranted to clarify the most beneficial candidates for PBS CIRT.
Conclusion
Our study presented the oncologic outcomes and toxicity profiles of CIRT for HCC in the setting of advanced PBS delivery technique and LEM-based treatment planning system, which were comparable to those of a series of Japanese studies derived from PS and MKM approaches. PBS CIRT was clinically feasible to treat HCC by applying necessary measures here that may combine with additional strategies such as robust optimization and rescanning to further mitigate interplay effects in the residual motion.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author (Zheng Wang) on reasonable request.
Ethics Approval and Informed Consent
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Shanghai Proton and Heavy Ion Center (approval number: 221122EXP-01). Informed consent was obtained from all individual participants included in the study.
Funding
This research received no external funding.
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
1. Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S94–S100. doi:10.1016/j.ijrobp.2009.06.092
2. Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31(13):1631–1639. doi:10.1200/JCO.2012.44.1659
3. Yu Y, Feng M. Radiotherapy for hepatocellular carcinoma. Semin Radiat Oncol. 2018;28(4):277–287. doi:10.1016/j.semradonc.2018.06.005
4. Kobeissi JM, Hilal L, Simone CB, et al. Proton therapy in the management of hepatocellular carcinoma. Cancers. 2022;14(12). doi:10.3390/cancers14122900
5. Abousaida B, Seneviratne D, Hoppe BS, et al. Carbon ion radiotherapy in the management of hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:1169–1179. doi:10.2147/JHC.S292516
6. Byun HK, Kim C, Seong J. Carbon ion radiotherapy in the treatment of hepatocellular carcinoma. Clin Mol Hepatol. 2023;29(4):945–957. doi:10.3350/cmh.2023.0217
7. Apisarnthanarax S, Bowen SR, Combs SE. Proton beam therapy and carbon ion radiotherapy for hepatocellular carcinoma. Semin Radiat Oncol. 2018;28(4):309–320. doi:10.1016/j.semradonc.2018.06.008
8. Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol. 2010;7(1):37–43. doi:10.1038/nrclinonc.2009.183
9. Tinganelli W, Durante M. Carbon ion radiobiology. Cancers. 2020;12(10). doi:10.3390/cancers12103022
10. Shibuya K, Ohno T, Terashima K, et al. Short-course carbon-ion radiotherapy for hepatocellular carcinoma: a multi-institutional retrospective study. Liver Int. 2018;38(12):2239–2247. doi:10.1111/liv.13969
11. Shibuya K, Katoh H, Koyama Y, et al. Efficacy and safety of 4 fractions of carbon-ion radiation therapy for hepatocellular carcinoma: a prospective study. Liver Cancer. 2022;11(1):61–74. doi:10.1159/000520277
12. Habermehl D, Debus J, Ganten T, et al. Hypofractionated carbon ion therapy delivered with scanned ion beams for patients with hepatocellular carcinoma – feasibility and clinical response. Radiat Oncol. 2013;8(1):59. doi:10.1186/1748-717X-8-59
13. Noda K. Beam delivery method for carbon-ion radiotherapy with the heavy-ion medical accelerator in Chiba. Int J Part Ther. 2016;2(4):481–489. doi:10.14338/IJPT-15-00041.1
14. Chang JY, Zhang X, Knopf A, et al. Consensus guidelines for implementing pencil-beam scanning proton therapy for thoracic malignancies on behalf of the PTCOG Thoracic and Lymphoma Subcommittee. Int J Radiat Oncol Biol Phys. 2017;99(1):41–50. doi:10.1016/j.ijrobp.2017.05.014
15. Karger CP, Peschke P. RBE and related modeling in carbon-ion therapy. Phys Med Biol. 2017;63(1):01TR02. doi:10.1088/1361-6560/aa9102
16. Ye SL. Expert consensus on standardization of the management of primary liver cancer. Zhonghua Gan Zang Bing Za Zhi. 2009;17(6):403–410.
17. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
18. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
19. Hong Z, Zhang W, Cai X, et al. Carbon ion radiotherapy with pencil beam scanning for hepatocellular carcinoma: long-term outcomes from a phase I trial. Cancer Sci. 2023;114(3):976–983. doi:10.1111/cas.15633
20. Kato H, Tsujii H, Miyamoto T, et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. 2004;59(5):1468–1476. doi:10.1016/j.ijrobp.2004.01.032
21. Kasuya G, Kato H, Yasuda S, et al. Progressive hypofractionated carbon-ion radiotherapy for hepatocellular carcinoma: combined analyses of 2 prospective trials. Cancer. 2017;123(20):3955–3965. doi:10.1002/cncr.30816
22. Yasuda S, Kato H, Imada H, et al. Long-term results of high-dose 2-fraction carbon ion radiation therapy for hepatocellular carcinoma. Adv Radiat Oncol. 2020;5(2):196–203. doi:10.1016/j.adro.2019.09.007
23. Kim N, Cheng J, Huang WY, et al. Dose-response relationship in stereotactic body radiation therapy for hepatocellular carcinoma: a pooled analysis of an Asian Liver Radiation Therapy Group Study. Int J Radiat Oncol Biol Phys. 2021;109(2):464–473. doi:10.1016/j.ijrobp.2020.09.038
24. Komatsu S, Fukumoto T, Demizu Y, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer. 2011;117(21):4890–4904. doi:10.1002/cncr.26134
25. Wakatsuki M, Makishima H, Mori Y, et al. Clinical outcomes of carbon-ion radiotherapy for large-sized (≥4cm) hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2023;117(2S):e348. doi:10.1016/j.ijrobp.2023.06.2418
26. Feng M, Suresh K, Schipper MJ, et al. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: a Phase 2 Clinical Trial. JAMA Oncol. 2018;4(1):40–47. doi:10.1001/jamaoncol.2017.2303
27. Durand-Labrunie J, Baumann AS, Ayav A, et al. Curative irradiation treatment of hepatocellular carcinoma: a multicenter Phase 2 trial. Int J Radiat Oncol Biol Phys. 2020;107(1):116–125. doi:10.1016/j.ijrobp.2019.12.004
28. Mizumoto M, Okumura T, Hashimoto T, et al. Evaluation of liver function after proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;82(3):e529–e535. doi:10.1016/j.ijrobp.2011.05.056
29. Hsieh CE, Venkatesulu BP, Lee CH, et al. Predictors of radiation-induced liver disease in eastern and western patients with hepatocellular carcinoma undergoing proton beam therapy. Int J Radiat Oncol Biol Phys. 2019;105(1):73–86. doi:10.1016/j.ijrobp.2019.02.032
30. Osmundson EC, Wu Y, Luxton G, et al. Predictors of toxicity associated with stereotactic body radiation therapy to the central hepatobiliary tract. Int J Radiat Oncol Biol Phys. 2015;91(5):986–994. doi:10.1016/j.ijrobp.2014.11.028
31. Chiba T, Tokuuye K, Matsuzaki Y, et al. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res. 2005;11(10):3799–3805. doi:10.1158/1078-0432.CCR-04-1350
32. Mizumoto M, Tokuuye K, Sugahara S, et al. Proton beam therapy for hepatocellular carcinoma adjacent to the porta hepatis. Int J Radiat Oncol Biol Phys. 2008;71(2):462–467. doi:10.1016/j.ijrobp.2007.09.056
33. Yoo GS, Yu JI, Park HC, et al. Do biliary complications after proton beam therapy for perihilar hepatocellular carcinoma matter? Cancers. 2020;12(9). doi:10.3390/cancers12092395
34. Yu JI, Park HC, Lim DH, et al. Do biliary complications after hypofractionated radiation therapy in hepatocellular carcinoma matter? Cancer Res Treat. 2016;48(2):574–582. doi:10.4143/crt.2015.076
35. Kanemoto A, Mizumoto M, Okumura T, et al. Dose-volume histogram analysis for risk factors of radiation-induced rib fracture after hypofractionated proton beam therapy for hepatocellular carcinoma. Acta Oncol. 2013;52(3):538–544. doi:10.3109/0284186X.2012.718094
36. Yeung R, Bowen SR, Chapman TR, et al. Chest wall toxicity after hypofractionated proton beam therapy for liver malignancies. Pract Radiat Oncol. 2018;8(4):287–293. doi:10.1016/j.prro.2017.12.007
37. Li H, Dong L, Bert C, et al. AAPM Task Group Report 290: respiratory motion management for particle therapy. Med Phys. 2022;49(4):e50–e81. doi:10.1002/mp.15470
38. Kirilova A, Lockwood G, Choi P, et al. Three-dimensional motion of liver tumors using cine-magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2008;71(4):1189–1195. doi:10.1016/j.ijrobp.2007.11.026
39. Park JC, Park SH, Kim JH, et al. Liver motion during cone beam computed tomography guided stereotactic body radiation therapy. Med Phys. 2012;39(10):6431–6442. doi:10.1118/1.4754658
40. Hsi W, Huang Z, Wang W, et al. SU-E-T-281: dose measurements of modulated spot-scanning particle beams with beam-gating of respiratory-phase. Med Phys. 2015;42(6):3397. doi:10.1118/1.4924643
41. Hiroshima Y, Wakatsuki M, Kaneko T, et al. Clinical impact of carbon‐ion radiotherapy on hepatocellular carcinoma with Child‐Pugh B cirrhosis. Cancer Med. 2023;12(13):14004–14014. doi:10.1002/cam4.6046
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.