Back to Journals » International Journal of General Medicine » Volume 14
Patients with Earlobe Crease May Associate with Lower Concentration of the Age-Suppressing Hormone Klotho
Authors Wang J, Zhu ZF, Liu FQ, Liu C, Ou-Yang AM, Chen WW, Wang EG, Wang XM
Received 23 September 2021
Accepted for publication 2 November 2021
Published 25 November 2021 Volume 2021:14 Pages 8797—8803
DOI https://doi.org/10.2147/IJGM.S300309
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jian Wang,1,* Zhan-Fang Zhu,2,* Fu-Qiang Liu,3 Cun Liu,4 Ai-Mei Ou-Yang,1 Wei-Wei Chen,1 En-Guo Wang,1 Xi-Ming Wang5,6
1Department of Radiology, Jinan Central Hospital Affiliated to Shandong University, Jinan, Shandong, 250013, People’s Republic of China; 2Department of Internal Medicine, The Hospital of Xi’an Jiaotong University, Xi’an, 710049, People’s Republic of China; 3Department of Cardiovascular, Shaanxi Provincial People’s Hospital, Xi’an, 710068, People’s Republic of China; 4Department of Ultrasound, Jinan Central Hospital Affiliated to Shandong University, Jinan, Shandong, 250013, People’s Republic of China; 5Department of Radiology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, 250021, People’s Republic of China; 6Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xi-Ming Wang Tel/Fax +86 531-68776846
Email [email protected]
Objective: Earlobe crease (ELC) has been considered as a skin sign of atherosclerosis, and its pathophysiological mechanism is still unclear. Our study aims to test the hypothesis that ELC patients with lower serum levels of the age-suppressing hormone Klotho, which is not only associated with premature aging but also with endothelial dysfunction, may be associated with atherosclerosis.
Methods: A total of 135 patients aged 40– 68 years underwent coronary angiography. According to the presence or absence of coronary heart disease (CAD) and ELC, they were divided into three groups: CAD group and ELC group (ELC group, n = 45); no ELC group (non-ELC group, n = 45). There was no ELC or CAD in the control group (control group, n = 45). Serum Klotho concentration was obtained by enzyme-linked immunosorbent assay (ELISA).
Results: The Klotho level in the ELC group was 365.6 ± 38.1 pg/mL, while the Klotho level in the non-ELC group was 568.8 ± 44.9 pg/mL. It is worth noting that the Klotho level of the ELC group was significantly lower than that of the non-ELC group (P < 0.001). The serum Klotho level of the control group was higher than that of the non-ELC group (593.3± 45.3 vs 568.8± 44.9 pg/mL, P = 0.702), but the difference was not statistically significant. Multiple logistic regression analysis showed that the Klotho level is a parameter that affects the appearance of ELC.
Conclusion: Serum Klotho levels were considerably lower in patients with ELC. We concluded that the perturbations of Klotho in patients might be associated with ELC and with CAD.
Keywords: earlobe crease, Klotho, atherosclerosis, premature aging
Introduction
Earlobe crease (ELC), also known as Frank’s sign, was originally identified by Frank in 1973.1 From a palaeopathological perspective, the first recorded evidence of this sign dates back to the classical world, in particular to Emperor Hadrian (76–138 CE).2 Two more cases from the Italian Renaissance (1431–1506) and the sculptures of Medici family have also been reported with the ELC.3,4 It is classified as a diagonal fold or wrinkle-like line extending from the tragus across the lobule to the rear edge of the auricle area of the ear. A considerable amount of epidemiological and meta-analysis studies has demonstrated that ELC is independently linked to coronary artery disease (CAD). Therefore, it has been recognized as a simple cutaneous marker in patients with CAD.5–14 The putative pathophysiologic mechanisms leading to such individual susceptibility remain unresolved.
Klotho, a 130-kD gene first recognized in 1997, encodes a novel protein, which is expressed in the renal distal tubular epithelial cell and is assumed to be a regulator of the human aging course.15,16 Klotho knockout mice (Klotho-/-) produce a phenotype similar to premature human aging, such as endothelial dysfunction, progressive atherosclerosis, and lifespan shortening.17 Circulating forms of Klotho (defined as soluble Klotho) and proteolytic cleavage of the extracellular domain of Klotho can be detected in blood and urine and cerebrospinal fluid.18 Soluble Klotho is related to the anti-apoptotic effect of vascular endothelial cells and cardiovascular protective properties.16,19–21 Recently, some clinical trials have shown that a lower concentration of soluble Klotho is independently correlated with a higher risk of cardiovascular morbidity and mortality.22–26
Because ELC was always regarded as a marker of premature degradation, we hypothesized that Klotho might be a bridge connecting ELC and CAD. Thus, we conducted a cross-sectional study to test the hypothesis that lower serum Klotho concentrations are associated with the presence of CAD in patients with diagonal earlobe crease.
Materials and Methods
Subjects
From May to October 2016, patients with ELC (n = 45) and without ELC (n = 45) who underwent coronary angiography and were afflicted with coronary heart disease in the Shaanxi Provincial People’s Hospital were enrolled as test subjects. Other patients (n = 45) without ELC and CAD were selected as the control group. Since the correlation between ELC and CAD is ambiguous in the patients older than 70, only patients younger than 70 were recruited. A brief medical questionnaire was administered to all the patients before the examination. The exclusion criteria were as follows: Severe chronic heart failure (New York Heart Association Grade 3 or higher), severe valvular heart disease, arrhythmia, chronic kidney disease, adrenal insufficiency or thyroid dysfunction, or malignant tumors, steroids, non-steroidal anti-inflammatory drugs or other immunosuppressive drugs. The study was conducted in accordance with the Declaration of Helsinki (revised in 2013). This study was approved by the Ethics Committee of Shaanxi Provincial People’s Hospital. Written informed consent was obtained from all participants.
Coronary Angiography
Coronary angiography was performed through the femoral artery or radial artery approach using the Judkins technique, and the recording rate is 15 frames/s. Significant CAD is defined as a stenosis of one or more major epicardial vessels exceeding 50%, and the control group is defined as a stenosis of each major epicardial vessel <50%.
Grading of ELC
In short, the earlobe structure is first drawn in a sitting position. the characteristics (eg, length, depth, width, and the number of creases) of each earlobe crease were recorded and evaluated. In each ear, a deep and clear ELC that extends completely through the earlobe is scored as 2 points, and an ELC that is marked as superficial or not completely through the earlobe is scored as 1 point, and 0 for ears in which no ELC was observed. The ELC score in each case represents the total score of both ears. The two-sided ELC in this study was defined as a score ≥3. If ELC is observed on at least one earlobe, we assign the patient to the ELC group (Figure 1). The two authors took digital photographs of the earlobes for independent evaluation later. With the participation of the third researcher, the differences were resolved through consensus.
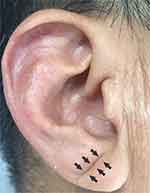 |
Figure 1 Typical example of the unilateral earlobe crease. The arrow shows earlobe crease. |
Biochemical Analyses
Fasting blood samples were collected on EDTA-aprotinin tubes and immediately placed in an ice bath. Serum lipids were measured via enzymatic procedures using an automated analyzer (AU 2700 Olympus, 1st Chemical Ltd., Japan), such as total cholesterol, triglycerides, and HDL-C. The Klotho concentration was determined using a validated sandwich ELISA with a Klotho specific antibody (Cusabio Biotech Co. Ltd., Wuhan, China). Five Klotho plasma samples were used to evaluate the intra- and inter-assay coefficients of variation, ranging from 3.5% to 4.7% (average 4.2%) and 4.8% to 6.2% (average 5.4%).
Statistical Analysis
Data are expressed as proportion n (%), ratio (A/B) and mean ± standard deviation (M ± SD). In addition, a one-way analysis of variance (ANOVA) was performed to calculate the difference between biochemical markers. Multivariate analysis was used to adjust age, gender, and BMI accordingly. We use multiple logistic regression analyses to determine the parameters that affect the appearance of the diagonal ELC. SPSS 16.0 for Windows was used for calculation. We use a two-tailed P value of <0.05 to evaluate the probability to describe statistical significance.
Result
Profiles of Study Subjects
Table 1 summarizes the population characteristics of all patients. The patients were similar in age, ranging from 40 to 68 years old (P>0.05). Risk factors for cardiovascular atherosclerosis, such as dyslipidemia, diabetes, obesity, and smoking status, were not significantly different between the groups. Although there was no significant difference in blood pressure, the ELC group used hypertension drugs more frequently.
 |
Table 1 Baseline Demographic and Clinical Characteristics of the Enrolled Patients |
As shown in Figure 2, the serum Klotho level in the ELC group was 365.6 ± 38.1 pg/mL, while the non-ELC group was 568.8 ± 44.9 pg/mL, and the ELC group was significantly lower than the non-ELC group (P < 0.001). The serum Klotho level of the control group was higher than that of the non-ELC group, but the difference was not statistically significant (593.3 ± 45.3 vs 568.8 ± 44.9 pg/mL, P = 0.702). Interestingly, when the patients were divided into two groups based on CAD, the serum Klotho level in the CAD group was significantly lower than that in the non-CAD group (CAD group, n = 90, 467.2 ± 31.2 pg/mL vs non-CAD group, n = 45, 593.3 ± 45.3 pg/mL, p = 0.02). After adjusting for age and gender, multiple logistic regression analysis showed that Klotho level is a parameter that affects the appearance of ELC (odds ratio = 0.997, 95% confidence interval (95% CI) = 0.996–0.999, P < 0.001). Therefore, a 100 pg/mL decrease in Klotho levels is associated with an average increase in the incidence of ELC by 30%.
 |
Figure 2 Comparison of serum Klotho levels between enrolled patients with and without diagonal ELC. Abbreviation: ELC, earlobe crease. |
Discussion
This research provides new insights for the development of ELC. Patients with ELC had significantly lower serum Klotho levels, indicating that the premature status may contribute to the progress of the disorder. In logistic regression analysis, a decrease of 100 pg/mL in Klotho level was associated with an average increase of 30% in the incidence of ELC.
Klotho is an age-suppressing protein that plays a role in many ways to control aging and decline during the aging process.27,28 The vascular phenotype of Klotho deficiency is characterized by endothelial dysfunction, progressive atherosclerosis, arteriosclerosis, and hypertension.15,29 In addition, the administration of recombinant soluble Klotho can prolong life and improve the phenotype associated with premature aging.30 Circulating Klotho is involved in maintaining endothelial integrity while also protecting against vascular permeability.18,31–33 Klotho could modulate endothelium-dependent dilation and increase excretion of nitric oxide metabolites, as well as ameliorate oxidative stress.29,34–37 Several studies have indicated that the disruption of Klotho homeostasis may be inseparable in the development of CAD. Navarro-González et al22 conducted a cross-sectional study, which reflected the lower soluble concentration of Klotho and the decrease of Klotho gene expression in the blood vessel wall. Kresovich and Bulka38 indicated that low serum klotho concentration is considered to be <666 pg/mL and that was found to be associated with a higher risk of death compared to higher klotho levels >985 pg/mL. In addition, the InCHIANTI (Aging in the Chianti Area) research, which involved 1023 subjects, observed that higher plasma Klotho concentrations were independently associated with a lower likelihood of cardiovascular disease among adults living in the community.23 Our study also found that the serum Klotho level in the CAD group is significantly reduced, consistent with previous studies, but the serum Klotho level seems to be maintained at a low level in all three groups. This may be because the three groups of patients in this study were not healthy people. The control group without ELC was not diagnosed with CAD, just because the results of coronary angiography are not sufficient to diagnose CAD. But the patients in control group were also suspected of having acute coronary syndrome for various reasons and accepted invasive coronary angiography. So from this perspective, the result of the serum Klotho level in our study is still consistent with the previous study. The ELC group had a significantly lower result than the non-ELC in the CAD group. If patients with ELC were excluded, no statistical significance was found between the CAD group and no CAD group. Therefore, we conclude that ELC, which was also an important predictive approach of CAD, may be associated with the perturbations of Klotho (Figure 3).
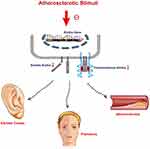 |
Figure 3 The potential pathophysiologic foundation for the relationship between ELC and CAD. Abbreviations: ELC, earlobe crease; CAD, coronary artery disease. |
A firm pathophysiologic foundation for the relationship between ELC and CAD was absent. Lucenteforte et al13 performed a meta-analysis, which included more than 31,100 subjects. This meta-analysis determined that 62% of patients with CAD have ELC, and the risk of CAD in patients with ELC was 3.3 times higher than that of the patients without ELC. A 35-years follow-up prospective cohort study of 10,885 people showed that ELC was associated with an increased risk of ischemic heart disease and myocardial infarction, but not with age and other cardiovascular risk factors.11 Meanwhile, a study conducted by Shoenfeld et al39 found that the biopsy specimens of the earlobe of ELC patients had some representative precocious variations, such as elastin degeneration, atrophic elastic fibers, and the thickness of the anterior artery wall. These were also observed in the coronary arteries of patients with CAD. Additionally, Higuchi et al40 demonstrated a shorter telomere length in the MetS patients with ELC, a useful indirect marker of an accelerated aging process.41 In the meantime, there are several systematic reviews disproving such association.42,43 There is also emerging research linking ELC to the mechanical traction secondary to visceral obesity of the face.44 Furthermore, congenital earlobe creases were notes in neonates with Beckwith-Wiedemann syndrome.45 Although the relationship between ELC and CAD still cannot be drawn a definite conclusion, for evidence which are for and against are both obtained, and the association between the ELC and atherosclerosis has not been established in this study. From our respective, we are more inclined to think that ELC is a sign of CAD, and consequently, we could hypothesize that some agents that accelerate premature degradation may act as the bridge, the Klotho, between ELC and CAD.
The advantage of this study is that subjects were recruited according to age matching, chronic kidney disease was excluded, and multiple logistic regression analysis was performed. Thus, confusion due to these exposures should be minimized. Moreover, coronary angiography was performed to define the CAD. However, a few limitations of the current analysis are worth discussing. First, the present study did not explore the differentiation of unilateral versus bilateral ELC, and the shapes of the ELC whether they were attached (soldered) or free were not focused on. Second, although the baseline demographic of the enrolled patients about the gender and age shows no statistical differences, it should be noticed that ELC group had the highest male/female ratio (M/F: 32/13) compared to controls (M/F: 24/21), and the participants of the ELC group were the oldest of all groups. For age and gender are strongly associated with the ELC, it may suggest that more sample size is needed. Additionally, the results may not be applicable to an unselected community population for all the participants are not healthy people. Further studies are required to validate our findings in a larger and more diverse sample. Moreover, although a statistical relationship appears to exist, the direct causal link between Klotho and ELC could not be clarified.
In conclusion, serum Klotho levels were significantly lowered in patients with ELC, and we provide a fresh insight into the putative mechanisms of ELC. We concluded that the perturbations of Klotho in patients might be associated with ELC and with CAD. Our findings may have potential clinical and public health implications.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article. The abstract of this article was presented at ESC Congress on 25–29 August, 2018 in Munich.
Funding
This study was supported by a grant from the Taishan Scholars Project (XM. Wang), Project 81871354 and 81571672 supported by NSFC, Academic Promotion Programme of Shandong First Medical University (2019QL023).
Disclosure
All authors declare no conflict of interest.
References
1. Frank ST. Aural sign of coronary-artery disease. N Engl J Med. 1973;289(6):327–328.
2. Petrakis NL. Diagonal earlobe creases, type A behavior and the death of Emperor Hadrian. West J Med. 1980;132(1):87–91.
3. Galassi FM, Borghi C, Ballestriero R, et al. Palaeopathology of the earlobe crease (Frank’s sign): new insights from renaissance art. Int J Cardiol. 2017;236:82–84. doi:10.1016/j.ijcard.2017.02.128
4. Bianucci R, Lippi D, Zucchini E, et al. Supplementary argumentations on authors’ response to Baudouin and Simon “The Medici earlobe crease”. Eur Ann Otorhinolaryngol Head Neck Dis. 2021;138(4):321–323. doi:10.1016/j.anorl.2021.04.012
5. Wu X-L, Yang D-Y, Zhao Y-S, et al. Diagonal earlobe crease and coronary artery disease in a Chinese population. BMC Cardiovasc Disord. 2014;14:43. doi:10.1186/1471-2261-14-43
6. Wang Y, Mao L-H, Jia E-Z, et al. Relationship between diagonal earlobe creases and coronary artery disease as determined via angiography. BMJ Open. 2016;6(2):e008558. doi:10.1136/bmjopen-2015-008558
7. Shmilovich H, Cheng VY, Rajani R, et al. Relation of diagonal ear lobe crease to the presence, extent, and severity of coronary artery disease determined by coronary computed tomography angiography. Am J Cardiol. 2012;109(9):1283–1287. doi:10.1016/j.amjcard.2011.12.024
8. Rodríguez-López C, Garlito-Díaz H, Madroñero-Mariscal R, et al. Earlobe crease shapes and cardiovascular events. Am J Cardiol. 2015;116(2):286–293. doi:10.1016/j.amjcard.2015.04.023
9. Evrengül H, Dursunoğlu D, Kaftan A, et al. Bilateral diagonal earlobe crease and coronary artery disease: a significant association. Dermatology. 2004;209(4):271–275. doi:10.1159/000080847
10. Tranchesi Júnior B, Barbosa V, de Albuquerque CP, et al. Diagonal earlobe crease as a marker of the presence and extent of coronary atherosclerosis. Am J Cardiol. 1992;70(18):1417–1420. doi:10.1016/0002-9149(92)90292-7
11. Christoffersen M, Frikke-Schmidt R, Schnohr P, et al. Visible age-related signs and risk of ischemic heart disease in the general population: a prospective cohort study. Circulation. 2014;129(9):990–998. doi:10.1161/CIRCULATIONAHA.113.001696
12. Elliott WJ, Powell LH. Diagonal earlobe creases and prognosis in patients with suspected coronary artery disease. Am J Med. 1996;100(2):205–211. doi:10.1016/S0002-9343(97)89460-0
13. Lucenteforte E, Romoli M, Zagli G, et al. Ear lobe crease as a marker of coronary artery disease: a meta-analysis. Int J Cardiol. 2014;175(1):171–175. doi:10.1016/j.ijcard.2014.04.025
14. Elliott WJ. Ear lobe crease and coronary artery disease: 1000 patients and review of the literature. Am J Med. 1983;75(6):1024–1032. doi:10.1016/0002-9343(83)90883-5
15. Kuro-O M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi:10.1038/36285
16. Nagai R, Saito Y, Ohyama Y, et al. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci. 2000;57(5):738–746. doi:10.1007/s000180050038
17. Lindberg K, Amin R, Moe OW, et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25(10):2169–2175. doi:10.1681/ASN.2013111209
18. Martín-Núñez E, Donate-Correa J, Muros-de-fuentes M, et al. Implications of Klotho in vascular health and disease. World J Cardiol. 2014;6(12):1262–1269. doi:10.4330/wjc.v6.i12.1262
19. Lewin E, Olgaard K. The vascular secret of Klotho. Kidney Int. 2015;87(6):1089–1091. doi:10.1038/ki.2015.80
20. Saito Y, Yamagishi T, Nakamura T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248(2):324–329. doi:10.1006/bbrc.1998.8943
21. Saito Y, Kuroo M, Nabeshima Y, et al. [The protective role of Klotho gene on vascular endothelium]. Nihon Rinsho Jpn J Clin Med. 1999;57(7):1514–1518. Japanese.
22. Navarro-González JF, Donate-Correa J, Muros de Fuentes M, et al. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart. 2014;100(1):34–40.
23. Semba RD, Cappola AR, Sun K, et al. Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59(9):1596–1601. doi:10.1111/j.1532-5415.2011.03558.x
24. Keles N, Caliskan M, Dogan B, et al. Is low serum Klotho level associated with alterations in coronary flow reserve? Echocardiography. 2016;33(6):881–888. doi:10.1111/echo.13176
25. Su X-M, Yang W. Klotho protein lowered in elderly hypertension. Int J Clin Exp Med. 2014;7(8):2347–2350.
26. Keles N, Caliskan M, Dogan B, et al. Low serum level of Klotho is an early predictor of atherosclerosis. Tohoku J Exp Medicine. 2015;237(1):17–23. doi:10.1620/tjem.237.17
27. Kim J-H, Hwang K-H, Park K-S, et al. Biological role of anti-aging protein Klotho. J Lifestyle Med. 2015;5(1):1–6. doi:10.15280/jlm.2015.5.1.1
28. Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36(2):174–193. doi:10.1210/er.2013-1079
29. Chung C-P, Chang Y-C, Ding Y, et al. α-Klotho expression determines nitric oxide synthesis in response to FGF-23 in human aortic endothelial cells. PLoS One. 2017;12(5):e0176817. doi:10.1371/journal.pone.0176817
30. Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–1833. doi:10.1126/science.1112766
31. Markiewicz M, Panneerselvam K, Marks N. Role of Klotho in migration and proliferation of human dermal microvascular endothelial cells. Microvasc Res. 2016;107:76–82. doi:10.1016/j.mvr.2016.05.005
32. Mencke R, Hillebrands J-L. The role of the anti-ageing protein Klotho in vascular physiology and pathophysiology. Ageing Res Rev. 2017;35:124–146. doi:10.1016/j.arr.2016.09.001
33. Saito Y, Nakamura T, Ohyama Y, et al. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276(2):767–772. doi:10.1006/bbrc.2000.3470
34. Yao Y, Wang Y, Zhang Y, et al. Klotho ameliorates oxidized low density lipoprotein (ox-LDL)-induced oxidative stress via regulating LOX-1 and PI3K/Akt/eNOS pathways. Lipids Health Dis. 2017;16(1):77. doi:10.1186/s12944-017-0447-0
35. Maekawa Y, Ishikawa K, Yasuda O, et al. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35(3):341–346.
36. Wang Y, Kuro-O M, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell. 2012;11(3):410–417. doi:10.1111/j.1474-9726.2012.00796.x
37. Richter B, Haller J, Haffner D, et al. Klotho modulates FGF23-mediated NO synthesis and oxidative stress in human coronary artery endothelial cells. Pflugers Arch. 2016;468(9):1621–1635. doi:10.1007/s00424-016-1858-x
38. Kresovich JK, Bulka CM. Low serum klotho associated with all-cause mortality among a nationally representative sample of American adults. J Gerontol Series a Biol Sci Med Sci. 2021. doi:10.1093/gerona/glab308
39. Shoenfeld Y, Mor R, Weinberger A, et al. Diagonal ear lobe crease and coronary risk factors. J Am Geriatr Soc. 1980;28(4):184–187. doi:10.1111/j.1532-5415.1980.tb00514.x
40. Higuchi Y, Maeda T, Guan J-Z, et al. Diagonal earlobe crease are associated with shorter telomere in male Japanese patients with metabolic syndrome. Circ J. 2009;73(2):274–279. doi:10.1253/circj.CJ-08-0267
41. Samani NJ, Boultby R, Butler R, et al. Telomere shortening in atherosclerosis. Lancet. 2001;358(9280):472–473. doi:10.1016/S0140-6736(01)05633-1
42. Davis TM, Balme M, Jackson D, et al. The diagonal ear lobe crease (Frank’s sign) is not associated with coronary artery disease or retinopathy in type 2 diabetes: the Fremantle Diabetes Study. Aust N Z J Med. 2000;30(5):573–577. doi:10.1111/j.1445-5994.2000.tb00858.x
43. Więckowski K, Gallina T, Surdacki A, et al. Diagonal earlobe crease (Frank’s sign) for diagnosis of coronary artery disease: a systematic review of diagnostic test accuracy studies. J Clin Med. 2021;10(13):2799. doi:10.3390/jcm10132799
44. Abrahim M. The pleating effect explains the cardioauricular connection. J Oral Maxillofac Surg. 2021;79(2):273. doi:10.1016/j.joms.2020.10.011
45. Tobias JD, Lowe S, Holcomb GW
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
