Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
PD-L1 is Fascinating but IDO Needs Attention in Non-HCV and Non-HBV-Associated Hepatocellular Carcinoma Patients
Authors Asghar K, Bashir S , Ali Rana I, Abu Bakar M, Farooq A , Hassan M , Asif Z , Afzal M , Masood I , Ishaq M , Tahseen M, Bilal S, Mehmood S , Kanwal N, Ud Din I, Loya A
Received 23 February 2023
Accepted for publication 20 May 2023
Published 17 June 2023 Volume 2023:10 Pages 921—934
DOI https://doi.org/10.2147/JHC.S409741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Manal Hassan
Kashif Asghar,1 Shaarif Bashir,2 Iftikhar Ali Rana,2 Muhammad Abu Bakar,3 Asim Farooq,1 Muhammad Hassan,1 Zukhruf Asif,1 Mahnoor Afzal,1 Iqra Masood,4 Muhammad Ishaq,2 Muhammad Tahseen,2 Sundus Bilal,5 Shafqat Mehmood,5 Nosheen Kanwal,6 Islah Ud Din,6 Asif Loya2
1Department of Basic Sciences Research, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Punjab, Pakistan; 2Department of Pathology, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Punjab, Pakistan; 3Department of Cancer Registry and Clinical Data Management, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Punjab, Pakistan; 4Department of Clinical Research, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Punjab, Pakistan; 5Department of Internal Medicine (Gastroenterology), Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Punjab, Pakistan; 6Department of Radiology, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Punjab, Pakistan
Correspondence: Kashif Asghar, Department of Basic Sciences Research, Shaukat Khanum Memorial Cancer Hospital and Research Centre, 7-A Block R-3, Johar Town, Lahore, Punjab, 54000, Pakistan, Tel +92-42-35905000, Fax +92-42-35945206, Email [email protected]
Background/Aim: Hepatocellular carcinoma (HCC) is one of the most common forms of liver cancer that is modulated by the immune system. Programmed cell death ligand-1 (PD-L1) has emerged as a novel therapeutic target in various cancers. Indoleamine 2,3-dioxygenase (IDO) is an immunosuppressive enzyme that is associated with poor prognoses in various cancer types. The aim of this study was to investigate the PD-L1 expression, and clinicopathological features of non-HCV and non-HBV-associated HCC patients, including IDO expression.
Patients and Methods: In this study, immunohistochemical analysis was performed to analyze the expression of PD-L1 and IDO. Formalin-fixed paraffin-embedded HCC tumor tissues (n=50) were obtained from the pathology department, at Shaukat Khanum Memorial Cancer Hospital and Research Centre (SKMCH&RC) in Lahore, Pakistan between 2005 and 2022. All the patients were HBV and HCV negative. Furthermore, it was a rare group of patients with no previous history of any viral hepatitis. In addition, for categorical and continuous variables chi-square or Fisher exact test and Mann–Whitney U-test was performed.
Results: Of 50 tissue specimens, PD-L1+ was observed in 21 [high: 12 (24%), low: 9 (18%)] and PD-L1- was observed in 29 HCC patients. IDO+ was observed in all 50 specimens [high: 42 (84%), low: 8 (16%)]. Additionally, both PD-L1 and IDO had high expression in 11 (22%) patients. While both PD-L1 and IDO had low expression in 2 (4%) patients. Furthermore, in IDO+/PD-L1- group, 20 (69%) out of 29 patients died while in the IDO+/PD-L1+ group, 9 (43%) out of 21 patients died.
Conclusion: Evaluation of IDO and PD-L1 expression may add therapeutic advantage in non-HCV and non-HBV-associated HCC patients that overexpress IDO. Further validation in a larger cohort is warranted.
Keywords: programmed cell death ligand-1, indoleamine 2, 3-dioxygenase, non-HCV HCC, non-HBV HCC, immune checkpoint molecules
Introduction
Hepatocellular carcinoma (HCC) is one of the most aggressive malignancies of the liver and is becoming the leading cause of cancer-related mortality and morbidity worldwide.1 It continues to be a global public health and economic burden, with a projected peak incidence of more than 1 million deaths by 2030.2 The incidence rate of HCC is growing worldwide, including in Pakistan.3 Hepatitis C virus (HCV) and Hepatitis B virus (HBV) infections primarily contribute to HCC development and its progression.4 HCV and HBV account for the majority of HCC incidence worldwide.4 However, some studies have also reported non-HCV and non-HBV HCC cases that confirm the non-viral etiological factors responsible for HCC development.5–8 HCC may evade the anti-cancer immune response due to the presence of a diverse range of immunity and immune tolerance in the liver.9 However, the actual underlying immunosuppressive mechanisms are still unknown.
Checkpoint blockades have emerged as a paradigm-shifting therapeutic modality in immunotherapy for HCC. In various malignancies, programmed death ligand-1 (PD-L1) checkpoint blockades have shown considerable clinical efficacy.10–12 PD-L1 protein expression on the surface of tumor cells is crucial for these cells to evade immunosuppression.13 The overexpression of PD-L1 on tumor cells can avoid T-cell cytolysis and facilitate cancer formation.14–16 Several studies have revealed a significant association of PD-L1 overexpression with antitumor immunity, tumor aggressiveness, and poor prognosis in HCC patients.17,18 However, a lot of cancer patients failed to respond to the PD-L1 checkpoint blockades.10 Immune checkpoint inhibitors (ICIs) such as pembrolizumab, nivolumab, durvalumab, atezolizumab, and others have been evaluated in HCC patients, but single-agent ICI trials have not yielded promising results.19 On the other hand, combination therapies involving ICIs have shown more favorable outcomes.20 The Phase III IMbrave150 trial has established a new standard of care for advanced HCC patients with a combination of bevacizumab and atezolizumab.19 This combination has resulted in significant benefits in clinical outcomes such as objective response rate (ORR), progression-free survival (PFS), and overall survival (OS).19 Despite the success of combination therapies, the lack of validated biomarkers of response in HCC immunotherapy remains a significant issue. PD-L1 expression, tumor mutational burden (TMB), microsatellite instability (MSI) status, gut microbiota, and other potential biomarkers need further exploration to identify patients who are most likely to benefit from immunotherapy.21 The heterogeneous tumor immune microenvironment in HCC may also account for the inconsistent outcomes observed with ICIs. Hence, the need for reliable markers of response is crucial.22
PD-L1 expression in predicting the response to immunotherapy in HCC remains controversial.23 Shrestha et al reported that only 65 out of 751 HCC patients expressed PD-L1, indicating the need for further research to determine whether PD-L1 expression can be used as a predictor of ICI efficacy in HCC patients.24
Thus, targeting immune checkpoints is an emerging field of research for novel cancer therapies.25–27 One such potential target is indoleamine 2, 3-dioxygenase (IDO), a checkpoint protein that contributes to an immunosuppressive tumor microenvironment.28 IDO is a heme-containing enzyme that degrades L-tryptophan into kynurenine.29 Local deprivation of tryptophan impedes the cytotoxicity of T-cells, resulting in the inhibition of T-cell immune responses by inducing regulatory T-cell differentiation.30,31 High IDO expression has been reported in many cancers, including breast, colorectal, ovarian, and gastric cancer.32–35 It is also associated with cancer metastasis and poor prognosis in HCC patients.36–40
PD-L1 expression is crucial in predicting which tumors are responsive to anti-PD-L1 immunotherapy. IDO overexpression is associated with poor prognosis in several tumors. Therefore, determining the expressions of PD-L1 and IDO might help to predict which individual patients may benefit from anti-IDO/anti-PD-L1 therapy or combinational therapies. In the current study, we investigated the expression of PD-L1 and IDO in HCC patients by immunohistochemical analysis.
Materials and Methods
Study Material
Formalin-fixed paraffin-embedded tissue samples of non-HCV and non-HBV HCC patients (n=50) were obtained from the archives of the Department of Pathology, Shaukat Khanum Memorial Cancer Hospital and Research Centre (SKMCH&RC) in Lahore, Pakistan, between 2005 and 2022. The patients chosen for the study were treatment-naive. The tumor tissue block for each sample was reviewed and confirmed by the histopathologist. The most appropriate tissue block was selected if the sample contained multiple tumors. The histologic cell types were allocated according to the criteria given in the WHO classification. All the clinicopathological and radiological parameters of non-HCV and non-HBV HCC patients were collected from the medical records of the hospital information system (HIS). The current retrospective study was approved by the institutional review board (IRB) of SKMCH & RC. IRB granted the waiver for informed consent for this study, which is in accordance with the Declaration of Helsinki. The patient data accessed complied with relevant data protection and privacy regulations.
Immunohistochemistry
Two sections of formalin-fixed paraffin-embedded (FFPE) tumor specimens of the same patients were cut at a thickness of 4 µm. IDO staining was performed as described in41 using an anti-Indoleamine 2, 3-dioxygenase antibody (Abcam, # ab55305); heat-mediated epitope retrieval with a Tris-EDTA buffer was performed. The immunoreactivity was detected by using the Dako EnVision kit (K5007). Normal human tonsils served as a positive control. PD-L1 immunoreactivity was assessed by an immunohistochemical assay for formalin-fixed, paraffin-embedded tumor specimens.42,43 Slides were stained using an autostainer Link 48 (Dako Denmark) as per the manufacturer’s protocol. Slides were deparaffinized and antigen was retrieved simultaneously with the target retrieval solution (#GV805 Dako). PD-L1 antibody (clone 22C3; Cat# M3653) and an automated staining procedure developed by DAKO. PD-L1 labeling was visualized using Envision Flex detection kit DAKO (K8000). Normal human tonsils served as a positive control. Slides were visualized by an optical microscope (Provis AX-70, Olympus, Melville, NY).
Scoring
Pathologists assessed all the results. They performed a blind histopathologic evaluation. The discrepancies between the pathologists were examined mutually to reach a consensus and the mean score of both of them was considered a decisive score. The total IDO immunostaining scores were calculated as described earlier.38 The intensity was scored for IDO as negative (0), weak (1), moderate (2), or strong (3). The percentage of tumor cells with positive staining (range, 0–9) were classified as diffuse (3+, 50–75%), focal (2+, 25–50%), sporadic (1+, 5–25%), and negative (0, 0%). The immunohistochemical expression of PD-L1 was calculated as described earlier.44 PD-L1 staining intensity was assessed as strong (3), moderate (2), weak (1), or negative (0). The percentage of tumor cells with positive staining was categorized according to the following formula: PD-L1 expression score (H score) (range, 0–9)=0×% of non-stained tumor cells +1×% of weakly stained tumor cells +2×% of moderately stained tumor cells +3×% of strongly stained tumor cells.
Statistical Analysis
Statistical analysis was performed by using SPSS software (version 20.0; SPSS, Chicago, IL, USA). Frequency and percentage were used for categorical variables while the median and range (min-max) were used for continuous variables. Bivariate analysis was done using chi-square or Fisher exact test (where necessary). For continuous explanatory variables such as age, the Mann–Whitney U-test was performed. Statistical significance was defined as a two-tailed P-value of 0.05.
Results
Clinicopathological Profiles of Non-HCV and Non-HBV-Associated HCC Patients
Over the last 17 years (2005 to 2022), we identified 50 patients of non-HCV and non-HBV-associated HCC at SKMCH&RC. Among these, 39 were male and 11 were female. Of the 50 patients, the majority 42 (84%), belonged to the Punjab province. The mean age of patients was 65 years. As per the medical history of the patients, 16 (32%) were smokers and 4 (8%) were consuming alcohol. Diabetes was observed as the major comorbidity (54%) in the current data set. Out of 50 patients, 29 (58%) had died as of 2022. In addition, further clinicopathological characteristics of patients are given in Table 1 and Table 2.
 |
Table 1 Demographics and Baseline Characteristics of Patients with PD-L1 |
 |
Table 2 Demographics and Baseline Characteristics of Patients with IDO |
Immunohistochemical Staining of PD-L1 and IDO in Tissue Samples
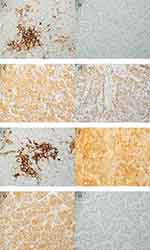
To evaluate the expression of PD-L1 and IDO, formalin-fixed paraffin-embedded (FFPE) non-HCV and non-HBV-associated HCC tissues (n=50) were used for immunohistochemical analysis. Patients were categorized into PD-L1+ (42%) and PD-L1- (58%) groups as shown in Figure 1A and B. In addition, the PD-L1+ group was further classified into high (24%) and low (18%). IDO+ was observed in all 50 tissue specimens. High IDO expression was observed in 42 (84%) and low IDO expression was observed in 8 (16%) tissue specimens as shown in Figure 1C and D. Additionally, both PD-L1 and IDO had high expression in 11 (22%) patients as shown in Figure 1E and F. While IDO+ and PD-L1- expressions are shown in Figure 1G and H. There was no statistically significant association between PD-L1 and IDO expression with age, sex, histology, or smoking and alcoholic status in these patients (Table 1 and Table 2).
Discussion
In the present study, we examined the PD-L1 expression, and clinicopathological and radiological features of non-HCV and non-HBV-associated HCC patients, including IDO expression. To evaluate the expression of PD-L1 and IDO, immunohistochemistry was performed on the HCC tissues. In recent years, it has been observed that the number of patients with non-HCV and non-HBV-associated HCC has increased, however, the incidence and the number of patients with viral-associated HCC tended to decrease.8 Furthermore, Toyoda et al revealed that the patient’s survival improved significantly in the viral HCC group as compared to the non-viral HCC group.45 One of the reasons could be less efficacy of surveillance in non-viral HCC patients. In addition, there is a lack of measures to preserve liver function in non-viral HCC.45
The immune system plays a pivotal role in regulating cancer progression.46 Over the past few years, immuno-oncology has been a paradigm shift in cancer therapy, including HCC.47 Although there are several treatment options for HCC, such as surgery, trans-arterial chemoembolization, radioembolization, radiofrequency ablation, and chemotherapy, however, they will only assist a limited percentage of patients.48 The capability of immunotherapy to elicit efficient anti-tumor responses makes it remarkably well-suited for the treatment of HCC.48 Immune checkpoint molecules have considerably transformed the clinical management of HCC.49 Several studies have demonstrated the role of PD-L1 in HCC.14–18 Moreover, the role of IDO has also been identified in HCC.36–40 Keeping the previously reported data in the view, we investigated the expression of PD-L1 and IDO in non-HCV and non-HBV-associated HCC patients. This rare group of patients was identified from the hospital record for the last seventeen years.
Jung et al observed overexpression of PD-L1 in 27% of HCC specimens.17 In our data set, PD-L1 was overexpressed in 24% of HCC specimens. These percentages were lower as compared to the other malignancies, including cancers of the lung (50%), esophagus (44%), and stomach (42%).17,50 Furthermore, Jung et al reported PD-L1+ expression in 11[high: 2 (18.2%), low: 9 (81.8%)] non-HCV and non-HBV-associated HCC patients.17 We identified PD-L1+ in 21 [high: 12 (24%), low: 9 (18%)] and PD-L1- in 29 (58%) non-HCV and non-HBV-associated HCC patients. However, no significant association was found between PD-L1 expression and clinicopathological characteristics: age, gender, histological grading, and liver function tests. Our results were consistent with a previous study conducted by Jung et al.17
Pan et al observed overexpression of IDO in 35.5% of HCC specimens.38 In our data set, IDO was overexpressed in 84% of HCC specimens. Moreover, Pan et al reported IDO expression in 22 [high: 5 (22.7%), low: 17 (77.2%)] in only non-HBV-associated HCC patients.38 We identified IDO expression in all 50 [high: 42 (84%), low: 8 (16%)] non-HCV and non-HBV-associated HCC patients. Nevertheless, no significant association was found between IDO expression and clinicopathological features: age, gender, histological grading, and liver function tests. Our results were in compliance with Pan et al except for the gender.38
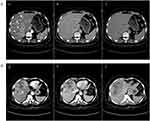
We further analyzed the clinicopathological and radiological characteristics of non-survivor HCC patients along with PD-L1/IDO expression as shown in Table 3. We evaluated that in the IDO+/PD-L1- group, 20 (69%) out of 29 patients died. All the non-survivor HCC patients were IDO+ [high: 24 (82.7%), low: 5 (17.3%)]. IDO was overexpressed in HCC specimens with histological grading of moderate to poor differentiation. Low IDO expression was only observed in the well-differentiated HCC specimens. Diabetes mellitus is a global endemic and one of the major risk factors for HCC.51,52 In the current study, out of 29 non-survivor HCC patients, 14 (48.3%) had diabetes. 13 (92.8%) out of 14 diabetic non-survivor HCC patients had high IDO expression. Smoking and alcohol consumption are also considered risk factors for HCC.53,54 In our data set, 10 non-survivor HCC patients were smokers, while only one patient had a history of alcohol consumption. Out of 10 smokers, 8 (80%) had high IDO expression. Liang et al reported that tobacco smoke induces IDO expression, which leads to immunosuppression and cancer progression.55 Early HCC can be managed by surgical resection.56 Nevertheless, multifocal HCC is challenging to treat with frequent recurrences that influence its outcome.56 We identified 9 multifocal HCCs among the non-survivors (Figure 2). 7 (77.7%) out of 9 had high IDO expression. IDO overexpression is associated with poor prognosis and metastasis in HCC patients.38,40 We observed metastasis in 8 out of 29 non-survivors among which 7 (87.5%) had high IDO expression. Our results are in compliance with those previously published by Pan et al.38
 |
Table 3 Clinicopathological and Radiological Characteristic of Non-Survivor HCC Patients Along with PD-L1/IDO Expression |
There were some limitations in the current study. The sample size was small because all the patients were HBV and HCV-negative. Furthermore, it was a rare group of patients with no previous history of any viral hepatitis. The data of this retrospective study was collected over the last 17 years (2005 and 2022) from a specialized cancer care hospital in Pakistan. Another limitation was the retrospective nature of our data and lack of awareness to seek medical care and early diagnosis among the patient population. Gao et al examined PD-L1 expression in 240 HCC patients. Among them, only 16 were both HBV and HCV negative.18 Pan et al observed IDO expression in 138 HCC patients out of which only 22 patients were negative for HBV.38 We have analyzed the expression of IDO and PD-L1 in 50 non-HCV and non-HBV-associated HCC patients. To the best of our knowledge, although there have been studies on IDO expression status and its immunosuppressing effect in HCC,36,38,57 this is the first study to examine its expression along with PD-L1.
Conclusion
In conclusion, we report that among non-survivors, non-HCV, and non-HBV-associated HCC patients, PD-L1 was negative while IDO was overexpressed in the majority of patients. PD-L1 expression is controversial in predicting which tumor subtypes might be responsive to anti-PD-L1 immunotherapy. Hence, it is suggested to investigate PD-L1 expression prior to treatment to determine which individual patients may benefit from therapy. Recently, IDO has gained attention as a novel immunotherapeutic and prognostic marker in cancer. Combinational therapy is a cornerstone of cancer therapy as it enhances therapeutic efficacy more than the mono-therapeutic approach. IDO and PD-L1 inhibitors might be a promising strategy for HCC management. Nevertheless, further multicenter studies on larger cohorts are warranted to fortify these findings.
Data Sharing Statement
The data generated in the present study may be requested from the corresponding author.
Ethics Approval and Informed Consent
The institutional review board (IRB) of SKMCH&RC approved the current retrospective study (#EXMPT-09-03-18-01). IRB granted the waiver for informed consent for this study, which is in accordance with the Declaration of Helsinki. The patient data accessed complied with relevant data protection and privacy regulations.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sim HW, Knox J. Hepatocellular carcinoma in the era of immunotherapy. Curr Probl Cancer. 2018;42:40–48. doi:10.1016/j.currproblcancer.2017.10.007
2. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi:10.1056/NEJMra1713263
3. Hafeez Bhatti AB, Dar FS, Waheed A, Shafique K, Sultan F, Shah NH. Hepatocellular carcinoma in Pakistan: national trends and global perspective. Gastroenterol Res Pract. 2016;2016:5942306. doi:10.1155/2016/5942306
4. Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi:10.1001/jamaoncol.2017.3055
5. Hatanaka K, Kudo M, Fukunaga T, et al. Clinical characteristics of NonBNonC-HCC: comparison with HBV and HCV related HCC. Intervirology. 2007;50:24–31. doi:10.1159/000096309
6. Tateishi R, Okanoue T, Fujiwara N, et al. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort study. J Gastroenterol. 2015;50:350–360. doi:10.1007/s00535-014-0973-8
7. Utsunomiya T, Shimada M, Kudo M, et al. Nationwide study of 4741 patients with non-B non-C hepatocellular carcinoma with special reference to the therapeutic impact. Ann Surg. 2014;259:336–345. doi:10.1097/SLA.0b013e31829291e9
8. Nagaoki Y, Hyogo H, Ando Y, et al. Increasing incidence of non-HBV-and non-HCV-related hepatocellular carcinoma: single-institution 20-year study. BMC Gastroenterol. 2021;21:306. doi:10.1186/s12876-021-01884-5
9. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi:10.1038/nm.3394
10. Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41:868–876. doi:10.1016/j.ctrv.2015.11.001
11. Katsuya Y, Fujita Y, Horinouchi H, Ohe Y, Watanabe SI, Tsuta K. Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer. 2015;88:154–159. doi:10.1016/j.lungcan.2015.03.003
12. Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–1182. doi:10.1007/s00262-006-0266-z
13. Wei R, Guo L, Wang Q, Miao J, Kwok HF, Lin Y. Targeting PD-L1 protein: translation, modification and transport. Curr Protein Pept Sci. 2019;20(1):82–91. doi:10.2174/1389203719666180928105632
14. Paterson AM, Brown KE, Keir ME, et al. The programmed death-1 ligand 1: B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol. 2011;187:1097–1105. doi:10.4049/jimmunol.1003496
15. Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–314. doi:10.1007/s00262-004-0593-x
16. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapyPD-L1 IHC as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. doi:10.1158/1535-7163.MCT-14-0983
17. Jung HI, Jeong D, Ji S, et al. Overexpression of PD-L1 and PD-L2 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res Treat. 2017;49:246–254. doi:10.4143/crt.2016.066
18. Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi:10.1158/1078-0432.CCR-08-1608
19. Rizzo A, Ricci AD, Di Federico A, et al. Predictive biomarkers for checkpoint inhibitor-based immunotherapy in hepatocellular carcinoma: where do we stand? Front Oncol. 2021;11:803133. doi:10.3389/fonc.2021.803133
20. Viscardi G, Tralongo AC, Massari F, et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. 2022;177:175–185. doi:10.1016/j.ejca.2022.09.031
21. Rizzo A, Cusmai A, Gadaleta-Caldarola G, Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expert Rev Gastroenterol Hepatol. 2022;16(4):333–339. doi:10.1080/17474124.2022.2064273
22. Di Federico A, Rizzo A, Carloni R, et al. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs. 2022;31(4):361–369. doi:10.1080/13543784.2022.2009455
23. Yin X, Wu T, Lan Y, Yang W. Current progress of immune checkpoint inhibitors in the treatment of advanced hepatocellular carcinoma. Biosci Rep. 2022;42(2):BSR20212304. doi:10.1042/BSR20212304
24. Shrestha R, Prithviraj P, Anaka M, et al. Monitoring immune checkpoint regulators as predictive biomarkers in hepatocellular carcinoma. Front Oncol. 2018;8:269. doi:10.3389/fonc.2018.00269
25. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi:10.1016/j.cell.2015.03.030
26. Archilla-Ortega A, Domuro C, Martin-Liberal J, Muñoz P. Blockade of novel immune checkpoints and new therapeutic combinations to boost antitumor immunity. J Exp Clin Cancer Res. 2022;41:62. doi:10.1186/s13046-022-02264-x
27. Shiravand Y, Khodadadi F, Kashani SM, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. 2022;29:3044–3060. doi:10.3390/curroncol29050247
28. Song X, Si Q, Qi R, et al. Indoleamine 2, 3-dioxygenase 1: a promising therapeutic target in malignant tumor. Front Immunol. 2021;12:800630. doi:10.3389/fimmu.2021.800630
29. El-Fattah A, Eslam E. IDO/kynurenine pathway in cancer: possible therapeutic approaches. J Transl Med. 2022;20:347. doi:10.1186/s12967-022-03554-w
30. Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107:452–460. doi:10.1046/j.1365-2567.2002.01526.x
31. Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–473. doi:10.1016/s0167-5699(99)01520-0
32. Yu J, Du W, Yan F, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–3797. doi:10.4049/jimmunol.1201449
33. Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2, 3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–1151. doi:10.1158/1078-0432.CCR-05-1966
34. Okamoto A, Nikaido T, Ochiai K, et al. Indoleamine 2, 3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11:6030–6039. doi:10.1158/1078-0432.CCR-04-2671
35. Liu H, Shen Z, Wang Z, et al. Increased expression of IDO associates with poor postoperative clinical outcome of patients with gastric adenocarcinoma. Sci Rep. 2016;6:21319. doi:10.1038/srep21319
36. Li S, Han X, Lyu N, et al. Mechanism and prognostic value of indoleamine 2, 3-dioxygenase 1 expressed in hepatocellular carcinoma. Cancer Sci. 2018;109:3726–3736. doi:10.1111/cas.13811
37. Asghar K, Farooq A, Zulfiqar B, Rashid MU. Indoleamine 2, 3-dioxygenase: as a potential prognostic marker and immunotherapeutic target for hepatocellular carcinoma. World J Gastroenterol. 2017;23:2286–2293. doi:10.3748/wjg.v23.i13.2286
38. Pan K, Wang H, Chen MS, et al. Expression and prognosis role of indoleamine 2, 3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134:1247–1253. doi:10.1007/s00432-008-0395-1
39. Li T, Yang Y, Hua X, et al. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318(2):154–161. doi:10.1016/j.canlet.2011.12.020
40. Lin L, Yang DH, Huang Y, et al. Relationship between the expressions of indoleamine 2, 3-dioxygenase in hepatocellular carcinoma and clinicopathological parameters. Zhonghua Yi Xue Za Zhi. 2013;93:2186–2190.
41. Asghar K, Brain J, Palmer JM, et al. Potential role of indoleamine 2, 3-dioxygenase in primary biliary cirrhosis. Oncol Lett. 2017;14:5497–5504. doi:10.3892/ol.2017.6834
42. Ilie M, Khambata-Ford S, Copie-Bergman C, et al. Use of the 22C3 anti–PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms. PLoS One. 2017;12:e0183023. doi:10.1371/journal.pone.0183023
43. Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89:181–188. doi:10.1016/j.lungcan.2015.05.007
44. Igarashi T, Teramoto K, Ishida M, Hanaoka J, Daigo Y. Scoring of PD-L1 expression intensity on pulmonary adenocarcinomas and the correlations with clinicopathological factors. ESMO Open. 2016;1:e000083. doi:10.1136/esmoopen-2016-000083
45. Toyoda H, Kariyama K, Hiraoka A, et al. Improved survival of viral hepatocellular carcinoma but not non-viral hepatocellular carcinoma from 2000 to 2020: a multi-centre cohort study of 6007 patients from high-volume academic centres in Japan. Aliment Pharmacol Ther. 2022;56:694–701. doi:10.1111/apt.17088
46. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi:10.1126/science.1203486
47. Foerster F, Gairing SJ, Ilyas SI, Galle PR. Emerging immunotherapy for HCC: a guide for hepatologists. Hepatology. 2022;75(6):1604–1626. doi:10.1002/hep.32447
48. Pardee AD, Butterfield LH. Immunotherapy of hepatocellular carcinoma: unique challenges and clinical opportunities. Oncoimmunology. 2012;1:48–55. doi:10.4161/onci.1.1.18344
49. van Doorn DJ, Takkenberg RB, Klümpen HJ. Immune checkpoint inhibitors in hepatocellular carcinoma: an overview. Pharmaceuticals. 2020;14:3. doi:10.3390/ph14010003
50. McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013;2:662–673. doi:10.1002/cam4.106
51. Li X, Wang X, Gao P. Diabetes mellitus and risk of hepatocellular carcinoma. Biomed Res Int. 2017;2017:5202684. doi:10.1155/2017/5202684
52. Campbell PT, Newton CC, Freedman ND, et al. Body mass index, waist circumference, diabetes, and risk of liver cancer for US adults. Cancer Res. 2016;76:6076–6083. doi:10.1158/0008-5472.CAN-16-0787
53. Ambade A, Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int J Hepatol. 2012;2012:853175. doi:10.1155/2012/853175
54. Azzalini L, Ferrer E, Ramalho LN, et al. Cigarette smoking exacerbates nonalcoholic fatty liver disease in obese rats. Hepatology. 2010;51:1567–1576. doi:10.1002/hep.23516
55. Liang F, Wang G-Z, Wang Y, et al. Tobacco carcinogen induces tryptophan metabolism and immune suppression via induction of indoleamine 2,3-dioxygenase 1. Signal Transduct Target Ther. 2022;7(1):311. doi:10.1038/s41392-022-01127-3
56. Feo F, Pascale RM. Multifocal hepatocellular carcinoma: intrahepatic metastasis or multicentric carcinogenesis? Ann Transl Med. 2015;3:4. doi:10.3978/j.issn.2305-5839.2014.12.08
57. Brown ZJ, Yu SJ, Heinrich B, et al. Indoleamine 2, 3-dioxygenase provides adaptive resistance to immune checkpoint inhibitors in hepatocellular carcinoma. Cancer Immunol Immunother. 2018;67:1305–1315. doi:10.1007/s00262-018-2190-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.