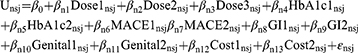Back to Journals » Patient Preference and Adherence » Volume 16
Patients’ Preferences for Sodium-Glucose Cotransporter 2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists
Authors Banjara B , Poudel N , Garza KB, Westrick S , Whitley HP , Redden D, Ngorsuraches S
Received 19 October 2022
Accepted for publication 15 December 2022
Published 28 December 2022 Volume 2022:16 Pages 3415—3428
DOI https://doi.org/10.2147/PPA.S391719
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Bidur Banjara,1,2 Nabin Poudel,1 Kimberly B Garza,1 Salisa Westrick,1 Heather P Whitley,3 David Redden,4 Surachat Ngorsuraches1
1Department of Health Outcomes Research and Policy, Auburn University, Harrison College of Pharmacy, Auburn, AL, USA; 2Cytel Inc, Waltham, MA, USA; 3Department of Pharmacy Practice, Auburn University, Harrison College of Pharmacy, Auburn, AL, USA; 4Department of Biomedical Affairs and Research, Auburn University, Edward via College of Osteopathic Medicine, Auburn, AL, USA
Correspondence: Surachat Ngorsuraches, Department of Health Outcomes Research and Policy, Auburn University, Harrison College of Pharmacy, 4306A Walker Building, Auburn, AL, 36849, USA, Tel +1 334 844 8357, Fax +1 334 844 8307, Email [email protected]
Purpose: To determine patients’ preferences for sodium-glucose cotransporter 2 inhibitors (SGLT-2is) and glucagon-like peptide-1 receptor agonists (GLP-1RAs).
Patients and Methods: A cross-sectional, web-based discrete choice experiment was conducted among US adults with type 2 diabetes mellitus (T2DM) in May 2021. Six attributes—the route and frequency of administration, the chance of reaching target HbA1c in six months, the percentage reduction in the risk of major adverse cardiovascular events (MACE), the chance of gastrointestinal side effects, the chance of genital infection, and out-of-pocket cost per month—were identified from literature review and consultation with patients and clinicians. A Bayesian efficient design was used to generate choice sets. Each choice set contained two hypothetical SGLT-2i and GLP-1 RA alternatives described by the attributes and an opt-out alternative. A total of 176 patients were asked to select the most preferred option from each choice set. Mixed logit (ML) and latent class (LC) models were developed. The conditional relative importance of each attribute was determined.
Results: The ML model showed the out-of-pocket cost had the highest conditional relative importance, followed by the chance of reaching the target HbA1c. The best LC model revealed two patient classes. All attributes were significantly important to the patients in both classes, except the chance of genital infection in class 2. Compared to the patients in class 2, the patients in class 1 were older (approximately 65 vs 56 years) and had a higher number of comorbidities (approximately three vs two).
Conclusion: T2DM patients placed different preference weights or importance across SGLT-2i and GLP-1 RA attributes. Preference heterogeneity was found among patients with different ages and numbers of comorbidities.
Keywords: diabetes, discrete choice experiment, patient preference, second-line antihyperglycemic agents
Introduction
Diabetes Mellitus (DM) affects millions of people around the world.1–3 In the US, over 34.2 million people had DM in 2018, with type 2 DM (T2DM) affecting the majority (90–95%).4 Studies showed that controlling blood glucose decreases microvascular complications and thus reduces related morbidity.5–7 The 2022 Standards of Medical Care in Diabetes emphasize comorbidities and a patient-centered approach to select first-line and subsequent therapies for treating T2DM.8 For individuals with T2DM with atherosclerotic cardiovascular disease (ASCVD), high-risk factors for ASCVD, heart failure (HF), and/or chronic kidney disease (CKD), sodium-glucose cotransporter-2 inhibitors (SGLT2is) or glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are preferentially recommended above other therapeutic antihyperglycemic options.8
In addition to lowering blood glucose, many clinical studies have demonstrated that SGLT-2is and GLP-1 RAs provide cardiovascular (CV) protection.9–12 For instance, Lo et al found that SGLT-2is significantly reduced the risk ratio (RR) of major adverse cardiovascular events (MACE), ie, cardiovascular death, stroke, or myocardial infarction, by 7%, reduced CV death by 11%, reduced RR for heart failure hospitalizations by 29%, and lowered RR for all-cause mortality by 10%.13 Similarly, another study reported that GLP-1 RAs reduced the hazard ratio (HR) for MACE by 12%, for all-cause mortality by 12%, and hospital admission from heart failure by 9%.14 Given the collective benefits along with the decrease in cardiovascular risks, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) updated their treatment guidelines for T2DM populations with high-risk to advise preferential use of SGLT-2is and GLP-1 RAs.15 Similarly, diabetes, cardiorenal, and/or metabolic diseases (DCRM) multispecialty practice recommended using SGLT-2is and long-acting GLP1-RAs among patients with T2DM with established or at high risk for ASCVD, CKD and/or HF to decrease the corresponding risks independent of their effects on glucose.16
The American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE) developed the comprehensive T2DM management algorithm.17 A central theme of the AACE/ACE algorithm is patient-centered diabetes therapies, which are also mirrored by the ADA.15 However, incorporating patients’ preferences of the newer antihyperglycemic agents (AHAs) in treatment decisions remains a challenge since these AHAs have a wide variety of treatment attributes, including benefits, risks, and route and frequency of administration.18–20 Also, while both GLP-1 RAs and SGLT-2is provided additional benefits, their costs were higher than the costs of other AHAs.20 A recent study reported that the median retail prices for a one-year supply of these two drug groups across Medicare Part D prescription drug plans in 2019 ranged from $3600 to $11,304, and the average beneficiary could spend at least $1000 annually for an SGLT-2is and higher than $1500 for a GLP-1 RA.21 Clinicians, therefore, are challenged to balance the benefits, risks, and costs of these treatments. To our best knowledge, the patient’s preferences for SGLT-2is and GLP-1 RAs have not been previously explored in the US Therefore, the objective of this study was to determine patients’ preferences for SGLT-2is and GLP-1 RAs.
Methods
A cross-sectional, web-based discrete choice experiment (DCE) questionnaire survey was used. This study design followed a DCE user’s guide and two reports from the ISPOR Good Research Practices Task Force.22–24 The Institutional Review Board (IRB) of Auburn University approved this study (Protocol number: 21–035 EX 2101) and is compliant with the Declaration of Helsinki. Informed consent was obtained from all participants using a script in accordance with the Auburn University IRB.
Selection of Study Attributes and Levels
The ISPOR good research practices were used to guide the elicitation of the attributes related to the AHAs that were important to patients.23 First, benefits and risks associated with SGLT-2is and GLP-1 RAs were obtained from the literature.13,14,25–28 These attributes of SGLT-2is and GLP-1 RAs were then consolidated with a) the results from in-depth interviews with five T2DM patients and b) the results of a best-worst scaling (BWS) object case study that asked 99 patients to rank the importance of the attributes for all second-line AHAs.29 As a result, this DCE study included a total of six attributes, ie, the route and frequency of administration, the chance of reaching target HbA1c (long-term blood glucose level) in six months, percentage (%) reduction in the risk of MACE (eg, heart attack, stroke, and death due to cardiovascular diseases), the chance of gastrointestinal (GI) side effects (eg, nausea, vomiting, and diarrhea), the chance of genital infection, and out-of-pocket cost per month. Since DCE should sufficiently vary the relevant attribute levels for modeling, an extreme range of attribute levels was recommended.24 This study identified the extreme ranges of the levels of all attributes, except the cost attribute, from the clinical literature.9–14 The highest and lowest wholesale acquisition costs (WAC) of SGLT-2is and GLP-1 RAs were used to determine the extreme range of the levels of cost attribute. These levels of attributes were equally spaced as much as possible for design purposes.22 The attributes and levels were confirmed by a clinical pharmacist who has provided DM care for over a decade. Table 1 shows the attributes and their levels included in this study.
 |
Table 1 Selected Attributes and Levels for the Discrete Choice Experiments (DCE) Survey Instrument |
Instrument Development
A self-administered, web-based questionnaire survey was developed. Primarily, the survey consisted of three sections, including questions about patients’ characteristics, their T2DM-related experience, and DCE choice sets. A single open-ended question about the patient’s experience taking the survey was also included. By using Ngene® software, an orthogonal and balanced design was used to develop DCE choice sets that were piloted with 30 patients. A multinomial logit model (MNL) was developed to produce prior parameters. Then, a Bayesian efficient design used the prior parameters to generate 36 choice sets from the list of attributes and their levels.30 These choice sets were divided into four blocks. Each block comprised nine choice sets and was used to develop one version of the questionnaire survey. Each choice set contained two unlabeled treatment alternatives described by the SGLT-2is’ and GLP-1 RAs’ attributes with various levels and an opt-out alternative. Graphics were also included to enhance understanding of all attributes and levels. For the % reduction in the risk of MACE, baseline risk was also included. Patients were asked to choose one of these alternatives in each choice set if their initial treatment (ie, metformin) alone did not help them achieve their treatment goal. Figure 1 shows an example of a choice set. Another choice set, which contained a dominant alternative (eg, highest chance of reaching target HbA1c, lowest GI side effect, and lowest cost), was added for a validity check. Patients who understood the DCE choice sets were expected to choose the dominant alternative of this choice set. Detailed instruction, the description of attributes and the meanings of their value, and one example of DCE choice sets were provided to ensure the patient’s understanding of the survey. A clinical expert and a survey expert reviewed the survey before it was piloted with 30 patients through the QualtricsXM panel. All responses to the single open-ended question for the patient’s experience of taking the survey did not indicate any difficulty in completing the survey. No major problem was identified.
 |
Figure 1 An example of a DCE choice set. Abbreviation: HbA1c, glycated hemoglobin. |
Data Collection
Patients aged 19 years or older, diagnosed with T2DM, and proficient in English were recruited through a national, online QualtricsXM panel, a market research company. Various approaches, including a good DCE research practice31 and a published practical guide,32 were used to determine the sample size in this study. Based on the approach from the published practical guide, a minimum of 156 patients were required for this study when the significance level was set at 0.05, and the statistical power level was 80%. The survey was launched in May 2021. One version of the questionnaire survey was randomly presented to each patient. Finally, a total of 176 patients agreed to participate in the survey and completed the survey. All of them correctly responded to the validity choice set.
Data Analysis
Descriptive analyses of patients’ characteristics and experiences with T2DM were conducted. The following utility function that individual n derived from alternative j in choice set s was developed. Nlogit® software was used to analyze the data.33
where β0 was the constant reflecting patients’ preferences for using an SGLT-2i or a GLP-1 RA relative to no treatment. βn1, βn2, βn3,…, βn13 were the coefficients or preference weights of the effect codes of the route and frequency of administration (Dose), the chance of reaching target HbA1c (HbA1c), the % reduction of the risk of major adverse cardiovascular events (MACE), the chance of gastrointestinal side effects (GI), the chance of a genital infection (Genital), the out-of-pocket cost per month (Cost), respectively, and εnsj was an error term. A mixed logit (ML) model was developed to estimate means and standard deviations of normally distributed variations for all preference weight estimates. Likelihood ratio tests were used to determine the final model. A Wald test was used to test for differences between adjacent levels of the study attributes. The level of statistical significance was set at 0.05. The difference of the preference weights between the highest and lowest preference weights of the same attribute was calculated to determine the conditional relative importance of each study attribute.
A latent class (LC) model was developed to examine preference heterogeneity. The LC model identified patient classes with similar preferences, and it estimated the probability of each patient in each class. Consistent Akaike Information Criterion (CAIC) value was used to determine the optimal number of classes of patients. Based on clinical reasoning, various covariates, eg, age, gender, comorbidity, years of T2DM experience, HbA1c level, and experience with SGLT-2is and GLP-1 RAs, were tested and incorporated or omitted in the LC model for predicting class membership. Their significance levels were set at 0.05. The class probability model from the LC model was used to assign the class membership. The class membership was based on the highest probability of the patients.
Results
Table 2 shows the characteristics and DM-related experiences of the patients. Their average age was 60.4 (SD=13.7) years old. The majority of them were White, non-Hispanic (86.9%), and female (54.6%). Almost half of them were retired (48.9%), reported an annual household income of $50,000 or higher (48.9%), and had Medicare for health insurance (49.5%). Approximately 47% of the patients had one or two comorbidities. The average number of years of T2DM diagnosis was about 13 years. Approximately 76% reported their HbA1c level, and the average % of HbA1c was 7.5. Less than half of the patients had previously used either SGLT-2is or GLP-1 RAs.
 |
Table 2 Participant’s Characteristics and T2DM Experiences |
Preference Weights of the SGLT-2i and GLP-1 RA Attributes
Based on the ML model, the significant alternative specific constant of this model was 1.64, showing that when the initial treatment (ie, metformin) alone did not help patients with T2DM achieve their treatment goal, they preferred using the treatment alternatives described by the SGLT-2is or GLP-1 RAs attributes, compared to the opt-out alternative. Figure 2 shows the preference weights of the attributes of SGLT-2is and GLP-1 RAs from the ML model. The estimated preference weights for all attributes had expected directions. Specifically, the preference weight for the oral, once-daily treatment was greater than for any injectable treatment. Also, the preference weights were greater for the SGLT-2is and GLP-1 RAs offering a higher chance of reaching target HbA1c, higher % reduction of the risk of MACE, lower chance of GI side effects, lower chance of a genital infection, and lower out-of-pocket cost per month.
While all adjacent levels of the chance of reaching target HbA1c and the out-of-pocket cost per month attributes were significantly different from one another, only some adjacent levels of the route and frequency of administration, the percentage reduction of MACE, and the chance of GI side effects were significant. The preference weight of the injectable, twice a day treatment was significantly lower than the injectable, once-daily treatments. Compared with a 0% reduction of MACE, patients preferred a 20% reduction of MACE. However, there was no significant difference between the SGLT-2is and GLP-1 RAs offering a 20% reduction and a 40% reduction of MACE. Only the difference between the preference weights of treatments causing a 30% chance and a 15% chance of GI side effects was significant for the chance of GI side effects. Also, only the difference between the preference weights of treatments causing an 8% chance and a 0% chance of genital infection was significant. Preference heterogeneity was found through significant standard deviations of the preference weights of all attributes. The conditional relative importance estimate of the out-of-pocket cost attribute was the highest (2.2), followed by the chance of reaching target HbA1c in six months (1.4), the route and frequency of administrations (0.8), % reduction in the risk of MACE (0.7), the chance of GI side effects (0.6), and the chance of genital infection (0.4).
Based on the CAIC values, the best LC model revealed two patient classes with different preferences. Only age and the number of comorbidities were significant in the class probability model of the final LC model. A total of 86 patients were assigned to class 1, and 90 patients were to class 2. The average ages of the patients in classes 1 and 2 were 64.7 and 56.2 years old, respectively. The average numbers of comorbidities of the patients in class 1 and class 2 were 3.1 and 2.0, respectively. Figure 3 shows the preference weights of attributes of SGLT-2is and GLP-1 RAs for the patients in these two classes. Both classes showed significant alternative specific constants (−1.2 and 2.6 for classes 1 and 2, respectively), indicating that only patients in class 2 preferred using the treatment alternatives described by the SGLT-2is or GLP-1 RAs attributes, compared to the opt-out alternative. Similar to the ML results, all attributes in both classes primarily had expected directions. For the patients in class 1, all adjacent levels of the chance of reaching target HbA1c, the chance of genital infection, and the out-of-pocket cost per month attributes were significantly different from one another. On the other hand, only the preference weight of the injectable, once a week treatment was significantly lower than the oral, once-daily treatment, and the preference weight of the injectable, twice a day was lower than the injectable, once-daily treatment. Only the difference between the preference weights of treatments offering a 20% reduction and a 0% reduction of MACE was significant. Also, there was a significant difference between the SGLT-2is and GLP-1 RAs causing a 30% chance and a 15% chance of GI side effects. For the patients in class 2, only the preference weight of the injectable, twice a day treatment was significantly lower than the injectable, once-daily treatments. Also, only the differences between the preference weights for SGLT-2is and GLP-1 RAs offering a 50% chance and a 10% chance of reaching target HbA1c, for treatments offering a 20% reduction and a 0% reduction of MACE, for treatments causing a 15% chance and a 1% chance of GI side effects, and for treatments with $500 and $1000 out-of-pocket per month were significant. On the other hand, no adjacent level of the chance of genital infection was significant.
In class 1, the conditional relative importance estimate of the out-of-pocket cost attribute was the highest (3.3), followed by the chance of reaching target HbA1c in six months (1.7), the route and frequency of administration (1.2), the chance of genital infection (0.9), % reduction in the risk of MACE (0.7), and the chance of GI side effects (0.6). In class 2, the highest conditional relative importance estimate was the out-of-pocket cost attribute (0.9), followed by the chance of reaching target HbA1c in six months (0.7), the chance of GI side effects, % reduction in the risk of MACE, and the route and frequency of administrations (0.4), and the chance of genital infection (0.2).
Discussion
This study used a DCE to examine patients’ preferences for SGLT-2is and GLP-1RAs as the second-line AHAs. While the average age of the patients in this study was similar to the patients from a national survey, the gender, race, and educational background were different.34 This study included higher percentages of males and Caucasians. Also, the patients in this study tended to have a higher education background. Their average years of T2DM experience, ability to report HbA1c levels, and positive responses to the single open-ended question regarding their experiences of taking the survey reflected that they understood T2DM and treatments and responded to the survey in this study well.
The study carefully selected treatment attributes by using the BWS results, a literature review, and interview responses from patients with T2DM and a clinical pharmacist. Overall, this study showed that the AHA attributes, including the route and frequency of administration, the chance of reaching target HbA1c in six months, % reduction in the risk of MACE, chances of GI side effects, chances of genital infection, and out-of-pocket cost per month, had expected influences on patients’ preferences. The findings of ML and LC models revealed preference heterogeneity among patients with T2DM in this study.
Overall, the intuitive findings from the ML model showed that the patients preferred using SGLT-2is or GLP-1RAs rather than opting out of a second-line AHA treatment when their first-line treatment (ie, metformin) could not help them achieve the treatment goal. The patients preferred higher benefits (ie, the chance of reaching target HbA1c in six months and % reduction in the risk of MACE), lower risks (ie, the chance of GI side effects, chance of genital infection), and lower cost of the SGLT-2is and GLP-1 RAs. Also, the coefficients of the route and frequency administration were sensible since the patients more often preferred the oral, once-daily treatment option. While these findings were generally consistent with the results of previous studies on T2DM patients’ preferences,35–43 the relative importance of these attributes could not be compared directly because the set of attributes and their levels were different.
The conditional relative importance estimates from the ML model showed that the patients weighed the study attributes differently. For instance, an increase in the chance of reaching target HbA1c in six months from 10% to 90% had a relative importance of approximately 1.4, while a decrease in the chance of GI side effects from 30% to 1% had a relative importance of 0.6 and a decrease in the chance of genital infection side effect from 16% to 0% had a relative importance of 0.4. Therefore, this change in the chance of reaching target HbA1c in six months was approximately two and three times more important than the changes in the chances of GI side effects and genital infection, respectively, in this study. Similarly, an increase in the percent reduction in the risk of MACE from 0% to 40% was approximately 1.2 to 1.8 times more important than the changes in the chances of GI side effects from 30% to 1% and genital infection side effect from 16% to 0%. This demonstrated that patients weighed the cardiovascular benefits more important than the chances of experiencing a GI or genital adverse event. It was noteworthy that a decrease in the patients’ out-of-pocket costs per month for these treatments to $0 had the highest impact on the patients’ preferences, compared to any changes between all levels of each attribute. Also, the relative importance of the route and frequency of administration reflected that this attribute might be as or more important than other benefits and risks of these treatments. These findings were consistent with previous studies that indicated the route and frequency of administration were significant while determining the T2DM patients’ preferences.36,37,41,42 Moreover, the existing preference heterogeneity in the ML model indicated that the patients with various (observed and unobserved) characteristics and underlying conditions might weigh the importance of these attributes differently.
The LC model identified two patient classes with different preferences for SGLT-2is and GLP-1 RAs. The patients in class 1 were approximately ten years older with three versus two comorbidities than those in class 2. Interestingly, based on the significant alternative specific constants of these two classes, the patients in class 1 or the older patients with a higher number of comorbidities did not prefer the addition of the second-line AHA treatments such as SGLT-2is and GLP-1 RAs, while the patients in class 2 did. One of the reasons could be that the patients in class 1 had already used several treatments for their comorbidities and did not want to add more treatments. The preference weights across all study attributes and levels in these two patient classes differed. The conditional relative importance estimates of these two classes also indicated that the preference weights for the patients in class 1 tended to vary more than the preference weights for the patients in class 2 when the levels of the study attributes changed. These results implied that the patients in class 1 were more selective in choosing the preferred SGLT-2is or GLP-1 RAs.
All study attributes, except the genital infection, had at least one level that was significantly important to the patients in both classes. The genital infection was not an important side effect to the patients in class 2, who tended to be younger patients. These findings were consistent with a recent review indicating that older adults, who tended to be in class 1 in this study, tolerated newer T2DM treatments well, except for the increased risk of genital infections from using SGLT-2is.44
The patients in class 1 preferred the oral, once-daily treatments to the injectable, once a week treatments, and they preferred the injectable, once-daily treatments to the injectable, twice a day treatments. These findings were somewhat similar to a study showing that patients preferred oral, once-daily treatments, but their preferences for oral, once-daily treatments and injectable, once a week treatments were comparable after the patients were informed.45 On the other hand, the patients in class 2 seemed to have indifferent preferences for the oral, once-daily, injectable, once a week, and injectable, once-daily treatments. In other words, the patients in class 2 were more flexible about the route and frequency of administration. One reason could be that they only needed to administer fewer treatments since they had a lower number of comorbidities.
Interestingly, while the patients in both classes preferred the higher level of the chance of reaching target HbA1c, only the difference between the preference weights of SGLT-2is and GLP-1 RAs offering a 50% chance and a 0% chance of reaching target HbA1c was significant for the patients in class 2. It was possible that their target HbA1c level was more achievable than the target of the patients in class 1. Also, the patients in both classes preferred the treatments with lower out-of-pocket costs. However, for the patients in class 2, only the difference between the preference weights for treatments with $500 and $1000 out-of-pocket per month was statistically significant. These results implied that if the out-of-pocket payment per month was under a certain amount, this payment might not affect the preferences of the younger patients or patients who had a lower number of comorbidities. It was also noteworthy that the out-of-pocket payment in this study was used to only determine the relative importance of the cost attribute compared to other attributes.
The patients in both classes had similar preferences for the percent reduction in the risk of MACE. Only the difference between the preference weights of treatments offering a 20% reduction and a 0% reduction of MACE was significant. One reason was that the patients might consider the reduction in the risk of MACE as only an additional benefit of these treatments that had a limited impact on their preferences. On the other hand, the patients in both classes had different views on the GI side effects. While only the difference between the preference weights of the treatments causing a 30% chance and a 15% chance of GI side effects was significant for the patients in class 1, the difference between the preference weights of the treatments causing a 15% chance and a 0% chance of GI side effects was significant for the patients in class 2. These results implied that the patients in class 2 might be more sensitive to the lower level of GI side effects. A reason could be they had less experience with the GI side effects than the patients in class 1, who possibly had more experience with the GI side effects due to the other treatments used for other conditions.
The findings of this study have various policy and clinical implications. Healthcare payers could incorporate patients’ preferences and the preference heterogeneity for these newer AHA treatments in the development of formularies and treatment guidelines. The patients then could access preferred treatments. For instance, while the percent reduction in the risk of MACE was promoted to be an important benefit of SGLT-2is and GLP-1 RAs, the healthcare payers should still focus on promoting the chance of reaching target HbA1c, the route and frequency of administration, and the out-of-pocket cost since these attributes highly affected the patients’ preferences. Another example would be that the providers would choose GLP-1 RAs over SGLT-2is for older patients with more comorbidities since SGLT-2is increases the risk of genital infection, which was a significant concern for this patient population. However, future studies should be warranted on how to successfully incorporate patients’ preferences into real practice and policy since the patients’ preferences are relatively new information in the healthcare field.
The study findings should be interpreted cautiously in light of at least four limitations. First, the samples were recruited from an online panel and might not represent the US patient population with T2DM as a whole. Second, the patients stated their preferences from hypothetical treatment choices in this study; their stated preferences might not reflect their real choices, where patients made decisions with emotional, financial, and clinical consequences. This study generated hypothetical treatment choices based on real-world treatment attributes and their levels to mitigate this limitation. Third, this study used a self-administered, web-based questionnaire survey to elicit patients’ preferences, which might be subjected to response bias due to misinterpretation of the attribute levels. However, various methods, including expert review, and the inclusion of a validity check choice set, were performed to minimize this bias. Another limitation was that this study included only six attributes. Although this study carefully selected the attributes, patients’ preferences might still be influenced by other treatment aspects. Therefore, future studies should include more patients, explore other treatment attributes, and verify stated preferences with revealed preferences from the real-world utilization of SGLT-2is and GLP-1 RAs.
Conclusion
Among various attributes of SGLT-2is and GLP-1 RAs, the route and frequency of administration, the chance of reaching target HbA1c, the risk reduction of MACE, the chance of gastrointestinal side effects, the chance of genital infection, and out-of-pocket cost per month were significant attributes for the preferences of patients with T2DM when choosing among AHA treatments. However, the results of the ML model suggested that preference heterogeneity existed, and the results of the LC model indicated patients with different ages and numbers of comorbidities tended to have different preferences for SGLT-2is and GLP-1 RAs.
Acknowledgments
This paper is based on the thesis of [Banjara B]. It has been published on the institutional website: https://etd.auburn.edu/bitstream/handle/10415/7910/MS_Thesis_BidurBanjara.pdf?sequence=2. The abstract of this paper was presented at the International Society for Pharmacoeconomics and Outcomes Research Conference (ISPOR), 2022 named “Patients’ Preferences for Newer Second-Line Pharmacological Agents in Type 2 Diabetes” as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Value in Health journal named “Patients’ Preferences for Newer Second-Line Pharmacological Agents in Type 2 Diabetes”: https://www.valueinhealthjournal.com/article/S1098-30152201676-X/fulltext
https://doi.org/10.1016/j.jval.2022.04.1475
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Classification of diabetes mellitus; 2019. Available from: https://apps.who.int/iris/handle/10665/325182.
2. Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Esposito K. Glycemic control, preexisting cardiovascular disease, and risk of major cardiovascular events in patients with type 2 diabetes mellitus: systematic review with meta-analysis of cardiovascular outcome trials and intensive glucose control trials. J Am Heart Assoc. 2019;8:e012356. doi:10.1161/JAHA.119.012356
3. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi:10.2337/dc10-S062
4. Centers for Disease Control and Prevention. National diabetes statistics report; 2020. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
5. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi:10.1056/NEJMoa0806470
6. UK Prospective Diabetes. Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK prospective diabetes study (UKPDS) group. Lancet. 1998;352:837–853. doi:10.1016/S0140-6736(98)07019-6
7. American Diabetes Association. Glycemic targets: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Supplement_1):S66–S76. doi:10.2337/dc20-S006
8. American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Supplement_1):S125–S43. doi:10.2337/dc22-S009
9. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi:10.1056/NEJMoa1607141
10. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi:10.1056/NEJMoa1603827
11. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi:10.1056/NEJMoa1811744
12. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380:347–357. doi:10.1056/NEJMoa1812389
13. Lo KB, Gul F, Ram P, et al. The effects of SGLT2 inhibitors on cardiovascular and renal outcomes in diabetic patients: a systematic review and meta-analysis. Cardiorenal Med. 2020;10:1–10. doi:10.1159/000503919
14. Hussein H, Zaccardi F, Khunti K, et al. Efficacy and tolerability of sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: a systematic review and network meta-analysis. Diabetes Obes Metab. 2020;22:1035–1046. doi:10.1111/dom.14008
15. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–2701. doi:10.2337/dci18-0033
16. Handelsman Y, Anderson JE, Bakris GL, et al. DCRM Multispecialty Practice Recommendations for the management of diabetes, cardiorenal, and metabolic diseases. J Diabetes Complications. 2022;36(2):108101. doi:10.1016/j.jdiacomp.2021.108101
17. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2020 executive summary. Endocr Pract. 2020;26:107–139. doi:10.4158/CS-2019-0472
18. McIntosh B, Cameron C, Singh SR, et al. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2011;5:e35–e48. doi:10.1007/s00125-005-0108-0
19. Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. N Engl J Med. 2010;303:1410–1418. doi:10.1001/jama.2010.405
20. McEwen LN, Casagrande SS, Kuo S, Herman WH. Why are diabetes medications so expensive and what can be done to control their cost? Curr Diab Rep. 2017;17:71. doi:10.1007/s11892-017-0893-0
21. Luo J, Feldman R, Rothenberger SD, et al. Coverage, formulary restrictions, and out-of-pocket costs for sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists in the medicare part D program. JAMA Netw Open. 2020;3:e2020969–e2020969. doi:10.1001/jamanetworkopen.2020.20969
22. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26:661–677. doi:10.2165/00019053-200826080-00004
23. Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14:403–413. doi:10.1016/j.jval.2010.11.013
24. Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16:3–13. doi:10.1016/j.jval.2012.08.2223
25. Hussein H, Zaccardi F, Khunti K, et al. Cardiovascular efficacy and safety of sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: a systematic review and network meta-analysis. Diabet Med. 2019;36:444–452. doi:10.1111/dme.13898
26. Lorenzi M, Ploug UJ, Langer J, et al. Liraglutide versus SGLT-2 inhibitors in people with type 2 diabetes: a network meta-analysis. Diabetes Ther. 2017;8:85–99. doi:10.1007/s13300-016-0217-4
27. McKee A, Al-Khazaali A, Albert SG. Glucagon-like peptide-1 receptor agonists versus sodium-glucose cotransporter inhibitors for treatment of T2DM. J Endocr Soc. 2020;4:bvaa037. doi:10.1210/jendso/bvaa037
28. Nuhoho S, Gupta J, Hansen BB, et al. Orally administered semaglutide versus GLP-1 RAs in patients with type 2 diabetes previously receiving 1–2 oral antidiabetics: systematic review and network meta-analysis. Diabetes Ther. 2019;10:2183–2199. doi:10.1007/s13300-019-00706-y
29. Banjara B Patients’ preferences for second-line pharmacological agents in type 2 diabetes [Thesis]. Auburn, AL: Auburn University; 2021.
30. ChoiceMetrics. Ngene 1.1. 1 user manual and reference guide; 2012. Available from: http://www.choice-metrics.com.
31. Hauber AB, González JM, Groothuis-Oudshoorn CGM, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19:300–315. doi:10.1016/j.jval.2016.04.004
32. de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8:373–384. doi:10.1007/s40271-015-0118-z
33. Greene WH. Nlogit reference guide: version 3.0. Plainview, NY: Econometric Software, Inc., 2002. Available from: https://www.limdep.com/products/nlogit.
34. Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999–2018. N Engl J Med. 2021;384:2219–2228. doi:10.1056/NEJMsa2032271
35. Brooks A, Langer J, Tervonen T, et al. Patient preferences for GLP-1 receptor agonist treatment of type 2 diabetes mellitus in Japan: a discrete choice experiment. Diabetes Ther. 2019;10:735–749. doi:10.1007/s13300-019-0591-9
36. Gelhorn HL, Bacci ED, Poon JL, et al. Evaluating preferences for profiles of glucagon-like peptide-1 receptor agonists among injection-naive type 2 diabetes patients in Japan. Patient Prefer Adherence. 2016;10:1337–1348. doi:10.2147/PPA.S109289
37. Gelhorn HL, Poon JL, Davies EW, et al. Evaluating preferences for profiles of GLP-1 receptor agonists among injection-naïve type 2 diabetes patients in the UK. Patient Prefer Adherence. 2015;9:1611–1622. doi:10.2147/PPA.S90842
38. Hauber AB, Han S, Yang JC, et al. Effect of pill burden on dosing preferences, willingness to pay, and likely adherence among patients with type 2 diabetes. Patient Prefer Adherence. 2013;7:937–949. doi:10.2147/PPA.S43465
39. Hauber AB, Mohamed AF, Johnson FR, Falvey H. Treatment preferences and medication adherence of people with type 2 diabetes using oral glucose-lowering agents. Diabet Med. 2009;26:416–424. doi:10.1111/j.1464-5491.2009.02696.x
40. Janssen EM, Longo DR, Bardsley JK, Bridges JF. Education and patient preferences for treating type 2 diabetes: a stratified discrete-choice experiment. Patient Prefer Adherence. 2017;11:1729–1736. doi:10.2147/PPA.S139471
41. Mansfield C, Sikirica MV, Pugh A, et al. Patient preferences for attributes of type 2 diabetes mellitus medications in Germany and Spain: an online discrete-choice experiment survey. Diabetes Ther. 2017;8:1365–1378. doi:10.1007/s13300-017-0326-8
42. Marchesini G, Pasqualetti P, Anichini R, et al. Patient preferences for treatment in type 2 diabetes: the Italian discrete-choice experiment analysis. Acta Diabetol. 2019;56:289–299. doi:10.1007/s00592-018-1236-6
43. Mol PG, Arnardottir AH, Straus SM, et al. Understanding drug preferences, different perspectives. Br J Clin Pharmacol. 2015;79:978–987. doi:10.1111/bcp.12566
44. Longo M, Bellastella G, Maiorino MI, et al. Diabetes and aging: from treatment goals to pharmacologic therapy. Front Endocrinol. 2019;10:45. doi:10.3389/fendo.2019.00045
45. Boye K, Ross M, Mody R, Konig M, Gelhorn H. Patients’ preferences for once-daily oral versus once-weekly injectable diabetes medications: the REVISE study. Diabetes Obes Metab. 2021;23:508–519. doi:10.1111/dom.14244
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.