Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 13
Patterns of Childhood Tuberculosis Diagnosis in Hawassa University Comprehensive Specialized Hospital, Hawassa, Sidama Regional State, Ethiopia
Authors Taye K , Tolesa N, Tadewos A , Ketema W
Received 6 July 2022
Accepted for publication 31 October 2022
Published 9 November 2022 Volume 2022:13 Pages 349—359
DOI https://doi.org/10.2147/PHMT.S380092
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Roosy Aulakh
Kefyalew Taye,1 Nagasa Tolesa,1,2 Agete Tadewos,3 Worku Ketema1
1Department of Pediatrics and Child Health, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia; 2Department of Pediatrics and Child Health, College of Medicine and Health Sciences, Dembi Dollo University, Dembi Dollo, Ethiopia; 3School of Medical Laboratory Science, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia
Correspondence: Worku Ketema, Department of Pediatrics and Child Health, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia, Email [email protected]
Background: Because of the pauci bacillary nature of childhood tuberculosis and the difficulties in obtaining proper sputum samples from young children, diagnosing childhood tuberculosis (TB) is difficult. Childhood TB needs early identification and care since it advances swiftly to more advanced stages. This study was aimed to determine the patterns of all forms of childhood tuberculosis diagnosis at Hawassa University Comprehensive Specialized Hospital in Hawassa, Ethiopia.
Methods: A retrospective cross-sectional study was conducted from February 1, 2017 to January 30, 2021 among 175 children diagnosed and treated for tuberculosis in the pediatric ward. Children medical charts and pediatrics ward logbook were used to extract pertinent data by structured checklists. SPSS version 23.0 was used for data entry and statistical analysis.
Results: Of 175 children, fever was the leading clinical symptoms and diagnosed in 166 (94.9%) children followed by weight loss (154, 88%), and cough (136, 77.7%). In twenty seven out of 88 (30.6%) children, gastric aspirate was positive for TB infection by Xpert MTB/Rif, while 3/40 (7.5%) were positive for TB using fine needle aspiration cytology (FNAC), 19/66 (28.8%) had suggestive TB by cerebrospinal fluid analysis (CSF), 10/29 (34.5%) were smear positive for TB and 70/162 (43.2%) were suspected for TB by chest X-ray.
Conclusion: Despite recent breakthroughs in quick microbiological detection, such as Xpert MTB/Rif, this study revealed that more than half of the children, 89/175 (51%), were treated for TB diseases solely based on clinical criteria. This will significantly underestimate the true nature of the illness or disease and make them vulnerable to mistreatment. As a result, in order to appropriately treat the disease and manage patients in our settings, getting a microbiological diagnosis of childhood tuberculosis requires improvement, and we call for expanded availability and use of a more sensitive and specific diagnostic technique to circumvent these concerns.
Keywords: childhood, diagnosis, tuberculosis
Introduction
The World Health Organization (WHO) projects that 1.2 million (12%) of the 10 million people infected with tuberculosis (TB) each year are children under the age of 15.1 Each year, 230,000 children die of tuberculosis, with virtually all of these deaths happening among children who did not receive treatment.2 Children have a significant case detection gap as 56% of all children and 65% of children under the age of five years with tuberculosis are “missed” each year.3 This age group is at the greatest risk of getting serious forms of tuberculosis, and delays in diagnosis can result in death.3
Despite recent breakthroughs in rapid diagnostics, getting a microbiological diagnosis of tuberculosis in children continues to be a problematic. Young children often have trouble generating sputum and may have paucibacillary disease, which can make existing laboratory testing for tuberculosis diagnosis less useful and accurate. Negative laboratory test results cannot conclusively rule out tuberculosis in this age group3–5 As a result, clinical diagnosis continues to be crucial in the treatment of childhood tuberculosis. Physical examination, clinical history, contact history, radiography, response to treatment, and other assessments should be used in conjunction with available laboratory diagnostic testing to support and confirm a TB diagnosis in young children. TB programs must continue to prioritize improvements in frontline healthcare worker competence and confidence in clinically screening and diagnosing children with tuberculosis.2–4,6,7
The WHO’s global End TB initiative intends for a significant decrease in TB incidence and mortality, as well as for no affected households to experience calamitous consequences as a result of TB. The political declaration of the 2018 TB-UNHLM of the United Nations General Assembly underlines the worldwide resolve to abolish the TB pandemic by the year 2030. The adoption of the Post-2015 SDGs by Ethiopia, as well as the WHO’s end TB objectives and the aim of eradicating leprosy, underscores the need to speed up equitable, people-centered TB and leprosy (TBL) screening, diagnosis, prevention, care, and treatment services6,8
In Ethiopia, TB is still a major public health issue that poses considerable risks to the population’s health by harming its productive age groups, placing a heavy burden on the healthcare system, and undermining household and individual economies. With an anticipated TB incidence rate of 140/100,000 people (157,000 people annually) and 21,000 (19/100,000 population) TB fatalities in 2018, Ethiopia is still one of the 30 countries with the highest TB burden worldwide. From 421 per 100,000 people in 2000 to 140 per 100,000 people in 2019, the annual average TB incidence has decreased by 8–9%. In recent decades, Ethiopia has also seen a drop in TB mortality, but at a slower rate than the country’s decline in incidence.6 Rapid and sensitive molecular tests for tuberculosis and drug resistance have recently become in use to supplement the already in use conventional tests, and the WHO calls for using these rapid techniques as the initial diagnostic test for tuberculosis detection and rifampicin (RIF) resistance to circumvent delays in initiating the recommended management.6,7 The Ethiopian national tuberculosis control programmes has recently implemented these recommendations.3,4 Previous research mostly focused on tuberculosis prevalence, and data on childhood tuberculosis diagnosis patterns are scarce. This study will bring attention to this issue, and the findings will be valuable in reducing concerns regarding tuberculosis diagnosis in children, particularly the use of existing bacteriologic confirmatory tests at HUCSH, Sidama, Ethiopia.
Methods
Study Setting and Study Population
The study was carried out at the HUCSH, which is situated 273 kilometers south of Addis Ababa in Hawassa City, the regional seat of the SNNPR and Sidama. Lake Hawassa in the east, Oromia in the west, Wondogenet woreda in the north and east, and Shebadeno in the south of Ethiopia are the limits of Hawassa city. The area of the city administration, which is made up of 32 kebeles and eight sub cities, is 157.2 square meters (km2). The local administration also oversees thirteen public health clinics and three public hospitals. The Hawassa University Comprehensive Specialized Hospital (HUCSH) was constructed 16 years ago by the Regional Health Bureau. The hospital was designed to serve a 3.5–5-million-person population, but it today covers the whole Sidama, SNNPR, and Oromia areas (HUSCH human recourse office). More than 20 departments in this tertiary hospital offer clinical and academic services. Children treated for any kind of tuberculosis (TB) (diagnosed by a clinician or confirmed bacteriologically) between the ages of 1 month and 14 years were the subjects of this retrospective cross-sectional study, which was carried out between February 1, 2017, and January 30, 2021. The age ranges of the study participants were less than or equal to 5, 5 to 10, and 10 to 14 years. The age, gender, vaccination history, contact history, duration of contact, duration of antibiotics, signs and symptoms, TB, chest radiograph findings, brain computerized tomography (CT)/magnetic resonance index (MRI), and microbiological diagnosis like sputum smear and Xpert MTB/Rif were all extracted from the medical chart and pediatric ward registry for each study child. Any individual exhibiting symptoms and/or indicators suggestive of tuberculosis was considered a TB suspect, particularly those who had coughs lasting two weeks or more. Under-fives were classified as severely wasted if their mid-upper arm circumference (MUAC) was less than 11.5 cm and moderately wasted if their MUAC was greater than or equal to 12.5 cm based on WHO severe acute malnutrition guideline.9
Data Collection and Assessments
The study subjects’ socio-demographic, clinical, and microbiological data were obtained from medical records of all patients suspected with TB in the pediatric ward using predefined checklists. In addition, pediatric ward registration book was also examined in order to find study-related data.
Inclusion and Exclusion Criteria
All patients aged 0 to 14 years and diagnosed to have tuberculosis from February 1, 2017 to January 30, 2021 were eligible for the study. However, those whose records were incomplete for the assessment of the disease pattern, and those whose medical records were absent in the cards room during tracing were omitted from the study.
Definition of Terms
Definite/Proven Case of Tuberculosis
A patient with two positive sputum smears (one positive sputum smear is sufficient for HIV positive people) or a Mycobacterium tuberculosis culture. A patient with Mycobacterium tuberculosis complex diagnosed from a clinical specimen, either by culture or a newer approach such as a molecular line probe assay, is also considered a definitive case of tuberculosis.7,10–13
Childhood Tuberculosis
Is a person between the ages of 0 and 14 who was diagnosed with tuberculosis.7,10–13
Presumptive TB
A presumed TB patient is someone who has symptoms and/or indicators of tuberculosis, particularly a cough lasting two weeks or more, loss of appetite, weight loss and weakness.14
TB Close Contact
TB Close contacts were defined as individuals who shared airspace (household, social, workplace, or school settings) with an individual with pulmonary TB for at least 15 hours a week during 1 or more weeks, or for at least 180 hours or more during the period when the person was infectious.15–17
Ethical Clearance
The study was ethically approved by the Institutional Review Board (IRB) of Hawassa University’s College of Medicine and Health Sciences. The Academic and Clinical Director’s office then granted permission to review data from patients’ records. This study adhered to the standards outlined in the Helsinki Declaration and its subsequent amendments. The ethics committee waived the requirement for informed permission since the research would be impossible to execute without it, had critical social significance, and posed only minor risks to the study subjects. However, the study participants’ personal information was maintained as private as possible.
Statistical Analysis
The data were coded and entered into SPSS version 23 for statistical analysis. While categorical variables were reported as frequency and percentage, continuous quantitative data were expressed as means and standard deviation.
Results
Socio-Demographic Data
The study comprised 175 complete records of suspected tuberculosis patients. One hundred and five (60%) were males, and the remaining 70/175 (40%) were females with a mean age of 6.7 years with range of 0 to 14 years. About 103/175 (58.9%) were less than or equal to 5 years old, 45/175 (25.7%) were 5–10 years old and 27/175 (15.4%) were 10–14 years old. In terms of religion, 78/175 (44.6%) of the total participants were Protestant, followed by 55/175 (31.4%) who were Muslim (Table 1).
 |
Table 1 Socio-Demographic Characteristics of the Study Participants |
Clinical Features of the Study Participants
Fever was the most prevalent clinical manifestations, and documented in 166/175 (94.9%) of children, followed by weight loss 154/175 (88%) and cough 136/175 (77.7%). A positive history of contact was reported in 85/175 (48.6%), with 65/85 (76.5%) had contacts with TB patients and the rest had contacts with chronic coughers. The majority of the children 141/175 (80.6%) had received BCG vaccination, and 70/175 (40%) were initiated on anti-TB treatment within the first week of admission (Table 2). Of the total participants, 8/175 (4.6%) children were negative for HIV, and 71/175 (40.6%) were severely malnourished having mid-upper arm circumference (MUAC) <11.5cm, and 34/175 (19.4%) had bilateral edema. 10/29 (34.5%) of the children tested by AFB smear were PTB positive, while 27/88 (30.6%) children were diagnosed to have TB disease by Xpert MTB/Rif from gastric aspirate. The chest x-ray was performed for 162/175 (92.6%), and the most common view was the Anteroposterior (AP) view, which was performed for about 158/162 (97.5%). About 70/162 (43.2%) were suspected for TB by chest X-ray. Regarding tuberculous lymphadenitis, it was done for around 40/175 (22.9%), and of these, the FNAC result revealed TB lymphadenitis in only 3/40 (7.5%) of the cases. Cerebrospinal fluid (CSF) analysis was performed in about 66/175 (37.7%) of the cases, with a positivity rate of 19/66 (28.8%), whilst imaging (brain CT scan) was indicated in 52/175 (29.7%), with the scanning reading suggestive of TB meningitis in around 31/52 (59.6%) of the imaged patients (Tables 2 and 3).
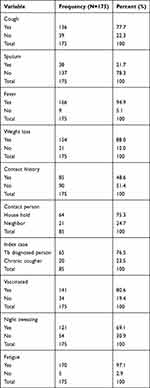 |
Table 2 Clinical Symptoms of the Study Participants |
 |
Table 3 Clinical Sign and Examination of the Study Participants |
Pattern of Tuberculosis in Relation to Some Variables
Smear positive TB, smear negative and clinically suspected TB were found in 19/29 (65.6%), 16/48 (33.3%) and 30/76 (39.5%) of female children, respectively. The majority of children aged less than or equal to 5 years had smear positive TB in about 17/29 (58.6%) of the cases, clinically suspected TB in about 46/76 (60.5%) of the cases (Table 4).
 |
Table 4 Pattern of Tuberculosis in Relation to Different Variables |
Discussion
In impoverished countries, such as Ethiopia, tuberculosis remains a major health issue. Five years’ worth of data were gathered for this study in order to examine the patterns in childhood TB diagnoses. According to the study, males had more TB than females. TB was found in 105 (60%) males and 70 (40%) females out of 175 subjects. This discovery of a high frequency of tuberculosis in males is consistent with findings from studies undertaken in India, Nepal, Kilimanjaro, Beijing, and Nigeria, all of which attribute their findings to higher male birth rates than females.13,18–22
In terms of religion, 78/175 (44.6%) of the total participants were Protestant, followed by 55/175 (31.4%) who were Muslim. The association between religion and tuberculosis has been extensively researched. Religion influences therapy, notably during the Muslim month of Ramadan, when they are not permitted to eat, drink, or take anything by mouth from dawn to dusk. As a result, recognizing religious barriers to accommodating religious or cultural issues affecting client service is necessary. As proven by several sources, religion, on the other hand, influences understandings of disease causation, screening (health seeking), a delay in seeking medical attention at hospitals, and immunization.23–25
Contact history was reported in 85/175 (48.6%) of the cases, of which 134/175 (76.5%) had contacts with TB patients and the rest had contacts with chronic coughers. However, contact history is less useful in countries with high TB endemicity.13,18 TB was discovered in 10/29 (34.5%) of cases and negative TB in 19/29 (65.5%) of cases, which is similar to this study in Nigeria and Indonesia.13 This could be due to the fact that the cavitary form of pulmonary tuberculosis is uncommon in children, with smear negative TB being more common in this age group.2,13,26
Since January 2020, the WHO has recommended Xpert MTB/Rif testing of stool samples as a primary diagnostic test for childhood pulmonary TB consideration.6 This approach, which is greatly being embraced by nationwide tuberculosis programmes such as Ethiopia, has the ability to enhance childhood tuberculosis bacteriological verification.3,4,7,14 Despite the fact that Xpert MTB/Rif was provided at no cost as part of the TB program, it was only used for 88/175 (50.3%) of the patients, with a 30.7% positivity rate. In this research, no one was diagnosed with rifampicin-resistant tuberculosis. This research’s use of Xpert MTB/Rif is significantly less than what is recommended (for all pediatric age groups)3–5,7 and also far less than another study done in Nigeria that reported rates (100%).13
However, the microbiological yield was modest (30.4%), which is consistent with earlier investigations and presumably reflects the paucibacillary nature of childhood tuberculosis.20,21
The suboptimal utilization of Xpert MTB/Rif might be because of shortages of reagents in the facility during the study period. It could also be due to a lack of a sufficient sample and the hassles experienced by caregivers due to the requirement that the patient fast for at least 6 hours. Furthermore, professionals’ preferences for clinical and/or imaging TB diagnosis will contribute to the underutilization of Xpert MTB/Rif. The low microbiologic yield in this study contrasted with the high yield seen in Marais et al study,18 with reasons mentioned for the high yield including rigorous inclusion criteria of only children with radiographic evidence of intrathoracic TB; carefulness in sample collection and processing; community-based approach; and the presence of advanced disease in most of their patients, but it was slightly higher than the study done in India.12,18,19,21 Furthermore, the disparity in TB detection rates between nations like Ethiopia may be influenced by the prevalence of the HIV epidemic, overpopulation, sensitivity variations in laboratory diagnostic techniques, and variability in the efficacy of prevention measures.2,5,12,18,19,27
Around 71 of 175 people (40.6%) had a mid-upper arm circumference (MUAC) of 11.5 cm or below, and 34/175 (19%) had bilateral edema. The correlation between tuberculosis and malnutrition has long been established. Undernutrition exacerbates TB, because undernutrition impairs immunity, increasing the chances of latent TB becoming an active disease. Malnutrition increases the chance of developing tuberculosis by threefold, putting a huge population at risk of developing the disease in high-TB-burden nations.2,6,22
Regarding tuberculous lymphadenitis, it was done for around 40/175 (22.9%), and of these, the FNAC result revealed TB lymphadenitis in only 3/40 (7.5%) of the cases, which is typically identified with fine-needle aspiration (FNA) of the lymph node and responds effectively to antituberculosis therapy, albeit the lymph nodes do not return to normal size for months or even years. A tuberculous adenitis diagnosis usually necessitates histologic or bacteriologic confirmation, which is best performed using FNA for culture, stain, and histology. If FNA fails to establish a diagnosis, an excisional biopsy of the affected node is recommended. Only about half the time does culture of lymph node tissue generate the organism.28–31
CSF analysis was performed in about 66/175 (37.7%) of the cases, with a positivity rate of 19/66 (28.8%), whilst imaging (brain CT scan) was indicated in 52/175 (29.7%), with the scanning reading suggestive of TB meningitis in around 31/52 (59.6%) of the imaged patients. Up to 20–50% of children will have a normal chest radiograph, and it demands a high level of suspicion on the part of the clinicians. The most essential laboratory test for tuberculous meningitis diagnosis is lumbar CSF investigation and culture. The leukocyte count in CSF typically varies from 10 to 500 cells/L. Although polymorphonuclears (PMNs) may be present at first, lymphocytes predominate in the vast majority of cases. CSF glucose levels are typically 40 mg/dL, but rarely 20 mg/dL. Because of the hydrocephalus and spinal block, the protein level is raised and may be exceptionally high (400–5000 mg/dL). The volume of the CSF sample is directly connected to the success of the microscopic inspection of acid-fast-stained CSF and mycobacterial culture. CSF and ADA polymerase chain reaction (PCR) testing can help in diagnosis.5,29–31
The diagnosis of tuberculous meningitis can be aided by radiographic examinations. Basilar enhancement and communicating hydrocephalus with signs of cerebral edema or early localized ischemia, which are more noticeable in the late stages of the illness, are the most common TB meningitis findings. One or more clinically silent tuberculomas, which most frequently form in the cerebral cortex or thalamus, are present in some small kids with tuberculous meningitis.30,31
In general, only a small percentage of children with tuberculosis are microbiologically proven in normal practices. Even for those children who are successfully screened, the inability to obtain a representative sample for confirmation diagnosis is a significant constraint. Children have more difficulty expectorating than adults and produce less sputum. Sputum is typically swallowed by kids, making it difficult to get high-quality sputum samples for laboratory tests to confirm TB diagnosis. Another constraint is the paucibacillary character of pulmonary tuberculosis in children versus adults, which makes existing TB diagnostic tests from respiratory samples less effective in children than in adults. Despite the availability of several diagnostic procedures, as discussed above, the availability of a suitable sample remains critical.19,32 It is strongly advised to increase the use of bacteriologic confirmation for the diagnosis of childhood tuberculosis, and all stakeholders must collaborate to narrow the aforementioned gaps.
This study has its own limitations. The fact that it is an analysis of retrospective records might make it prone to bias, and it is also confounded by incomplete records.
Conclusion
Despite recent breakthroughs in quick microbiological detection, such as Xpert MTB/Rif, this study found that more than half of the children, 89/175 (51%), were treated for TB diseases solely based on clinical criteria. This will significantly underestimate the true nature of the illness or disease and make them vulnerable to mistreatment. As a result, in order to appropriately treat the disease and manage patients in our settings, getting a microbiological diagnosis of childhood tuberculosis requires improvement, and we call for expanded availability and use of a more sensitive and specific diagnostic technique to circumvent these concerns.
Data Sharing Statement
All data are freely accessible.
Acknowledgments
We thank each person who helped with the completion of this paper.
Author Contributions
All authors greatly contributed to this paper, whether it be in the ideation, study design, and implementation, or data gathering, analysis, and interpretation, or in all of these areas. They also all participated in writing, revising, or critically reviewing the article and gave final approval of the version to be published. They also all agreed on the journal to which the article was submitted and agreed to be responsible for all aspects of the work.
Funding
This paper was not supported by any organization.
Disclosure
The authors affirm that they do not have any conflicts of interest in relation to this work.
References
1. World Health Organization. Global tuberculosis report 2020. Glob Tuberc Rep. 2020;2020:1.
2. Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health. 2017;5(9):e898–e906. doi:10.1016/S2214-109X(17)30289-9
3. Hajizadeh A, Lotfi T, Falzon D, et al. Recommendation mapping of the World Health Organization’s guidelines on tuberculosis: a new approach to digitizing and presenting recommendations. J Clin Epidemiol. 2021;134:138–149. doi:10.1016/j.jclinepi.2021.02.009
4. World Helath Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: xpert MTB. World Health Organization; 2013.
5. Atehortúa S, Ramírez F, Echeverri LM, Peñata A, Ospina S. Xpert MTB/RIF test performance assay in respiratory samples at real work settings in a developing country. Biomédica. 2015;35(1):125–130. doi:10.1590/S0120-41572015000100015
6. Ereso BM, Yimer SA, Gradmann C, Sagbakken M. Barriers for tuberculosis case finding in Southwest Ethiopia: a qualitative study. PLoS One. 2020;15(1):e0226307. doi:10.1371/journal.pone.0226307
7. Nathavitharana RR, Garcia-Basteiro AL, Ruhwald M, Cobelens F, Theron G. Reimagining the status quo: how close are we to rapid sputum-free tuberculosis diagnostics for all? EBioMedicine. 2022;78:103939. doi:10.1016/j.ebiom.2022.103939
8. Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385(9979):1799–1801. doi:10.1016/S0140-6736(15)60570-0
9. Guideline W. Updates on the management of severe acute malnutrition in infants and children. Geneva. 2013;2013:6–54.
10. Lawn SD, Kerkhoff AD, Nicol MP, Meintjes G. Underestimation of the true specificity of the urine lipoarabinomannan (LAM) point-of-care diagnostic assay for HIV-associated tuberculosis. J Acquir Immune Defic Syndr. 2015;69(4):e144. doi:10.1097/QAI.0000000000000672
11. Negash H, Legese H, Adhanom G, et al. Six years trend analysis of tuberculosis in Northwestern Tigrai, Ethiopia; 2019: a retrospective study. Infect Drug Resist. 2020;13:643. doi:10.2147/IDR.S239717
12. Yerramsetti S, Cohen T, Atun R, Menzies NA. Global estimates of paediatric tuberculosis incidence in 2013–19: a mathematical modelling analysis. Lancet Glob Health. 2022;10(2):e207–e15. doi:10.1016/S2214-109X(21)00462-9
13. Coghlan R, Gardiner E, Amanullah F, et al. Understanding market size and reporting gaps for paediatric TB in Indonesia, Nigeria and Pakistan: supporting improved treatment of childhood TB in the advent of new medicines. PLoS One. 2015;10(10):e0138323. doi:10.1371/journal.pone.0138323
14. Mafirakureva N, Klinkenberg E, Spruijt I, et al. Xpert Ultra stool testing to diagnose tuberculosis in children in Ethiopia and Indonesia: a model-based cost-effectiveness analysis. BMJ open. 2022;12(7):e058388. doi:10.1136/bmjopen-2021-058388
15. Chamie G, Kato-Maeda M, Emperador DM, et al. Spatial overlap links seemingly unconnected genotype-matched TB cases in rural Uganda. PLoS One. 2018;13(2):e0192666. doi:10.1371/journal.pone.0192666
16. Reichler MR, Khan A, Sterling TR, et al. Risk factors for tuberculosis and effect of preventive therapy among close contacts of persons with infectious tuberculosis. Clin Infect Dis. 2020;70(8):1562–1572. doi:10.1093/cid/ciz438
17. Reichler MR, Khan A, Sterling TR, et al. Risk and timing of tuberculosis among close contacts of persons with infectious tuberculosis. J Infect Dis. 2018;218(6):1000–1008. doi:10.1093/infdis/jiy265
18. Marais BJ, Gie RP, Schaaf HS, Beyers N, Donald PR, Starke JR. Childhood pulmonary tuberculosis: old wisdom and new challenges. Am J Respir Crit Care Med. 2006;173(10):1078–1090. doi:10.1164/rccm.200511-1809SO
19. Oludiran KA, Eziuka OR, Mayowa IO, Ajani BR, Kikelomo O. Bacteriology of childhood tuberculosis in Ibadan, Nigeria: a five-year review. Trop Med Health. 2008;2008:0808040014.
20. Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50(Supplement_3):S184–S94. doi:10.1086/651490
21. Thapa A, Gurung P, Ghimire G. Evaluation oF gene XPert mtb/riF assay For tHe detection oF mycobacterium tuberculosis in sPutum oF patients susPected oF pulmonary tuberculosis VisitinG national tuberculosis centre, tHimi, bHaKtaPur, Nepal. SAARC J Tuberc Lung Dis HIV/AIDS. 2016;13(1):16–22. doi:10.3126/saarctb.v13i1.16924
22. Gupta KB, Gupta R, Atreja A, Verma M, Vishvkarma S. Tuberculosis and nutrition. Lung India. 2009;26(1):9. doi:10.4103/0970-2113.45198
23. Abolaban H, Al Moujahed A. Muslim patients in Ramadan: a review for primary care physicians. Avicenna J Med. 2017;7(3):81–87. doi:10.4103/ajm.AJM_76_17
24. Mobolaji OI. Determinants of tuberculosis services acceptance among patients in Ibadan, Nigeria. Afr J Soc Work. 2014;4(1):1.
25. Tabong PT-N, Akweongo P, Adongo PB. Community beliefs about tuberculosis in Ghana: implications for the end tuberculosis global agenda. Cogent Med. 2021;8(1):1870069. doi:10.1080/2331205X.2020.1870069
26. Seddon JA, Shingadia D. Epidemiology and disease burden of tuberculosis in children: a global perspective. Infect Drug Resist. 2014;7:153. doi:10.2147/IDR.S45090
27. World Health Organization. Global tuberculosis report 2020. World Health Organization; 2020.
28. Berwal A, Chawla K, Vishwanath S, Shenoy VP. Role of multiplex polymerase chain reaction in diagnosing tubercular meningitis. J Lab Physicians. 2017;9(02):145–147. doi:10.4103/0974-2727.199633
29. Anderson ST, Kaforou M, Brent AJ, et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. N Eng J Med. 2014;370(18):1712–1723. doi:10.1056/NEJMoa1303657
30. Roberts I. Nelson’s textbook of pediatrics. In: Kliegman R, Stanton B, St J, Geme N, editors.
31. Loscalzo J, Fauci AS, Kasper DL, Hauser S, Longo D, Jameson JL. Harrison’s Principles of Internal Medicine. Vol. 1. McGraw Hill Professional; 2022.
32. Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet. 2005;365(9454):130–134. doi:10.1016/S0140-6736(05)17702-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
