Back to Journals » Cancer Management and Research » Volume 12
PD-L1 in Combination with CD8+TIL and HIF-1α are Promising Prognosis Predictors of Head and Neck Squamous Cell Carcinoma
Authors Zhou Z, Mu D, Zhang D, Zhang X , Ding X, Yang J, Bai X, Hu M
Received 19 October 2020
Accepted for publication 2 December 2020
Published 23 December 2020 Volume 2020:12 Pages 13233—13239
DOI https://doi.org/10.2147/CMAR.S285691
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Zihan Zhou,1,2 Dianbin Mu,3 Dexian Zhang,3 Xianbin Zhang,2 Xingchen Ding,2 Jia Yang,2 Xinbin Bai,4 Man Hu2
1Department of Oncology, Weifang Medical University, Weifang, Shandong, People’s Republic of China; 2Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China; 3Department of Pathology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China; 4Department of Oncology, Jining Cancer Hospital, Jining, Shandong, People’s Republic of China
Correspondence: Man Hu
Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jiyan Road 440, Jinan 250117, Shandong, People’s Republic of China
Tel +86-531-67626152
Fax +86-531-67626153
Email [email protected]
Objective: The aim of this study was to evaluate the prognosis effect of PD-L1 in combination with CD8+ tumor-infiltrating lymphocyte (TIL) or HIF-1α in head and neck squamous cell carcinoma (HNSCC).
Methods: A total of 63 patients who underwent surgical resection were included in this study. The level of PD-L1, CD8+ TIL, and HIF-1α was determined by immunohistochemical analysis. The survival of patients was evaluated by Kaplan–Meier analysis. The prognostic power of these parameters was evaluated by C-index.
Results: We observed that the survival of patients, who had a high level of PD-L1 in tumor cells, was significantly shorter than those who had a low level of PD-L1. However, the survival of patients who had a high level of PD-L1 in tumor microenvironment was significantly longer than patients with a low level of PD-L1 in tumor microenvironment. In addition, high level of CD8+ tumor-infiltrating lymphocyte or low level of HIF-1α level suggests a better prognosis. Moreover, we observed that PD-L1 in combination with CD8+ tumor-infiltrating lymphocyte and HIF-1α could significantly improve the prognostic effect of current TNM stage.
Conclusion: The results of this study suggest that the level of PD-L1, CD8+TIL, and HIF-1α are useful prognostic biomarkers for patients with HNSCC. Incorporating these biomarkers into current TNM stage of HNSCC improve the discriminatory capability of TNM stage.
Keywords: PD-L1, HIF-1α, CD8+TIL, HNSCC, prognosis
Introduction
Head and neck squamous cell carcinoma (HNSCC) is diagnosed in 550,000 patients annually worldwide and leads to 300,000 deaths.1 Notably, the tumor staging system is important for cancer patients. It can provide precise information which can be used to predict treatment response and prognosis.2 Currently, the tumor/node/metastasis (TNM) staging is the most widely used cancer staging system. In the TNM staging system, the T indicates the size and extent of the tumor; N indicates the number of lymph nodes, which are infected by tumor cells; and M indicated the metastasized organs.
Recently, some studies suggest that the level of PD-L1 in tumor cells (TC-PD-L1) or in tumor microenvironment cells (TMC-PD-L1) is a promising biomarker for the prognosis of HMSCC patients. For example, Minichsdorfer et al 3 reported that the accumulation of TC-PD-L1 significantly decreased the survival of patients. In addition, some randomized controlled trials have demonstrated that pembrolizumab, a PD-L1 antibody, could successfully improve the survival of HNSCC patients. However, the accumulation of TMC-PD-L1 significantly improved the survival of HNSCC patients.4,5 This suggests that the level of TC-PD-L1 and TMC-PD-L1 are promising biomarkers for improving individualized treatment. In addition, previous studies suggest CD8+ tumor-infiltrating lymphocytes (CD8+ TIL) and HIF-1α are also valuable biomarkers for the prognosis.6,7 Therefore, in this study, we evaluated the prognostic value of TC-PD-L1, TMC-PD-L1, CD8+ TIL, and HIF-1α. In addition, we investigated if incorporating these biomarkers into the TNM stage could improve the prognostic value of the TNM stage.
Patients and Methods
Patients
A total of 63 patients who suffered from tumor of larynx, oropharynx, hypopharynx, or multiple sites were included in this study. These patients underwent surgical resection at the Shandong Cancer Hospital and Institute, and the clinicopathological characteristics of these patients were obtained from the electronic record of patients. Written informed consents were obtained from all patients for the publication of this report, and the protocol of this retrospective study was approved by the Institutional Review Board of Shandong Cancer Hospital (approval number SDTHEC201903087). All procedures were in accordance with the principles of the Declaration of Helsinki and its later amendments or comparable ethical standards.
Immunohistochemistry (IHC) of TC-PD-L1, TMC-PD-L1, CD8+ TIL, and HIF-1α
The level of TC-PD-L1, TMC-PD-L1, CD8+TIL, and HIF-1α were evaluated by immunohistochemical staining as previously reported.8,9 Briefly, serial histological sections (4-μm thick) were deparaffinzed, and then endogenous peroxidase activity was blocked by antigen retrieval. After that the tissues were incubated with the following primary antibody for 60 minutes at room temperature: A rabbit monoclonal antibody against PD-L1 (code GT228007, Gene Tech, Shanghai, China), an antibody against CD8+TIL (code AB93278; Abcam, Tokyo, Japan; 1:250 dilution), and a rabbit monoclonal antibody against HIF-1α (code AB51608; Abcam, Tokyo, Japan; 1:100 dilution). Then, the samples were incubated with the second antibody, MaxVisionTM/HRP (Dako) for 30 minutes at room temperature.
Evaluation of Immunohistochemical Staining
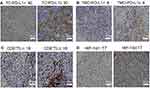
The IHC slides were evaluated by two pathologists in a blind way. In order to quantify the level of PD-L1, CD8+TIL, and HIF-1α, we randomly selected five fields and 200 cells/field. The level of these proteins was determined by the following formula: the number of positive cells/200 cells, and the mean value was determined. The optimal cutoffs of PD-L1, CD8+TIL, or HIF-1α were determined by the receiver operating characteristic (ROC) curve and Youden’s index. Figure 1 shows the representative staining patterns of PD-L1, CD8+TIL, and HIF-1α in the specimens.
Statistical Analysis
The survival of patients was determined by Kaplan-Meier curve and Log rank test. In order to determine the prognostic power of TNM stage and the biomarkers, the Harrell’s concordance index (C-index) was determined. The statistical analysis was performed by SPSS 23.0 (IBM SPSS Statistics) and the graph was operated by GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA). P-values of less than 0.05 were considered significant.
Results
The Clinicopathological Characteristics of Patients
In total, 63 patients were included in the present study and the clinicopathological characteristics of patients were summarized in Table 1. The tumors were located in laryngeal cancer (n=41), oropharyngeal cancer (n=12), and hypopharyngeal carcinoma (n=10), respectively. In order to determine the optimal cutoff of TC-PD-L1, TMC-PD-L1, CD8+ TIL,and HIF-1α, we performed a ROC curve and determined the optimal cutoff with the help of Youden’s index. We observed the optimal cutoff of TC-PD-L1, TMC-PD-L1, CD8+ TIL, and HIF-1α was 30%, 4%, 16%, and 17%, respectively.
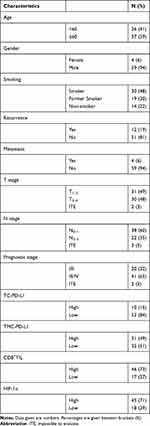 |
Table 1 Patient Characteristics |
PD-L1, CD8+TIL, and HIF-1α Regulate the Survival of HNSCC
To determine the prognostic effect of PD-L1, we evaluated the prognostic effect of PD-L1 in tumor cells (TC-PD-L1) and the PD-L1 in tumor microenvironment cells (TMC-PD-L1), respectively. This study proved that patients with negative TC-PD-L1 had significantly longer survival than patients with positive TC-PD-L1 (Figure 2A). However, patients with positive TMC-PD-L1 had significantly longer survival than those with negative TMC-PD-L1 (Figure 2B). The same as the TMC-PD-L1, positive CD8+TIL significantly increased the survival of patients, when compared to the survival of patients with negative CD8+TIL (Figure 2C). In order to evaluate the prognosis effect of HIF-1α, the patients with HIF-1α lower than 17% were defined as negative of HIF-1α, otherwise the patients were defined as positive HIF-1α. We observed that positive HIF-1α significantly decreased the survival of patients, when compared to negative HIF-1α (Figure 2D).
PD-L1, CD8+TIL, and HIF-1α are Predictors of HNSCC
To evaluate if incorporating PD-L1, CD8+TIL, and HIF-1α into TNM stage could improve the current TNM stage, we calculated the C-index of TC-PD-L1, TMC-PD-L1, CD8+TIL, and HIF-1α in combination with TNM stage. This study proved that PD-L1, CD8+TIL, and HIF-1α, in combination with TNM stage (C-index: 0.833, Figure 3A), were significantly higher than TNM stage (C-index: 0.580, Figure 3A). This suggests that incorporation of PD-L1, HIF-1α, and CD8+TIL into current TNM stage significantly improves the prognostic effect of TNM stage.
Discussion
In this study, we observed that the high level of PD-L1 in cancer cells significantly decreased the survival of HNSCC patients; however, the high level of PD-L1 in the tumor microenvironment significantly increased the survival of HNSCC patient. The mechanism of the controversial effect of PD-L1 in cancer cells and PD-L1 in the tumor microenvironment is still unclear. Previous studies prove that the expression of PD-L1 in immune cell suggests the level of pre-existing immunity, which has a strong prognostic utility and also can be used as a biomarker to evaluate the response to immunotherapies of cancer.10,11 However, it is well known that overexpression of PD-L1 in tumor cells suggests a poor prognosis of patients. The PD-L1 on tumor cells can bind to the receptor of PD-L1 on the activated T cells.12,13 This leads to the inhibition of the cytotoxic effect of T cells; and, in 2019, this concept was awarded the Nobel Prize.
Notably, the significance of PD-L1 expression in tumors is still controversial. Consistent with the present study, some previous study also demonstrates that a high level of PD-L1 in tumors suggests poor survival of patients with breast, gastric, or renal cancer. However, some studies suggest that a high level of PD-L1 in tumors significantly increases the survival of patients14,15 or failed to have an effect on the survival of patients.16,17 These controversial observations might be due to the fact that these studies did not distinguish the level of PD-L1 in tumor cells (TC-PD-L1) from the PD-L1 in microenvironment (TMC-PD-L1). Consistent with our study, Birtalan et al4 also reported the accumulation of TC-PD-L1 harmed the survival of HNSCC patients; however, the level of TMC-PD-L1 indicated a better prognosis.
Consistent with this study, previous studies demonstrated that the level of HIF-1α was associated with the prognosis of cancer patients. In addition, a previous study proved that, in tumor cells, the up-regulation of PD-L1 under hypoxia was dependent on HIF-1α.18 Noman et al19 reported that hypoxia rapidly led to the accumulation of PD-L1 by directly binding to HRE-4, a hypoxia response element in the PD-L1 proximal. This leads to immune escape for tumor cells. Thus, targeting PD-L1 in combination with HIF-1α might be a promising strategery for cancer immunotherapy. Indeed, we observed that patients with positive PD-L1 and HIF-1α in tumor cells had significantly shorter survival than patients with negative PD-L1 and HIF-1α (Figure 3B). Only two patients were in the TC-PD-L1+/HIF-1α− group (the green line), and one died, so the green line dropped suddenly at 40 months. In addition, a previous study proves that the expression of PD-L1 was positively correlated with the abundance of CD8+ TILs.20 This suggests that CD8+ TILs might also be a promising prognostic predictor of patients. Thus, we investigated the prognostic role of CD8+TIL in combination with the level of PD-L1 in the tumor microenvironment. We observed that the survival of TMC-PD-L1+ CD8+ patients was significantly better than TMC-PD-L1− CD8+and TMC-PD-L1− CD8− patients (Figure 3C).
Currently, TNM stage is still the most commonly used classification for staging and prognosis of HNSCC. This will help the clinicians to decide the optimal treatment for patients with different stage. However, previous studies and this study suggest that the biomarkers, such as PD-L1, CD8+TIL, and HIF-1α are promising molecules for the prognosis of HNSCC patients. Indeed, the study proved that the level of PD-L1, CD8+TIL, and HIF-1α in combination with TNM stage significantly improved the prognostic power of current TNM stage. However, this study is a retrospective study and the selective bias of clinicians should be considered. Therefore, the results of this retrospective study should be confirmed in prospective trials to assess the clinical significance of these biomarkers.
Conclusion
In conclusion, the results of this study suggest that the level of PD-L1, CD8+TIL, and HIF-1α are useful prognostic biomarkers for patients with HNSCC. Incorporating these biomarkers into the current TNM stage of HNSCC improve the discriminatory capability of TNM stage.
Acknowledgments
This work was funded by the National Key Research and Development Program of China (No. 2018YFE0114100), Key Research and Development Program of Shandong province, China (No. 2019GGX101057), Science Technology Program of Jinan (No. 201805051).
Disclosure
The authors report no conflicts of interest in this work. The abstract of this paper was presented at the ASCO in 2020, as an online publication with interim findings. The abstract was published in DOI: 10.1200/JCO.2020.38.15_suppl.e18545,38, no. 15_suppl in the Journal of Clinical Oncology.
References
1. Kann BH, Hicks DF, Payabvash S, et al. Multi-institutional validation of deep learning for pretreatment identification of extranodal extension in head and neck squamous cell carcinoma. J Clin Oncol. 2019:JCO1902031. doi:10.1200/jco.19.02031.
2. Budach V, Tinhofer I. Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: a systematic review. Lancet Oncol. 2019;20(6):e313–e326. doi:10.1016/s1470-2045(19)30177-9
3. Minichsdorfer C, Oberndorfer F, Krall C, et al. PD-L1 expression on tumor cells is associated with a poor outcome in a cohort of caucasian nasopharyngeal carcinoma patients. Front Oncol. 2019;9:1334. doi:10.3389/fonc.2019.01334
4. Birtalan E, Danos K, Gurbi B, et al. Expression of PD-L1 on immune cells shows better prognosis in laryngeal, oropharygeal, and hypopharyngeal cancer. Appl Immunohistochem Mol Morphol. 2018;26(7):e79–e85. doi:10.1097/pai.0000000000000590
5. Mehra R, Seiwert TY, Gupta S, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018;119(2):153–159. doi:10.1038/s41416-018-0131-9
6. Goode EL, Block MS, Kalli KR, et al. Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol. 2017;3(12):e173290. doi:10.1001/jamaoncol.2017.3290
7. Swartz JE, Pothen AJ, van Kempen PM, et al. Poor prognosis in human papillomavirus-positive oropharyngeal squamous cell carcinomas that overexpress hypoxia inducible factor-1alpha. Head Neck. 2016;38(9):1338–1346. doi:10.1002/hed.24445
8. Patel NR, Jain L, Mahajan AM, Hiray PV, Shinde SS, Patel PA. An immunohistochemical study of HIF-1 alpha in oral epithelial dysplasia and oral squamous cell carcinoma. Indian J Otolaryngol Head Neck Surg. 2019;71(4):435–441. doi:10.1007/s12070-019-01597-y
9. Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi:10.1126/scitranslmed.3003689
10. Corrales L, Matson V, Flood B, Spranger S, Gajewski TF. Innate immune signaling and regulation in cancer immunotherapy. Cell Res. 2017;27(1):96–108. doi:10.1038/cr.2016.149
11. Li YM, Yu JM, Liu ZY, Yang HJ, Tang J, Chen ZN. Programmed death ligand 1 indicates pre-existing adaptive immune response by tumor-infiltrating CD8(+) T cells in non-small cell lung cancer. Int J Mol Sci. 2019;20(20). doi:10.3390/ijms20205138
12. Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019;12(1):92. doi:10.1186/s13045-019-0779-5
13. Sun JY, Zhang D, Wu S, et al. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: mechanisms, predictive factors, and future perspectives. Biomark Res. 2020;8:35. doi:10.1186/s40364-020-00212-5
14. Chen SW, Li SH, Shi DB, et al. Expression of PD-1/PD-L1 in head and neck squamous cell carcinoma and its clinical significance. Int J Biol Markers. 2019;34(4):398–405. doi:10.1177/1724600819884722
15. Solomon B, Young RJ, Bressel M, et al. Prognostic significance of PD-L1(+) and CD8(+) immune cells in HPV(+) oropharyngeal squamous cell carcinoma. Cancer Immunol Res. 2018;6(3):295–304. doi:10.1158/2326-6066.cir-17-0299
16. Lin YM, Sung WW, Hsieh MJ, et al. High PD-L1 expression correlates with metastasis and poor prognosis in oral squamous cell carcinoma. PLoS One. 2015;10(11):e0142656. doi:10.1371/journal.pone.0142656
17. Muller T, Braun M, Dietrich D, et al. PD-L1: a novel prognostic biomarker in head and neck squamous cell carcinoma. Oncotarget. 2017;8(32):52889–52900. doi:10.18632/oncotarget.17547
18. Zhou L, Cha G, Chen L, Yang C, Xu D, Ge M. HIF1alpha/PD-L1 axis mediates hypoxia-induced cell apoptosis and tumor progression in follicular thyroid carcinoma. Onco Targets Ther. 2019;12:6461–6470. doi:10.2147/ott.s203724
19. Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–790. doi:10.1084/jem.20131916
20. Chen H, Xia B, Zheng T, Lou G. Immunoscore system combining CD8 and PD-1/PD-L1: a novel approach that predicts the clinical outcomes for cervical cancer. Int J Biol Markers. 2019;1724600819888771. doi:10.1177/1724600819888771
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.