Back to Journals » Journal of Hepatocellular Carcinoma » Volume 4
Pediatric hepatocellular carcinoma: challenges and solutions
Authors Schmid I, von Schweinitz D
Received 21 May 2016
Accepted for publication 15 November 2016
Published 16 January 2017 Volume 2017:4 Pages 15—21
DOI https://doi.org/10.2147/JHC.S94008
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ravi Murthy
Irene Schmid,1 Dietrich von Schweinitz,2
1Department of Pediatric Hematology and Oncology, 2Department of Pediatric Surgery, Dr. von Hauner Children`s Hospital, Ludwig-Maximilians-University, Munich, Germany
Abstract: Hepatocellular carcinoma (HCC) is a very rare entity in children, making it nearly impossible to orchestrate Phase II/III studies even as multinational cooperative trials. In contrast to adults, nearly 50% of the children have a response (α-fetoprotein decline and/or tumor shrinkage) to chemotherapeutic agents such as cisplatin and doxorubicin (PLADO), demonstrating that HCC in childhood can be chemotherapy sensitive. As a result, the main treatment options in pediatric HCC focus on systemic drug therapies and resection as the central therapy. In nonmetastatic patients with complete resection upfront, the 5-year event-free survival and overall survival has reached 80%–90%. In almost all reported studies, children received adjuvant chemotherapy (mostly PLADO), but it has never been proven that postoperative chemotherapy is superior to observation. No data are available for the effects of sorafenib. The 3-year survival is <20% in children with unresectable HCC independent of the chemotherapy given preoperatively. Currently, PLADO in combination with sorafenib is recommended with the goal of achieving operability status. Alternatively, data are promising for the combination of sorafenib with gemcitabine and oxaliplatin. For children with nonresectable and nonmetastastic liver tumors, it has been shown that the Milan criteria regarding liver transplantation are not applicable – individual decisions have to be made. Transarterial chemoembolization could be offered to patients with chemotherapy-resistant liver tumors for palliative care or potentially to achieve surgical resectability, and therefore cure. Information about the feasibility or effects of new agents or approaches as discussed in adult HCC patients is not available for childhood HCC. Research has to be done for characterizing the molecular and genomic mechanisms of pediatric HCC to support the development of novel therapeutic approaches and the implementation of personalized medicine.
Keywords: hepatocellular carcinoma, pediatric, sorafenib, cisplatin, doxorubicin
Introduction
Primary malignant liver tumors are rare in childhood with an incidence of about 1.6 cases per million children (0–14 years).1,2 While hepatoblastoma (HB) represents 80% of the hepatic-related cancer affecting children predominantly between 6 months and 3 years, hepatocellular carcinoma (HCC) is more uncommon, with incidence increasing with age. Only about 0.5%–1% of all pediatric tumors are HCC.1,2 In hepatoblastoma, event-free survival (EFS) and overall survival (OS) was increased from roughly 30% in the 1970s to 70%–90% these days, especially due to advances of chemotherapy regimens and surgical approaches.3 In HCC, the results with unresectable tumor especially are rather dismal.
Contrary to adults, in the majority of children or adolescents, no etiologic factors can be detected. However, in areas with a high prevalence of hepatitis B virus (HBV) infection rate, the lifetime risk of HCC in chronic HBV carriers is estimated to be 10%–25%.4 For example, in Taiwan and Hong Kong, 100% and 64%, respectively, of all children with HCC were chronic HBV carriers. It can be expected that universal newborn vaccination will have an effect in reducing the incidence of HCC.5,6 Only a minority of HCC cases are associated with cirrhosis or other chronic liver diseases such as glycogen storage disease type III, tyrosinemia type I, Wilson disease, or biliary atresia. This indicates that the pathogenesis of HCC in childhood is different compared with that in adults.7–9
Management of HCC remains difficult since complete surgical resection is fundamental for cure. However, in pediatric HCC, <20% of the patients are considered eligible for initial resection. Various studies have been conducted using different combinations of chemotherapeutic agents to reduce tumor load, thereby helping patients become suitable candidates for resection. Historically, HCC patients were treated with the same protocols as HB patients, and so, primarily, cisplatin, doxorubicin, carboplatin, 5-fluorouracil, and vincristine were used.1,10 However, to date, there is no convincing data that this approach results in a benefit for survival.
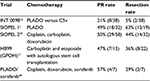
The 3-year EFS and OS for children with complete tumor excision upfront and two courses of carboplatin (200 mg/m2/d × 4) and etoposide (100 mg/m2/d × 4) were 72% and 89%, respectively, in the HB99 study (1999–2008)11 conducted by the German Society for Pediatric Oncology and Hematology (GPOH). However, the prognosis remained poor, with 3-year EFS and OS rates of 12% and 20% in those patients who had inoperable or metastatic disease. Intensifying preoperative chemotherapy with two courses of carboplatin and etoposide followed by two courses of high-dose carboplatin (500 mg/m2/d × 4) and etoposide (300 mg/m2/d × 4) with autologous stem cell transplantation did not translate into a satisfactory operability rate, EFS, or OS.
Similar findings were reported by the North American Intergroup Hepatoblastoma Study (INT-0098) (1989–1992).12 The 5-year EFS (OS) for pediatric patients (26/46 patients ≥10 years) with inoperable tumor upfront was 8% (23%), and for those with metastases it was 0% (19%). There was no difference based on whether the children received cisplatin (90 mg/m2 on d0) and doxorubicin (20 mg/m2/d × 4 from d2) or cisplatin (90 mg/m2 on d0), 5-fluorouracil (600 mg/m2 on d2), and vincristine (1.5 mg/m2 on d2).
Importantly, the first International Society of Pediatric Oncology Liver Tumor Study (SIOPEL-1 study, 1990–1994)1 demonstrated that HCC in childhood (4–15 years, median: 12 years) can be chemotherapy sensitive. They proved this by showing that 49% of the children responded to cisplatin (80 mg/m2 on d1) and doxorubicin (30 mg/m2/d on d2+3) (PLADO). However, taking into consideration that complete resection is the cornerstone of cure, only 36% had complete tumor excision, and so the 5-year EFS was only 17%. The next attempt (SIOPEL-2 study, 1995–1998)10,13 tried was rapidly switching between cisplatin (80 mg/m2 on d1) and carboplatin (500 mg/m2 on d1)/doxorubicin (30 mg/m2/d on d2+3) (SuperPLADO) every 14 days, but this did not improve the response rate after preoperative chemotherapy (46%), and therefore also not 3-year OS (22%).
Thus, to date, for children with inoperable liver tumor and/or with metastases, the complete resection (and so the EFS and OS) have not improved although different strategies have been attempted.
Sorafenib is an inhibitor of several tyrosine protein kinases such as VEGFR, PDGFR, and Raf kinases.14–16 In preclinical models, sorafenib demonstrated antitumor activity alone and in combination with, for instance, doxorubicin, gemcitabine, and cisplatin.17,18 In adult patients with advanced HCC, sorafenib significantly improved both time to tumor progression and OS from a median of 2.8 to 5.5 months and from 7.9 to 10.7 months, respectively, compared with placebo. The most important grade 3 adverse effects were diarrhea, hand–foot skin reaction, and fatigue.19 Therefore, sorafenib has become the standard therapy for adult patients with HCC.20 Furthermore, in a randomized, double-blind, Phase II study combining sorafenib with doxorubicin, the progression-free survival (PFS) was significantly better in patients receiving sorafenib and doxorubicin than in those receiving doxorubicin and placebo (median: 4.8 vs 8.6 months).21,22 Moreover, tumor reduction was achieved in 62% vs 29% of the patients. This effect could be due to the fact that combining sorafenib with doxorubicin translated into an increased mean values of doxorubicin Cmax and area under the curve by 33% and 21%, respectively.23 In the recent study with 12 children (seven with unresectable liver tumor, age 7–16 years), it was demonstrated that sorafenib (244–602 mg/m2/d, median: 288 mg/m2/d) added to PLADO is a promising new therapeutic option with hand–foot skin reaction being the most relevant toxicity.24 With this combination, four of the seven children with inoperable liver tumor achieved a partial response (PR), two a stable disease, and one a progression. Three patients were alive without evidence of tumor after complete tumor excision at 12 months (with second-line chemotherapy after two courses sorafenib and PLADO), 12 months, and 18 months (both patients had six courses sorafenib and PLADO), respectively, after primary diagnosis. The elevated α-fetoprotein levels seen in four patients at diagnosis markedly declined after two courses of therapy. Since then, some pediatric liver tumor specialists have recommended PLADO with sorafenib as a “standard” chemotherapy.
Thus, there are big challenges to be solved for pediatric patients with HCC, namely: 1) What is the standard-of-care in children with newly diagnosed HCC with complete resection upfront: observation vs sorafenib vs PLADO vs PLADO and sorafenib? 2) What are the therapeutic options in newly diagnosed patients with unresectable tumors and/or metastatic disease? 3) Are there new approaches on the horizon for HCC in children? 4) Must the Milan criteria for a liver transplantation be strictly adhered to? and 5) Does transarterial chemoembolization (TACE) play a role in pediatric patients?
Standard-of-care with complete resection upfront: observation vs sorafenib vs PLADO vs PLADO and sorafenib?
Katzenstein et al12 reported an 88% 5-year EFS in patients with completely resected HCC receiving either cisplatin, 5-fluorouracil, and vincristine or PLADO (n=8). The German HB99 study11 used two cycles of carboplatin/etoposide postoperatively, which translated into 5-year EFS and OS probabilities of 72% and 89% (n=14), respectively. Thus, there seems to be no difference in survival based on the chemotherapy used. Whether postoperative sorafenib has a survival benefit remains unclear. In adults, it was recently shown that sorafenib is not effective as an adjuvant treatment following resection or ablation.25 An enhanced chemotherapeutic response to sorafenib and PLADO was demonstrated in the small series of patients with advanced HCC (PR in four out of seven). However, further data regarding sorafenib are urgently needed in the pediatric HCC population.24
The problem is that it is impossible to realize Phase II to III studies in an entity as rare as HCC in childhood. In the SIOPEL 1, 2, and 3 studies1,10 recruiting patients between 1990 and 2004, 15/121 had an HCC with complete resection at diagnosis. Internationally, the estimated number of primary resectable patients would be about 10/year. With such low numbers, a study randomizing patients after upfront complete surgical resection to observation vs sorafenib vs PLADO vs PLADO and sorafenib, and even to a two-arm study, will never be feasible within an adequate amount of time.
Thus, only recommendations can be given. Pediatric liver tumor specialists currently recommend that children with HCC should receive PLADO with or without sorafenib, as more intensive regimens have not yielded better results. But the role of postoperative chemotherapy and the amount (PLADO for two or four cycles? sorafenib at all or for 6 or 12 months?) for a stage I disease that has demonstrated chemotherapeutic sensitivity in pediatric patients are unknown.
Current therapeutic options in newly diagnosed patients with unresectable tumors and/or metastatic disease
Pediatric patients with unresectable or primarily metastatic HCC do not survive unless the disease can be rendered resectable. Given preexisting evidence that pediatric HCC is chemotherapy responsive in nearly 50% of the patients (Table 1), PLADO has been established as the standard chemotherapy. Intensification of platinum and doxorubicin agents, as in the SIOPEL 2 and 3 studies,13 did not result in improved survival. However, 5-year EFS rates still remain between 10%–34% since response mostly does not translate into complete surgical resection. Better tumor shrinkage is needed to facilitate surgery. Hopefully, sorafenib in addition to PLADO improves the resectability rate, EFS, and OS.24
In a recently published multicenter study from France, 204 adults with advanced HCC received gemcitabine (1,000 mg/m² on d1) and oxaliplatin (100 mg/m² on d2) (GEMOX), with promising response and tumor control rates of 22% and 66%, respectively.26 In 44% of the patients, grade 3–4 toxicities were reported, especially neutropenia, thrombocytopenia, neurotoxicity, and diarrhea. In a retrospective survey within the international liver tumor community, the response to GEMOX was 30% in heavily pretreated pediatric patients (personal communication).
Adding GEMOX to sorafenib (n=83) increased 4-month PFS rate from 54% to 61% and median OS from 13 to 13.5 months.27 Williet et al28 described a 61 year old man with HCC and lymph node metastasis treated with sorafenib and GEMOX, who achieved a PR and drop of α-fetoprotein to normal levels. The treatment resulted in a curative surgery. The experience within the GPOH with GEMOX given every 14 days with sorafenib in-between further supports that this regimen is worth being evaluated in a prospective study in pediatric patients.
But still, new effective drugs besides the conventional chemotherapeutic ones (eg, PLADO, GEMOX, and sorafenib) are definitely needed with the goal to achieve a higher response rate, thus translating into a higher surgical resection rate.
New agents in pediatric HCC
Since it is strongly believed that HCC in children is a different biologic disease, results from studies in the adult population cannot simply be translated to children.7,8 The better response to chemotherapy in pediatric patients may be due to the much higher rate of “de novo” tumors and normal liver function. In addition, in older children and in young adolescents, an entity called transitional liver cell tumor has been observed, which is made up of chemotherapy-sensitive hepatoblastoma-like cells, cells similar to those of HCC, and intermediate cell forms.29 Young people more often present the fibrolamellar histologic variant.30 It was thought that this variant has a more favorable prognosis, but recently it was shown that the long-term OS is similar to that for HCC.31
Since HCCs are highly vascularized tumors with increased levels of VEGF, antiangiogenic approaches represent a potential new therapeutic strategy. In adults, but not in children, different antiangiogenic agents besides sorafenib have been tested in clinical studies, eg, sunitinib, brivanib, bevacizumab, and ramucirumab.32 For example, sunitinib was proven to be nonsuperior when randomized with sorafenib and was highly toxic, with side effects including thrombocytopenia and neutropenia.33 Bevacizumab was the most promising agent, showing an objective response in six of 46 patients (13%) and a PFS rate of 65% at 6 months.34 In combination with erlotinib (EGFR inhibitor), a response rate of 25% was reported.35 Despite the initial promising results, there were no plans for a Phase III study with bevacizumab.
EGFR inhibitors (eg, erlotinib or cetuximab),32 mTor inhibitors (eg, sirolimus),36 and MEK inhibitors (eg, selumetinib)37 as single agents have not demonstrated significant antitumor activity.
The HGF /c-MET pathway has been identified as having an important role in tumor progression, angiogenesis, and appearance of metastases in HCC. Silencing the c-Met expression in cell lines and preclinical models was shown to inhibit HCC growth.38 However, only in patients with a high MET expression, tivantinib, a selective tyrosine kinase inhibitor, achieved a significantly longer median time to progression (1.4 vs 2.7 months), PFS (1.4 vs 2.2 months), and overall survival (3.8 vs 7.2 months).39 Cabozantinib (XL184), a dual tyrosine kinase inhibitor with activity against c-MET, VEGFR2, and RET, demonstrated a tumor control rate at week 12 of 71% in a randomized Phase II study.40 A 10 year old child was treated with relapsed HCC in complete remission with cabozantinib as maintenance therapy for 12 months. Two months later, the child relapsed again in the lung.
Immune checkpoint blockade has been presented as a new encouraging therapeutic option for various malignancies including HCC. Blocking of PD-1 with a specific antibody has been shown to amplify T-cell function and enhance antitumor effects.41 HCC is known to be an inflammation-associated cancer and can therefore be immunogenic.42 Upregulation of PD-1 and the PD-1 immune checkpoint ligand (PD-L1) in HCC is associated with poor prognosis.43,44 In a Phase I/II study, PD-1 blockade with nivolumab showed complete responses in two out of 39 patients (5%) and PRs in seven (18%) patients. OS rate was 72% at 6 months.45 These initial data support the continued exploration of nivolumab in HCC. The abovementioned 10 year old boy was treated for the second relapse with nivolumab and achieved a significant but temporary clinical response.
Current studies in adult cancer are ongoing, combining immune checkpoint inhibitors with targeted therapy or chemotherapeutic agents such as anthracyclines or gemcitabine since these can also modulate T-cell proliferation and promote immunogenic cell death.41,46,47 This makes sense as monotherapy with targeted agents demonstrated an improved response rate but a limited time to tumor progression, whereas checkpoint blockade monotherapy seems to have a lower response rate but an extended PFS.48
Unfortunately, information about the feasibility or effects of those new agents is not available for childhood HCC. Therefore, Phase I/II trials are urgently needed in childhood HCC.
Liver transplantation across the Milan criteria?
The indication for liver transplantation in adults is restricted to the Milan criteria, ie, the evidence of a single tumor <5 cm in size or no more than three foci with each not exceeding 3 cm and no vascular invasion or extrahepatic involvement.49 The practice guidelines of the American Association for Transplantation and the North American Society for Pediatric Gastroeneterology, Hepatology, and Nutrition recommend that the indication for liver transplantation in childhood HCC must be discussed individually for each patient. In principle, liver transplantation should be considered in children with no extrahepatic tumor or gross vascular invasion on radiological imaging, irrespective of the size of the lesions or the number of the lesions.50 Successful transplantations have been done in children with more liberal criteria, even in patients with large, multifocal HCC, microscopic blood vessel involvement, or limited extrahepatic tumor.51–53
A meta-analysis showed lower relapse rate and longer EFS and OS in patients treated with sirolimus compared with calcineurin inhibitor following liver transplantation for HCC.54
To conclude, the Milan criteria are not applicable for children with HCC. Individual decisions for a liver transplantation have to be made.
TACE in pediatric HCC patients
Palliative TACE is a standard procedure in adults with solitary or multifocal HCC without extrahepatic metastases. However, in children, only few cases were reported. Back in 2,000, Malogolowkin et al55 reported that all eleven children (18 months–14 years old) with unresectable, chemotherapy-resistant liver tumors (three with HCC) responded – five (one with HCC) went on to surgical resection and three survived. The conclusion was that TACE with a suspension of cisplatin, doxorubicin, and mitomycin mixed with lipiodol is feasible, well-tolerated, and effective in achieving surgical resectability in pediatric patients. These encouraging results were confirmed by Czauderna et al56 (five patients, 1–12 years old, one with HCC).
Thus, TACE could be offered to patients with chemotherapy-resistant liver tumor for palliative care or even with the goal of achieving surgical resectability and cure.
Conclusion
Research has to be done to characterize the molecular and genomic mechanisms of pediatric HCC to support the development of novel therapeutic approaches and the implementation of personalized medicine. At the moment, it would be worth initiating clinical studies to evaluate bevacizumab combined with standard chemotherapy (PLADO or GEMOX with sorafenib), c-met inhibitors like cabozantinib in tumors with high MET expression, and immune checkpoint blockade agents like nivolumab.
Disclosure
The authors report no conflicts of interest in this work.
References
Czauderna P, Mackinlay G, Perilongo G, et al. Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol. 2002;20:2798–2804. | ||
Litten JB, Tomlinson GE. Liver tumors in children. Oncologist. 2008;13:812–820. | ||
Czauderna P, Lopez-Terrada D, Hiyama E, Häberle B, Malogolowkin MH, Meyers RL. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr. 2014;26:19–28. | ||
McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma, present and future. Cin Liver Dis. 2011;15:223–243. | ||
Chen WJ, Lee JC Hung WT. Primary malignant tumour of the liver in infants and children in Taiwan. J Pediatr Surg. 1988;23:457–461. | ||
Chan KL, Fan ST, Tam PK, Chiang AK, Chan GC, Ha SY. Paediatric hepatoblastoma and hepatocellular carcinoma: retrospective study. Hong Kong Med J. 2002;8:13–17. | ||
Chen JC, Chen CC, Chen WJ, Lai HS, Hung WT, Lee PH. Hepatocellular carcinoma in children: clinical review and comparison with adult cases. J Pediatr Surg. 1998;33:1350–1354. | ||
Czauderna P. Adult type vs. Childhood hepatocellular carcinoma–are they the same or different lesions? Biology, natural history, prognosis, and treatment. Med Pediatr Oncol. 2002;39:519–523. | ||
Buendia MA. Genetic alterations in hepatoblastoma and hepatocellular carcinoma: common and distinctive aspects. Med Pediatr Oncol. 2002;39:530–535. | ||
Czauderna P, Maibach R, Aronson D, et al. Hepatocellular carcinoma in children: results of the second prospective study of the International Society of Pediatric Oncology (SIOP): SIOPEL 2. Med Pediatr Oncol. 2003;41:269. | ||
Schmid I, Albert MH, Häberle B, et al. HB99–Hepatozelluläre Karzinome: Behandlungsergebnisse und neue Konzepte [HB99 hepatocellular carcinomas: treatment results and new concepts]. Monatsschr Kinderheilkd. 2008;156:412. German. | ||
Katzenstein HM, Krailo MD, Malogolowkin MH, et al. Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children’s Cancer Group intergroup study. J Clin Oncol. 2002;20:2789–2797. | ||
Murawski M, Weeda VW, Maibach R, et al. Hepatocellular carcinoma in children: does modified platinum- and doxorubicin-based chemotherapy increase tumor resectability and change outcome? Lessons learned from the SIOPEL 2 and 3 studies. J Clin Oncol. 2016;34:1050–1056. | ||
Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. | ||
Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. | ||
Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. | ||
Carter CA, Chen C, Brink C, et al. Sorafenib is efficacious and tolerated in combination with cytotoxic or cytostatic agents in preclinical models of human non-small cell lung carcinoma. Cancer Chemother Pharmacol. 2007;59:183–195. | ||
Vincent P, Zhang X, Chen C, et al. Chemotherapy with the raf kinase inhibitor BAY43-9006 in combination with irinotecane, vinorelbine, or gemcitabine is well tolerated and efficacious in preclinical xenograft models. Proc Am Soc Clin Oncol. 2002;21:a1900. | ||
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. | ||
Peck-Radosavljevic M, Greten TF, Lammer J, et al. Consensus on the current use of sorafenib for the treatment of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2010;22:391–398. | ||
Abou-Alfa GK, Johnson P, Knox J, et al. Final results from a Phase II (PhII), randomized, double-blind study of sorafenib plus doxorubicin (SflD) versus placebo plus doxorubicin (PflD) in patients (pts) with advanced hepatocellular carcinoma. Presented at: Gastrointestinal Cancers Symposium: American Society of Clinical Oncology; 2008; Orlando, FL: a128. | ||
Takimoto CH, Awada A. Safety and anti-tumor activity of sorafenib (Nexavar) in combination with other anti-cancer agents: a review of clinical trials. Cancer Chemother Pharmacol. 2008;61:535–548. | ||
Richly H, Schultheis B, Adamietz IA, et al. Combination of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma: results from a Phase I extension trial. Eur J Cancer. 2009;45:579–587. | ||
Schmid I, Häberle B, Albert, MH, et al. Sorafenib and cisplatin/doxorubicin (PLADO) in pediatric hepatocellular carcinoma. Pediatr Blood Cancer. 2012;58:539–544. | ||
Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a Phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354. | ||
Zaanan A, Williet N, Hebbar M, et al. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: a large multicenter AGEO study. J Hepatol. 2013;58:81–88. | ||
Assenat E, Boige V, Thézenas S. Sorafenib (S) alone versus S combined with gemcitabine and oxaliplatin (GEMOX) in first-line treatment of advanced hepatocellular carcinoma (HCC): final analysis of the randomized Phase II GONEXT trial (UNICANCER/FFCD PRODIGE 10 trial). J Clin Oncol. 2013;31(Suppl):abstract 4028. | ||
Williet N, Dubreuil O, Boussaha T, et al. Neoadjuvant sorafenib combined with gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma. World J Gastroenterol. 2011;17:2255–2258. | ||
Zimmermann A, Perilongo G, editors. Pediatric Liver Tumors. Berlin, Heidelberg: Springer-Verlag; 2011. ISBN 978-3-642-14503-2. | ||
Lau CS, Mahendraraj K, Chamberlain RS. Hepatocellular carcinoma in the pediatric population: a population based clinical outcomes study involving 257 patients from the Surveillance, Epidemiology, and End Result (SEER) Database (1973-2011). HPB Surg. 2015;2015:670728. | ||
Weeda VB, Murawski M, McCabe AJ, et al. Fibrolamellar variant of hepatocellular carcinoma does not have a better survival than conventional hepatocellular carcinoma – results and treatment recommendations from the Childhood Liver Tumour Strategy Group (SIOPEL) experience. Eur J Cancer. 2013;49:2698–2704. | ||
Zhu AX. New agents on the horizon in hepatocellular carcinoma. Ther Adv Med Oncol. 2013;5:41–50. | ||
Cheng A, Kang Y, Lin D, et al. Phase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2011;29(Suppl):abstract 4000. | ||
Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992–2998. | ||
Thomas MB, Morris JS, Chadha R, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27:843–850. | ||
Zhu A, Abrams T, Miksad R, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094–5102. | ||
O´Neil B, Goff LW, Kauh JS, et al. Phase II study of the mitogen-activated protein kinase 1/2 inhibitor selumetinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2011;29:2350–2356. | ||
Goyal L, Muzumdar MD, Zhu AX. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res. 2013;19:2310–2318. | ||
Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebocontrolled Phase 2 study. Lancet Oncol. 2013;14:55–63. | ||
Cohn A, Kelley R, Yang T, et al. Activity of cabozantinib (XL184) in hepatocellular carcinoma patients (pts): results from a Phase II randomized discontinuation trial (RDT). J Clin Oncol. 2012;30(Suppl 4):abstract 261. | ||
Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–251. | ||
Hato T, Goyal L, Greten TF, Duda DG, Zhu AX. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology. 2014;60:1776–1782. | ||
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. | ||
Zheng P, Zhou Z. Human cancer immunotherapy with PD-1/PD-L1 blockade. Biomark Cancer. 2015;7(Suppl 2):15–18. | ||
El-Khoueiry AE, Melero I, Crocenzi TS, et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J Clin Oncol. 2015;33(Suppl):abstract LBA101. | ||
Zitvogel L, Apetoh L, Ghiringhelli F, et al. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. | ||
Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. | ||
Prieto PA, Reuben A, Cooper ZA, Wargo JA. Targeted therapies combined with immune checkpoint therapy. Cancer J. 2016;22:138–146. | ||
Mazzaferrro V, Bhoori S, Sposito C, Bongini M, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17(Suppl 2):44–57. | ||
Squires RH, Ng V, Romero R, Ekong U, et al. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American Association for the Study of Liver Diseases, American Society of Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Hepatology. 2014;60:362–398. | ||
Ismail H, Broniszczak D, Kaliciński P, et al. Liver transplantation in children with hepatocellular carcinoma. Do Milan criteria apply to pediatric patients? Pediatr Transplant. 2009;13(6):682–692. | ||
Malek MM, Shah SR, Atri P, et al. Review of outcomes of primary liver cancers in children: our institutional experience with resection and transplantation. Surgery. 2010;148:778–782. | ||
Beaunoyer M, Vanatta JM, Ogihara M, et al. Outcomes of transplantation in children with primary hepatic malignancy. Pediatr Transplant. 2007;11:655–660. | ||
Menon KV, Hakeem AR, Heaton ND. Meta-analysis: recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37:411–419. | ||
Malogolowkin MH, Stanley P, Steele DA, Ortega JA. Feasibility and toxicity of chemoembolization for children with liver tumors. J Clin Oncol. 2000;18:1279–1284. | ||
Czauderna P, Zbrzezniak G, Narozanski W, et al. Preliminary experience with arterial chemoembolization for hepatoblastoma and hepatocellular carcinoma in children. Pediatr Blood Cancer. 2006;46:825–828. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.