Back to Journals » Infection and Drug Resistance » Volume 16
Pediatric Mycotic Pseudoaneurysm of the Ascending Aorta: A Case Report
Authors Song JG, Yang ZX, Shi N, Zhu M
Received 21 September 2023
Accepted for publication 18 November 2023
Published 4 December 2023 Volume 2023:16 Pages 7405—7411
DOI https://doi.org/10.2147/IDR.S441449
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Jia-Guang Song,1,2,* Zi-Xin Yang,2,* Na Shi,3 Mei Zhu2
1Department of Ultrasound, Shandong Provincial Hospital, Shandong University, Jinan, 250021, People’s Republic of China; 2Department of Ultrasound, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, 250021, People’s Republic of China; 3Department of Ultrasound, Linyi Mental Health Center, Linyi, 276001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mei Zhu, Department of Ultrasound, Shandong Provincial Hospital Affiliated to Shandong First Medical University, No. 324 Jingwu Road, Jinan, Shandong, 250021, People’s Republic of China, Tel +86 15653101616, Fax +86 53168778113, Email [email protected]
Abstract: Mycotic pseudoaneurysm of the ascending aorta is extremely uncommon, particularly in children with no prior cardiac surgery or trauma. We report a rare case of a mycotic pseudoaneurysm of the ascending aorta in a 2-year-old girl with no history of cardiac surgery. Investigations revealed a methicillin-resistant Staphylococcus aureus infection and significant pericardial effusion in the child who presented with persistent fever and altered mental state. Cardiac ultrasound revealed a disruption in the aortic wall and a tumor-like structure. Contrast-enhanced computed tomography confirmed an ascending aortic pseudoaneurysm with thrombus. The child underwent successful surgical treatment without implants. This case emphasizes the diagnostic significance of imaging, particularly the advantages of ultrasound in pediatric settings, and the need for timely and accurate diagnosis using appropriate imaging modalities in children.
Keywords: aorta, mycotic, pseudoaneurysm
Introduction
Pseudoaneurysm refers to a complete or partial rupture of the arterial wall, with blood escaping through the rupture and surrounding adjacent tissues, to form an aneurysmal sac.1 Pseudoaneurysms are typically caused by inflammation, trauma, and medical factors.2 Mycotic pseudoaneurysm of the ascending aorta is caused by microorganisms that originate from endogenous bacteremia or infected neighboring tissues, the primary pathogens are Staphylococcus aureus, salmonella, and Streptococcus.3 The ascending aorta cannulation site is the most common site of mycotic pseudoaneurysm following cardiac surgery.4 Mycotic pseudoaneurysm of the ascending aorta is extremely uncommon, particularly in children with no prior cardiac surgery or trauma. Here, we present a case of mycotic pseudoaneurysm of the ascending aorta initially detected by ultrasound.
Case Presentation
This study followed the Declaration of Helsinki and was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University. Consent for publication was obtained from the legal guardian of the patient.
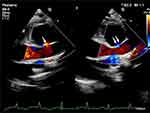
The patient, a 2-year-old girl, was admitted due to persistent fever for eight days and poor mental status for three days. Upon examination, her heart rate was 150 beats per minute, her heart sounds were distant, and no pathological murmurs were detected. Blood tests revealed a white blood cell count of 18.29×10^9/L, an absolute neutrophil count of 13.44×10^9/L, C-reactive protein (CRP) of 163.54 mg/L, N-terminal pro-B-type natriuretic peptide (NT-ProBNP) of 1673 pg/mL, and an erythrocyte sedimentation rate (ESR) of 90 mmol/h. Cardiac ultrasound revealed moderate to substantial pericardial effusion with fibrinous exudation. Ultrasound of the heart revealed a moderate to substantial pericardial effusion with fibrinous exudation. Over the course of 24 hours, an emergency subxiphoid pericardiocentesis with catheter drainage yielded 330 mL of brownish-red pericardial fluid with fibrinous debris. The pericardial fluid culture revealed methicillin-resistant S. aureus infection. A chest computed tomography (CT) scan revealed bilateral pleural effusion with atelectasis and drainage of purulent secretions from both pleural cavities. Following one week of drainage, the chest and pericardial drainage tubes were removed. Under ultrasound guidance, a puncture was performed at the right sternal border at the fourth intercostal space to aspirate 55 mL of encapsulated pericardial effusion from the right side of the right atrium. Cardiac ultrasound detected significant pericardial adhesion and a small amount of encapsulated pericardial effusion on the 12th day of hospitalization. In addition, a discontinuity in the echogenicity of the aortic wall with a diameter of approximately 0.42 cm was observed 2 cm above the aortic valve. A tumor-like structure measuring approximately 2.95 cm × 2.73 cm, with a thick wall and low-echoic material deposition within was present in this region (Figure 1). Bidirectional low-velocity blood flow signals were observed entering and exiting the pseudoaneurysm at the rupture site (Figure 2). Contrast-enhanced CT confirmed the presence of an ascending aortic pseudoaneurysm with intraluminal thrombus formation (Figures 3–5). Follow-up ultrasound imaging revealed an enlarging pseudoaneurysm, measuring 4.05 cm × 3.00 cm, and an increased rupture site diameter of 0.60 cm. One month after admission, the infection was completely under control, and the child underwent surgery. Intraoperatively, substantial pericardial edema, extensive adhesion, and extensive fibrinous exudation were observed in the pericardial cavity. A large protrusion resembling a tumor was discovered on the left anterior wall of the ascending aorta, with a wide base and tight adhesion to the surrounding tissues (Figure 6). Exploration of the pseudoaneurysm revealed partial thrombus and a 0.8 cm hole at the base of the pseudoaneurysm (Figure 7), surrounded by a smooth intimal surface. The pediatric patient underwent ascending aortic pseudoaneurysm resection, aortic repair, and pericardial decortication without foreign material or synthetic graft implantation. Pathological examination of the resected aortic wall confirmed the diagnosis of mycotic pseudoaneurysm of the ascending aorta (Figure 8). One week after surgery, a follow-up ultrasound revealed that the pseudoaneurysm had resolved.
 |
Figure 5 Three-dimensional reconstructed CTA image showing a large, sac-like protrusion on the left anterior wall of the mid-ascending aorta. Abbreviations: AAo, ascending aorta; P, pseudoaneurysm. |
 |
Figure 6 Intraoperative photograph showing a large tumor-like protrusion on the left anterior wall of the ascending aorta. Arrow: a large tumor-like protrusion. |
 |
Figure 7 Intraoperative photograph demonstrating the 0.8 cm hole at the base of the pseudoaneurysm. Arrow: rupture site at the base of the pseudoaneurysm. |
 |
Figure 8 Histopathological image of the resected aortic wall, validating the diagnosis of ascending aortic mycotic pseudoaneurysm. |
Discussion
In this case, the pericardial and pleural punctures and drainages performed on the pediatric patient were guided by ultrasound, with the puncture sites and trajectories strategically placed away from the root of the ascending aorta within the mediastinum. The extracted fluid was brownish-red effusion, not fresh red blood, effectively ruling out the possibility of iatrogenic injury. Additionally, the absence of a history of cardiac surgery in the child and the identification of Staphylococcus aureus in the pericardial fluid suggest that bacteremia or infective pericardial effusion is the most likely pathogenesis leading to the mycotic pseudoaneurysm of the ascending aorta. Fever, an elevated white blood cell count, an elevated ESR, and CRP are the most frequent clinical manifestations of pseudoaneurysms.5 Rheumatic fever can present with similar cardiac inflammation and laboratory findings, but it often also shows signs of involvement in other systems, such as migratory polyarthritis, subcutaneous nodules, erythema marginatum, and chorea. Since the clinical symptoms and laboratory tests of infective pseudoaneurysms are nonspecific, diagnosis primarily relies on imaging studies.
Standard imaging characteristics of mycotic pseudoaneurysm of the ascending aorta include a discontinuity in the aortic wall, a penetrating aortic ulcer, and a round or ovoid aneurysmal mass outside the aortic lumen, which is usually solitary and communicates with the ascending aorta via an identifiable opening. Additionally, the opening is frequently small and located on the anterior lateral wall of the ascending aorta, and the aneurysmal sac is frequently accompanied by a circular or irregular mural thrombus. Digital subtraction angiography (DSA) is effective in distinguishing between true and false aneurysms, and it can help determine the size and origin of the aneurysm. However, it is more intrusive. Computed tomography angiography (CTA) is the most common method for preoperative evaluation and postoperative follow-up as it can accurately assess the size, morphology, presence of mural thrombus, and adjacent relationship with surrounding tissues for true and false aneurysms.6 However, neither technique is appropriate for patients with iodine contrast allergy, renal insufficiency, or radiation exposure concerns. Magnetic resonance imaging (MRI) allows for clear visualization of soft tissue structures and evaluation of the relationship between pseudoaneurysms and surrounding tissues, but it requires a longer examination time and has equipment limitations. Ultrasound offers real-time, noninvasive, inexpensive, and radiation-free benefits and can sensitively detect blood flow between the aneurysmal sac and ascending aorta. It can also differentiate between abscesses, cysts, and tumors, but its capabilities are limited by the acoustic window.7 DSA, CT, MRI, and ultrasound all have their respective advantages and disadvantages when diagnosing and assessing mycotic pseudoaneurysm of the ascending aorta. In clinical practice, the most appropriate examination method should be selected based on the specific condition of the patient and equipment availability. In this case, the patient was a child, and as there were no acoustic window restrictions, ultrasound was the method of choice for the imaging diagnosis. Its convenience and cost-effectiveness also made it the primary imaging tool for follow-up in this patient.
Conclusion
Mycotic pseudoaneurysm of the ascending aorta can be diagnosed in patients through various imaging modalities. For pediatric patients, ultrasound is the preferred diagnostic method for mycotic pseudoaneurysm of the ascending aorta.
Ethics Statement
The consent was obtained for publication included the case details and the accompanying images.
Funding
This study was supported by the key R & D program of Shandong Province (2022CXGC010504) and (2018GSF118112).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Han Y, Zhang Y, Gong G, et al. Mycotic pseudoaneurysm of thoracic aortic caused by salmonella paratyphi a: a case report. Infect Drug Resist. 2023;16:4171–4176. doi:10.2147/IDR.S416783
2. Singh A, Sanchez-Garcia W, Maughan R, et al. Mycotic pseudoaneurysm formation at the cannulation site in the ascending aorta. Cureus. 2021;13(11):e19283. doi:10.7759/cureus.19283
3. Mangel TP, Balmforth D, Oo A. A case report of an unusual mycotic pseudoaneurysm of the ascending aorta. J Cardiothorac Surg. 2022;17(1):239. doi:10.1186/s13019-022-01989-2
4. Zivkovic I, Stankovic S, Krasic S, et al. Surgical treatment of pseudoaneurysm of the ascending Aorta in a patient with sepsis. Braz J Cardiovasc Surg. 2021;36(2):261–264. doi:10.21470/1678-9741-2020-0258
5. Kugler S, Pólos M, Király Á, et al. Pseudoaneurysm of the ascending aorta: case report of a donor-derived Pseudomonas infection in a heart transplant recipient. BMC Infect Dis. 2021;21(1):847. doi:10.1186/s12879-021-06557-y
6. Beslic S, Beslic N, Beslic S, et al. Diagnostic imaging of traumatic pseudoaneurysm of the thoracic aorta. Radiol Oncol. 2010;44(3):158–163. doi:10.2478/v10019-010-0026-8
7. Gimenez DC, Valencoso Gilabert O, Gimenez Ruiz J, et al. Ultrasound and magnetic resonance angiography features of post-traumatic ulnar artery pseudoaneurysm: a case report and review of the literature. Skeletal Radiol. 2009;38(9):929–932. doi:10.1007/s00256-009-0715-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.