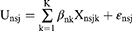Back to Journals » Patient Preference and Adherence » Volume 17
A Preference-Based Value Assessment of the Fear of COVID-19 Contagion
Authors Poudel N , Ngorsuraches S
Received 20 September 2023
Accepted for publication 6 December 2023
Published 18 December 2023 Volume 2023:17 Pages 3435—3448
DOI https://doi.org/10.2147/PPA.S431148
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Nabin Poudel, Surachat Ngorsuraches
Department of Health Outcomes Research and Policy, Harrison College of Pharmacy, Auburn University, Auburn, AL, USA
Correspondence: Surachat Ngorsuraches, Department of Health Outcomes Research and Policy, Harrison College of Pharmacy, Auburn University, 4306A Walker Building, Auburn, AL, 36849, USA, Tel +1 334 844 8357, Fax +1 334 844 8307, Email [email protected]
Purpose: To assess the preference-based value of the fear of COVID-19 contagion.
Patients and Methods: We conducted a web-based, cross-sectional discrete choice experiment among 544 US adults. We used a Bayesian efficient design to generate choice sets. Each choice set comprised two hypothetical COVID-19 vaccine options characterized by seven attributes: chance of COVID-19 infection, chance of having severe symptoms from COVID-19 infection, vaccine protection duration, chance of mild to moderate adverse events from vaccination, chance of serious adverse events from vaccination, chance of future exposure to COVID-19 after vaccination, and out-of-pocket cost. We used mixed logit (ML) and latent class (LC) models to analyze data. Furthermore, we calculated the willingness-to-pay for eliminating the chance of future exposure to COVID-19, shedding light on the value attributed to the fear of contagion.
Results: The ML model demonstrated all attributes, including the chance of future exposure to COVID-19, were statistically significant. The participants were willing to pay approximately $13,046 to eliminate the chance of future exposure to COVID-19 or their fear of contagion when COVID-19 was still pandemic. The LC model unveiled two participant classes with distinct preference weights for the chance of future exposure to COVID-19 and out-of-pocket cost attributes. Nevertheless, the chance of future exposure to COVID-19 exposure held a significant degree of importance in both classes.
Conclusion: The chance of future exposure to COVID-19 exposure or fear of contagion was a significant element in the value assessment of COVID-19 vaccines. Further studies should be conducted to verify the value of fear of contagion and include it in the value assessment of healthcare technologies for infectious diseases.
Keywords: COVID-19, discrete choice experiment, fear of contagion, patient preference
Introduction
Fear is characterized as an adaptive reaction in the presence of a potential threat or stimuli.1 Specifically, fear of contagion is the anxiety over the perceived risk of contracting infectious diseases.2 This response plays an integral role in the stress-coping process during a public health emergency, such as the COVID-19 pandemic.3,4 A previous study suggested four psychological dimensions of fear amid the COVID-19 pandemic: bodily, interpersonal, cognitive, and behavioral.5 One or more of these domains are measured using various instruments, such as the Fear of COVID-19 scale (FCV-19S), Fear perception and magnitude of the issue (MED-COVID19) scale, Scale of COVID-19 Related Psychological Distress in the healthy public (CORPD), and COVID-19 Phobia Scale (C19P-S).6
The rapid spread of virus increased fear of COVID-19, affecting not only the infected individuals but also the individuals with underlying chronic diseases, healthy individuals, family members, frontline service (hospitals and emergency departments) providers, and the economy.7,8 For instance, due to social distancing measures put in place to control the transmission and reduce mobility, numerous businesses had to close their doors, and many consumers either faced mandated stay-at-home orders or chose to limit their economic activities voluntarily around the globe.9 This possibility of widespread job losses and income reductions for many individuals resulted in the increase in fear of COVID-19 contagion.10
In response to the COVID-19 pandemic, pharmaceutical companies rapidly expanded research efforts on vaccines and treatments (healthcare technologies).11 To reduce the risk of viral transmission and to ensure the continuity of care, there was also an expanded use of technologies, eg, telepsychiatry and vaccines.12 Such healthcare technologies, which have the potential to mitigate the anxiety linked to the prospect of future disease spread, may hold significant value for individuals.13 A recent study indicated that positive information about COVID-19 vaccines fostered optimism and reduced concerns, instilling a belief in a quicker return to a normal way of life.14 ISPOR Special Task Force also identified the reduction in fear of contagion with the availability of vaccine or treatment alternatives as an element relevant in the value assessment of healthcare technologies for infectious diseases.13
Cost-effectiveness analysis (CEA) with quality-adjusted life years (QALYs) has been most frequently used to assess the value of these technologies.13 It tends to include the benefits beyond individuals who receive the technologies (a.k.a. externality) and is especially relevant because these technologies limit the spread of infectious diseases to others.13 When adopting a societal perspective, CEA considers all these external benefits. In the case of COVID-19 vaccines, CEA was utilized to gauge their economic value.15,16 For instance, a study used a CEA with QALY to assess the value of the COVID-19 vaccine. The findings indicated that the COVID-19 vaccine would be cost-effective for people aged 50 or above at a cost-effectiveness (CE) threshold of less than $50,000 per QALY.15 Another study used a similar CEA with the same CE threshold to determine the value-based price of remdesivir.16 However, the analyses did not include the benefits associated with diminishing the fear of COVID-19 contagion with new technologies.17
ISPOR Special Task Force suggested that a survey technique can elicit an individual’s willingness-to-pay (WTP) to eliminate the chance of disease exposure, reflecting the value of fear of contagion.13 However, no quantitative measure valuing this fear existed. Without such information, the opportunity to add the fear of contagion in value frameworks would remain unfulfilled. Thus, the objective of this study was to quantify the value attributed to the fear of contagion by determining the WTP for eliminating the chance of future exposure to COVID-19 exposure, using the COVID-19 vaccine as a case study.
Materials and Methods
Previously, the revealed preference (RP) technique, based on an individual’s actual choices and actions in the marketplace, and the contingent valuation (CV) technique, directly asking individuals in a survey how much they are willing to pay, were used to value fear in different contexts.18,19 However, the RP technique tended not to disentangle the individual’s WTP to avert a feared outcome from their WTP to avoid fear itself. Although CV, a stated preference method, could also be employed to determine the monetary valuation of changes in the risk of a feared outcome and the number of fear days (fear avoidance),18,19 an alternative stated preference approach, the discrete choice experiment (DCE) approach, offers several advantages compared to the CV: less cumbersome, less costly, more informative, and are suitable for multidimensional tradeoff.20,21
Thus, this study used a discrete choice experiment (DCE), a survey-based stated preference technique, to measure the value of fear of COVID-19 contagion.22 DCE entails presenting individuals with various hypothetical choice scenarios or alternatives.23 These alternatives are described using multiple attributes and levels. By analyzing the choices made by participants across different alternatives, researchers can infer the relative importance of various attributes and levels, gaining insights into individuals’ preferences. Importantly, if out-of-pocket cost is incorporated as one of the attributes, DCE allows the estimate of the marginal rate of substitution (MRS) between study attributes and out-of-pocket cost, a monetary value of the attribute commonly referred to as WTP from the individual or societal prospectives.24,25 This study elicited the WTP for eliminating the chance of future exposure to COVID-19, reflecting the value of fear of contagion. DCE was previously used to quantify the value of hope, one of the novel value elements defined by the ISPOR Special Task Force in cancer care.26 This study was designed based on the DCE user guide and two reports issued by ISPOR Good Research Practices Task Force.27,28 Since this study was a cross-sectional survey and collected deidentified participants’ information, the Institutional Review Board (IRB) at Auburn University approved this study to be exempt from human subject review. All participants provided informed consent in accordance with the Declaration of Helsinki.
Attribute and Level Identification
First, we conducted a literature review and identified five attributes related to COVID-19 vaccines and their potential levels. The primary source of information on attributes and levels includes the US Food and Drug Administration (FDA) factsheet on the emergency use authorization of Janssen,29 Moderna,30 and Pfizer-BioNTech31 COVID-19 vaccine, and other related publications.32–37 These attributes included the chance of COVID-19 infection, the chance of having severe symptoms if you are infected with COVID-19, vaccine protection duration, the chance of mild to moderate adverse events from vaccination, and the chance of serious adverse events from vaccination. The chance of future exposure to COVID-19 as a result of vaccination and out-of-pocket were purposely added to examine the value of fear of contagion. Based on the DCE user guide and two reports issued by the ISPOR Good Research Practices Task Force, we asked conveniently sampled five adults from the general public and two clinical experts in infectious diseases to review and confirm that these seven attributes were important to adults in the general public.27,28
The number of COVID-19 infections per 100,000 people was used to describe the chance of COVID-19 infection. The chance of having severe symptoms if you are infected with COVID-19 was set as the proportion of people who, upon contracting COVID-19, face severe outcomes such as hospitalization, admission to intensive care units (ICUs), required mechanical ventilation (machine-assisted breathing), or death. The vaccine protection duration was described as the number of months people remain protected from being infected with COVID-19. The chance of mild to moderate adverse events following vaccination was defined as the percentage of people who suffer from minor events such as pain in the injection site, fatigue, headache, or chills. Meanwhile, the percentage of people who suffer from life-threatening complications such as serious allergic reactions (anaphylaxis) or the formation of a blood clot in the veins (venous thromboembolism) represented the chance of serious adverse events from vaccination. The minimum and maximum possible levels of these attributes were identified from the literature. Additional levels were introduced between these two existing levels for each attribute to maintain equally spaced intervals to facilitate the study’s design.38 A uniformly distributed attribute level can be beneficial when interpreting the estimated effects of numerical attributes. This study described the chance of future exposure to COVID-19 as the possibility of contacting the infected person. For logical reasons, this study used three categorical levels, ie, high chance (COVID-19 remains a pandemic), medium chance (COVID-19 is no longer a pandemic), and no chance (COVID-19 disappears) to present the possibility of COVID-19 exposure. These levels for the possibility of COVID-19 exposure were confirmed by previous studies.39–44
Since this study was the first to value the fear of contagion, limited guidance about the levels of the cost attribute was available. This study adapted a technique from a previous study45 to establish the levels of out-of-pocket cost attribute. As part of our pilot study, a binary choice survey was sent to 30 people to assess their WTPs to eliminate the possibility of future exposure to COVID-19. This part of the survey contained six binary choice questions (Supplemental Material Annex 1). Each question included a choice between a lower possibility of exposure with zero cost or a higher possibility of exposure with nonzero cost. Since a US study reported a maximum WTP of about $15,500 for an intervention that reduced the chance of COVID-19 infection,46 this study randomly varied the nonzero costs of $500, $1000, $2000, $4000, $8000, and $16,000 across different binary choice questions. The results from the binary choice questions showed almost half of the respondents were not willing to pay $500, while one-third of these people were willing to pay $16,000 to eliminate the possibility of COVID-19 exposure. Therefore, the lowest level of $0 and the highest level of $16,000 were selected. Table 1 shows all study attributes and their levels.
 |
Table 1 Attributes and Levels for the DCE Survey Instrument |
DCE Instrument Development
We designed a self-administered, web-based survey questionnaire and implemented it using QualtricsXM. Ngene® software was employed to create an orthogonal and balanced design for developing DCE choice sets. These choice sets were then piloted with a group of 30 participants from the public (with or without a diagnosis of COVID-19) recruited by QualtricsXM. Subsequently, a multinomial logit model (MNL) was constructed to establish preliminary parameters. Next, a Bayesian efficient design was employed to sample a subset from the complete range of attribute and level combinations.27,47 This algorithm for Bayesian efficient design involved an iterative process that assessed statistical efficiency across different design options. The calculation of statistical efficiency was based on Halton draws derived from prior parameters obtained from the pilot DCE study.
The design produced 36 choice sets, which were organized into four distinct blocks. Each choice set featured two options describing hypothetical COVID-19 vaccines, varying in terms of the study attributes and their associated levels. Since this study intended to estimate the marginal rate of substitution between the chance of future exposure to COVID-19 and out-of-pocket cost, an opt-out alternative was not necessarily included.48,49 Besides texts and numbers describing all attributes and levels, graphics were used to enhance participants’ understanding of these attributes and levels. Participants in the study were tasked with selecting their preferred option from each choice set. Figure 1 presents an example of a choice set. Additionally, a validity check choice set was included in every questionnaire. This validity check choice set featured a dominant alternative (eg, the lowest chance of future exposure and lowest cost). Participants who understood the DCE choice sets were expected to choose this dominant alternative. Validated “cheap talk” text, which encouraged participants to provide honest and thoughtful answers, was also included to mitigate hypothetical bias.50 Questions pertaining to participants’ traits and their experiences concerning COVID-19 and COVID-19 vaccines were incorporated into the questionnaire. Also, an open-ended question asking how the participants thought about the survey was included.
 |
Figure 1 A choice set example. |
Using a purposive sampling technique, three experts (one adult from the general public, one clinical expert in infectious disease, and one social scientist) were asked to verify the content validity of the questionnaire survey. The survey was then examined for participant’s understanding using the “think aloud” method with five purposively sampled adults with various education levels (eg, with and without a college degree) from the general public. All of them indicated they understood the survey well. Also, they appropriately interpreted the attributes, levels, and graphics in the DCE choice sets. A preliminary study was conducted with 50 individuals by QualtricsXM before the main survey was launched. They received an incentive at the amount they previously agreed to participate as a QualtricsXM panelist. Since no significant issue was identified, there was no need to change the original survey instrument. Specifically, none suggested they had difficulty understanding and completing the survey.
Data Collection
The study population consisted of the general public, ie adults who were 18 years old or above and could read English, from the US. This study asked QualtricsXM to recruit study participants nationally. Data were collected from February 16, 2022, to March 9, 2022. QualtricsXM is a private research firm specializing in web-based data collection and collaborates with more than 20 web-based panel providers to deliver a varied pool of high-quality respondents. Following the methodology outlined in a published practical guide,51 this study determined the sample size using a significance level of 0.05, a statistical power level of 0.8, and prior parameter estimates derived from the preliminary study. Upon consideration of the estimated sample size and various methodologies, which included a good DCE research practice27 and sample-size efficiency (S-efficiency),52 this study found that at least 250 persons were required. After completing the survey, participants received a prearranged incentive agreed upon with QualtricsXM.
Data Analysis
Descriptive analyses were carried out, and individuals’ responses to each choice set in the DCE were observed and analyzed in accordance with random utility theory (RUT). Utilizing Nlogit® software, a mixed logit model (ML) was constructed to calculate the means and standard deviations of normally distributed variations for all preference weights in a utility function.
The utility function (Unsj) was
where n = individual adult, s = choice set, j = alternative, k = attribute, Xnsjk = the full vector of study attributes relating to individual n and alternative j on the choice set s, βnk = the vector of individual-specific coefficients of attribute k, and ε = an error term.
The final model was determined by likelihood ratio tests. A Wald test was used to examine the variations between consecutive levels of the study attributes, with a predefined level of statistical significance at 0.05. The difference between the highest and lowest preference weights for the same attribute was computed to determine the conditional relative importance of each study attribute. The marginal WTP for each level of the chance of exposure to COVID-19 attribute was calculated by dividing the mean coefficient of each level by the mean coefficient of the cost attribute. Krinsky and Robb method was applied to estimate 95% confidence intervals of these WTPs.53
Next, the main-effects of the latent class model (LCM), which distinguished groups of individuals with similar preferences, was developed. To ascertain preference estimates for various groups, the LCM model was fit to c classes by using Consistent Akaike Information Criteria (CAIC). A utility function for alternative 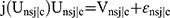 was estimated. Data were coded using the effect code for categorical attributes, and other attributes were treated as continuous. The level of statistical significance was 0.05. The conditional relative importance of the chance of future exposure and cost attributes was estimated.
was estimated. Data were coded using the effect code for categorical attributes, and other attributes were treated as continuous. The level of statistical significance was 0.05. The conditional relative importance of the chance of future exposure and cost attributes was estimated.
Results
A total of 623 participants completed the survey and provided accurate responses to the validity choice set. Of these, 79 participants with missing information were excluded, resulting in a final sample of 544 participants. Table 2 shows the study participants’ characteristics and COVID-19 related experiences. The mean age among these participants was 57.2 (SD=17.0) years old. Most of them were female (56.1%), White, non-Hispanic (88.2%), and married (57.9%). Almost half of these participants (43.8%) had less than $50,000 per year for their household incomes, and 44.3% had 4-year college or graduate or professional degree. Approximately 38% were either fully employed, self-employed, or employed part-time, and about 32% had private health insurance. Most participants had two or more comorbidities (60.7%) and good or better health status (79%). Approximately 76% of the participants were fully COVID-19 vaccinated. More than 10% of the participants diagnosed with COVID-19 manifested symptoms such as fever, chills, shortness of breath, fatigue, muscle aches, and headaches, and only 13.8% were hospitalized. Most of their loved ones or relatives (74.3%) were previously infected by COVID-19, but only 25.6% and 18.9% were hospitalized or died due to COVID-19 infection. Almost 80% experienced a loss of employment or income due to COVID-19.
 |
Table 2 Participants’ Characteristics and COVID-19 Related Experiences |
Preference Weights of the Study Attributes from the ML Model
Figure 2 displays the preference weights assigned to the study attributes by the ML model. In this model, all attribute levels were treated as categorical variables (See details in Supplemental Material Annex 2). The estimated preference weights of all attributes exhibited the anticipated trends. Specifically, attributes such as a lower chance of COVID-19 infection, lower chance of having severe COVID-19 symptoms, longer protection duration, lower chance of mild to moderate adverse events from vaccination, lower chance of serious adverse events from vaccination, lower chance of future exposure to COVID-19 as a result of vaccination, and lower out-of-pocket cost had higher preference weights compared to other levels of these attributes. While statistical significance was observed among all adjacent levels of the protection duration, the chance of mild to moderate adverse events, the chance of serious adverse events from vaccination, the chance of future exposure to COVID-19 as a result of vaccination, and out-of-pocket cost attributes, only some adjacent levels of the chance of COVID-19 infection and the chance of having severe COVID-19 symptoms were significant. Only the difference between the preference weights of the vaccines with the chances of COVID-19 infection at 500 and 1000 out of 100,000 people was significant. Similarly, a significant difference in preference weights between vaccines with a 0% and 5% chance of having severe COVID-19 symptoms was observed.
 |
Figure 2 Preference weights of study attributes from the mixed logit model. *Significant at 5% alpha. |
The out-of-pocket cost (6.3) attribute had the highest conditional relative importance estimate, followed by the chance of future exposure to COVID-19 as a result of vaccination (3.8), the chance of low to moderate adverse events from vaccination (1.4), the vaccine protection duration (1.2), the chance of COVID-19 infection and the chance of severe adverse events from vaccination (0.9), and the chance of severe symptoms (0.5). We observed significant standard deviations in the preference weights for all attributes, indicating the presence of preference heterogeneity. In summary, all attributes significantly affected the preferences for COVID-19 vaccines.
Value of Fear of Contagion
Table 3, derived from the ML model, presents the estimated WTP values for different levels of chance future exposure to COVID-19 as a result of vaccination. The study participants were unwilling to pay for high (-$6135) and medium (-$775.6) chances of COVID-19 exposure. Conversely, they expressed a WTP of $6910.7 for no chance of exposure. Thus, the participants were willing to pay approximately $13,046 to eliminate the chance of future exposure to COVID-19 or their fear of contagion when COVID-19 was still pandemic.
 |
Table 3 Estimated WTPs for Different Chances of COVID-19 Exposure |
Preference Weights of the Chance of Future Exposure and Cost Attributes from the LC Model
According to the CAIC values, the optimal LC model identified two distinct participant classes with varying preferences (See details in Supplemental Material Annex 3). To focus on the value of fear of contagion, Figure 3 highlights the preference weights of the chance of future exposure to COVID-19 as a result of vaccination and out-of-pocket cost attributes for the participants in these two classes. In both classes, preference weights for both attributes aligned with the expected directions. In class 1, there were significant differences between all consecutive levels of these two attributes. Conversely, in class 2, the out-of-pocket cost attribute had an adjacent level that was not significantly different from another level. More precisely, there was no significant difference in preference weights between the out-of-pocket cost levels of $8000 and $16,000. In class 1, the conditional relative importance estimate of the out-of-pocket cost (6.7) was higher than the estimate of the chance of future exposure to COVID-19 as a result of vaccination (4.0). Conversely, in class 2, the conditional relative importance estimate of the chance of future exposure to COVID-19 as a result of vaccination (2.5) was higher than the estimate for the out-of-pocket cost (0.7). In summary, the chance of future exposure and out-of-pocket cost were relatively important for both classes.
 |
Figure 3 Preference weights of chance of future exposure and cost attributes from the latent class model. *Significant at 5% alpha. |
Discussion
Although fear related to COVID-19 was much described7,54–56 and measured using different scales,6 the quantitative value for fear previously was not yet established. This study intended to pave the way to include the value of fear of contagion in value frameworks when dealing with health technology for infectious diseases. Specifically, the study used a DCE to quantify the value of fear of contagion using the COVID-19 vaccine as a case study. The results showed the study participants highly valued the lower chances of future exposure to COVID-19 as a result of vaccination compared with other vaccines’ benefits and risks, eg, the chance of COVID-19 infection, the chance of having severe symptoms if you are infected with COVID-19, vaccine protection duration, the chance of mild to moderate adverse events from vaccination, and the chance of a serious adverse event from vaccination. The conditional relative importance estimates from the ML model demonstrated that change in the chance of future exposure to COVID-19 as a result of vaccination was approximately three to eight times more important than the changes in the chance of mild to moderate adverse events from vaccination, vaccine protection duration, the chance of COVID-19 infection, the chance of serious adverse events from vaccination, and the chance of having severe COVID-19 symptoms. While these results were intuitive since the participants would expect that the vaccines would subsequently eliminate their future COVID-19 exposures, the results reinforced the significance of accounting for the fear of disease transmission as an important value element when evaluating the value of healthcare technologies for infectious diseases.
The findings also indicated that the participants were willing to pay approximately $13,046 to eliminate the chance of COVID-19 exposure or alleviate their fear of contagion during the pandemic phase (high chance of exposure). They were willing to pay approximately $7687 to eliminate the possibility of COVID-19 exposure from when COVID-19 was no longer pandemic (medium chance of exposure). While the value of fear of COVID-19 contagion has never been examined, this WTP amount could be compared with the results from various relevant studies. This WTP for fear of COVID-19 contagion was higher than the WTP for the COVID-19 vaccine (approximately $237 to $319) in a US study57 and also higher than an incremental net benefit (INB) of approximately $170 to $340 for COVID-19 vaccines and vaccination strategies reported in a systematic review of 85 modeling studies.58 One reason could be that these previous studies did not capture the possibility of future exposure to COVID-19 as a benefit of vaccines or vaccination strategies. However, the same systematic review reported an INB of approximately $1631 to $6599 for COVID-19 treatments.58 Also, a study estimated a maximum WTP of about $15,500 per person with a range of $8000 to $28,000 for morbidity and mortality risk reductions of COVID-19 infection.46 These estimates somewhat reflected the reasonable estimation of WTP amount for the fear of COVID-19 contagion in this study.
The ML results also indicated that preference heterogeneity among the participants existed. The main-effects LCM showed two classes of participants with different preference weights of the chance of future exposure to COVID-19 and out-of-pocket cost attributes. While the out-of-pocket cost attribute was more important for the participants in class 1, the chance of future exposure to COVID-19 was more important for the participants in class 2. However, the chance of future exposure to COVID-19 exposure was relatively important in both classes. In other words, the changes in the chance of future exposure to COVID-19 were consistently important to the participants with different preferences. These results implied that the fear of contagion was an important element in the value assessment of COVID-19 vaccines.
This study added empirical evidence to support the value of fear of contagion as a new element in the value assessment of healthcare technologies for infectious diseases. However, a concern, which was also raised by some participants in the open-ended question and was worth mentioning, was whether the value of fear of contagion would increase the prices of technologies. It was noteworthy that the results of this study should not be used to justify the technologies’ prices. Instead, this study intended to explain the decisions when technologies, which might be worthwhile, were not cost-effective, based on economic evaluation results such as CEA with QALY. For instance, a previous study published in 2021 showed the COVID-19 vaccine was not cost effective ($340,000 per QALY) for people in the US aged 18 to 49 years and had no serious medical condition.15 The decision that made COVID-19 vaccines available for these people would not be justified if it was based on this CEA alone. Instead, if the fear of contagion was valued, as shown in this study, the decision to make the COVID-19 vaccine available to people in this age range would be more justifiable.
This study possesses various notable strengths. For instance, this empirical study quantified the value associated with the fear of contagion, determining the WTP for eliminating the risk of future exposure to COVID-19. This information presents an opportunity to integrate the value of fear of contagion into existing value frameworks, such as CEA, thereby comprehensively capturing the value of emerging technologies for infectious disease. The study findings can aid decision-makers in efficiently allocating resources and policymaking by identifying and adjusting healthcare technologies that offer optimal value to the population relative to their costs. This involves tailoring interventions to align with the preferences and priorities of the target population. Also, this study determined the trade-offs individuals are willing to accept among various levels of fear, offering a quantitative measure of the value people assign to alleviating the fear of contagion. Moreover, heterogeneity in values concerning fear of contagion in this study provides an opportunity to tailor effective communication strategies for individuals with different preferences.
This study encountered four major limitations. Firstly, participant recruitment was conducted through an online panel generally white and with a higher level of education, potentially resulting in a sample that does not accurately mirror the broader US population. For instance, it was possible that this study could underestimate the value of fear of COVID-19 contagion since almost half of them were retired and had relatively low household incomes. Also, both ML and LCM results exhibited preference heterogeneity among the participants. Therefore, individuals with different characteristics and COVID-19 related experiences might value the fear of contagion differently. A larger and more representative sample size should be used to confirm the results of this study. Second, this study used a stated preference approach, where participants expressed their opinions rather than made actual choices and had no financial obligation, such responses might or might not reflect their real choices. However, the DCE, a rigorous method increasingly used in health economics, was used.21 Also, while this study intended to assess the value of fear of contagion, other attributes of COVID-19 vaccines were included to make the hypothetical choices in this study resemble real choices. Third, this study used only short descriptions of the study attributes instead of a comprehensive tutorial to increase the participant’s understanding of the attributes since this approach would allow the participants to finish the survey in approximately 12 minutes, which would cause less burnout to the participants. However, the validity and understanding of the survey were carefully tested using various approaches, including the experts’ reviews, the think aloud method, and the pilot study. Based on an open-ended question in the main study asking how the participants thought about the survey, only less than 1% of all respondents reported the survey was difficult confirming the validity of our findings. Also, the participants should be familiar with COVID-19 and COVID-19 vaccines since they were widely discussed during the pandemic. Another limitation was that this study assumed the linear continuous specifications of the chance of future exposure and cost when the WTPs were calculated from the ML model. However, the results from the model showed relatively straight lines among the level changes of these two attributes and therefore supported this assumption was appropriate.
Conclusions
In conclusion, our study revealed $13,046 as the value for eliminating the fear of contagion, suggesting the perceived importance of fear of contagion to be equivalent to other vaccine attributes. Future studies should validate this finding across diverse study populations and settings. Our study has an important implication for health technology assessment across countries with the potential to incorporate the value of fear of contagion into the value assessment of future healthcare technologies for infectious diseases.
Acknowledgments
The abstract of this paper was presented at the International Society for Pharmacoeconomics and Outcomes Research Conference (ISPOR), 2022 named “Patients’ Preferences for Newer Second-Line Pharmacological Agents in Type 2 Diabetes” as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Value in Health journal named “Valuing the Fear of COVID-19 Contagion”: https://doi.org/10.1016/j.jval.2022.04.719.
Author Contributions
All authors played a substantial role in the research reported, encompassing contributions to conception, study design, execution, data acquisition, analysis, and interpretation, as well as involvement in drafting, revising, and critically reviewing the article. They collectively provided final approval for the publication’s version, selected the journal for submission, and committed to being accountable for all facets of the study.
Funding
This work was supported by the “PhRMA Foundation”.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mertens G, Gerritsen L, Duijndam S, Salemink E, Engelhard IM. Fear of the coronavirus (COVID-19): predictors in an online study conducted in March 2020. J Anxiety Disord. 2020;74:102258. doi:10.1016/j.janxdis.2020.102258
2. Meisenhelder JB, LaCharite C. Fear of contagion: the public response to AIDS. Image J Nurs Sch. 1989;21(1):7–9. doi:10.1111/j.1547-5069.1989.tb00089.x
3. Azim D, Kumar S, Nasim S, Arif TB, Nanjiani D. COVID-19 as a psychological contagion: a new Pandora’s box to close? Infect Control Hosp Epidemiol. 2020;41(8):989–990.
4. Meisenhelder JB, LaCharite CL. Fear of contagion: a stress response to acquired immunodeficiency syndrome. ANS Adv Nurs Sci. 1989;11(2):29–38. doi:10.1097/00012272-198901000-00007
5. Schimmenti A, Billieux J, Starcevic V. The four horsemen of fear: an integrated model of understanding fear experiences during the Covid-19 pandemic. Clin Neuropsychiatr. 2020;17(2):41–45. doi:10.36131/CN20200202
6. Muller AE, Himmels JPW, Van de Velde S. Instruments to measure fear of COVID-19: a diagnostic systematic review. BMC Med Res Methodol. 2021;21(1):82. doi:10.1186/s12874-021-01262-5
7. Costanza A, Macheret L, Folliet A, et al. COVID-19 related fears of patients admitted to a psychiatric emergency department during and post-lockdown in Switzerland: preliminary findings to look ahead for tailored preventive mental health strategies. Medicina. 2021;57(12):12. doi:10.3390/medicina57121360
8. Şimşir Z, Koç H, Seki T, Griffiths MD. The relationship between fear of COVID-19 and mental health problems: a meta-analysis. Death Stud. 2022;46(3):515–523. doi:10.1080/07481187.2021.1889097
9. Nouvellet P, Bhatia S, Cori A, et al. Reduction in mobility and COVID-19 transmission. Nat Commun. 2021;12(1):1090. doi:10.1038/s41467-021-21358-2
10. Blandin A, Bick A Real-time labour market estimates during the 2020 coronavirus outbreak. Available from: https://cepr.org/voxeu/columns/real-time-labour-market-estimates-during-2020-coronavirus-outbreak#:~:text=Our%20estimates%2C%20summarised%20in%20Bick,April%20(see%20Figure%201.
11. Kamal-Bahl S, Willke RJ, Puckett JT, Doshi JA The case for using novel value elements when assessing COVID-19 vaccines and therapeutics. Health Affairs Blog. Available from: https://www.healthaffairs.org/content/forefront/case-using-novel-value-elements-assessing-covid-19-vaccines-and-therapeutics.
12. Amerio A, Odone A, Marzano L, et al. Covid-19: the last call for telepsychiatry. Acta Biomed. 2020;91:3.
13. Lakdawalla DN, Doshi JA, Garrison LP, Phelps CE, Basu A, Danzon PM. Defining elements of value in health care-a health economics approach: an ISPOR special task force report [3]. Value Health. 2018;21(2):131–139. doi:10.1016/j.jval.2017.12.007
14. Andersson O, Campos-Mercade P, Meier AN, Wengström E. Anticipation of COVID-19 vaccines reduces willingness to socially distance. J Health Econ. 2021;80:102530. doi:10.1016/j.jhealeco.2021.102530
15. Kohli M, Maschio M, Becker D, Weinstein MC. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine. 2021;39(7):1157–1164. doi:10.1016/j.vaccine.2020.12.078
16. Emond SK, Pearson SD Alternative policies for pricing novel vaccines and drug therapies for COVID-19. Available from: https://icer.org/wp-content/uploads/2021/04/Alternative-Policies-for-Pricing-Novel-Vaccines-and-Drug-Therapies-for-COVID-19-_-ICER-White-Paper.pdf.
17. The Council of Economic Advisers. Evaluating the effects of the economic response to COVID-19. 2020. Available from: https://trumpwhitehouse.archives.gov/wp-content/uploads/2020/08/Evaluating-The-Effects-of-The-Economic-Response-to-COVID-19.pdf.
18. Adler MD. Fear assessment: cost-benefit analysis and the pricing of fear and anxiety. Chi Kent L Rev. 2004;79:977.
19. Fisman DN, Mittleman MA, Sorock GS, Harris AD. Willingness to pay to avoid sharps-related injuries: a study in injured health care workers. Am J Infect Control. 2002;30(5):283–287. doi:10.1067/mic.2002.124586
20. Hanley N, Mourato S, Wright RE. Choice modelling approaches: a superior alternative for environmental valuatioin? J Econ Surv. 2001;15(3):435–462. doi:10.1111/1467-6419.00145
21. Mahieu P-A, Andersson H, Beaumais O, Crastes R, Wolff F-C Is choice experiment becoming more popular than contingent valuation? A systematic review in agriculture, environment and health. FAERE Working Paper, 2014.12.
22. Green C, Gerard K. Exploring the social value of health-care interventions: a stated preference discrete choice experiment. Health Econ. 2009;18(8):951–976. doi:10.1002/hec.1414
23. Soekhai V, de Bekker-Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–226. doi:10.1007/s40273-018-0734-2
24. Cleland J, Porteous T, Skåtun D. What can discrete choice experiments do for you? Med Educ. 2018;52(11):1113–1124. doi:10.1111/medu.13657
25. Sudeh C-S, Arne Risa H, Nicola M, et al. What patients want from primary care consultations: a discrete choice experiment to identify patients’ priorities. Ann Fam Med. 2008;6(2):107. doi:10.1370/afm.816
26. Reed SD, Yang JC, Gonzalez JM, Johnson FR. Quantifying value of hope. Value Health. 2021;24(10):1511–1519. doi:10.1016/j.jval.2021.04.1284
27. Hauber AB, González JM, Groothuis-Oudshoorn CGM, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–315. doi:10.1016/j.jval.2016.04.004
28. Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16(1):3–13. doi:10.1016/j.jval.2012.08.2223
29. U.S. Food & Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers) emergency use authorization (EUA) of the Janssen Covid-19 vaccine to prevent coronavirus disease 2019 (Covid-19). Available from: https://www.fda.gov/media/144637/download.
30. U.S. Food & Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers) emergency use authorization (EUA) of the Moderna covid-19 vaccine to prevent coronavirus disease 2019 (Covid-19). Available from: https://www.fda.gov/media/144637/download.
31. U.S. Food & Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers) emergency use authorization (EUA) of the Pfizer-BioNTech covid-19 vaccine to prevent coronavirus disease 2019 (Covid-19). Available from: https://www.fda.gov/media/144413/download.
32. Centers for Disease Control and Prevention. COVID data tracker. Available from: https://covid.cdc.gov/covid-data-tracker/#datatracker-home.
33. Havers FP, Pham H, Taylor CA, et al. COVID-19-associated hospitalizations among vaccinated and unvaccinated adults 18 years or older in 13 US States, January 2021 to April 2022. JAMA Int Med. 2022;182(10):1071–1081. doi:10.1001/jamainternmed.2022.4299
34. Kow CS, Hasan SS. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology. 2021;29(4):1075–1090. doi:10.1007/s10787-021-00839-2
35. Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. NEJM. 2020;383(27):2603–2615. doi:10.1056/NEJMoa2034577
36. The White House. COVID-19 press briefing. Available from: https://www.whitehouse.gov/wp-content/uploads/2021/08/COVID-Press-Briefing_24August2021_for-transcript.pdf.
37. Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21(10):626–636. doi:10.1038/s41577-021-00592-1
38. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–677. doi:10.2165/00019053-200826080-00004
39. Cartaxo ANS, Barbosa FIC, De souza bermejo PH, Moreira MF, Prata DN, Xue B. The exposure risk to COVID-19 in most affected countries: a vulnerability assessment model. PLoS One. 2021;16(3):e0248075. doi:10.1371/journal.pone.0248075
40. Centers for Disease Control and Prevention. Interim Guidance for Managing Healthcare Personnel with SARS-CoV-2 Infection or Exposure to SARS-CoV-2. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html.
41. COVID-19 Event Risk Tool. USA risk assessment by county. Available from: https://covid19risk.biosci.gatech.edu/.
42. Eisenstein M What’s your risk of catching COVID? These tools help you to find out. Available from: https://www.nature.com/articles/d41586-020-03637-y.
43. Ioannidis JPA. Benefit of COVID-19 vaccination accounting for potential risk compensation. Npj Vaccines. 2021;6(1):99. doi:10.1038/s41541-021-00362-z
44. Sun Z, Di L, Sprigg W, Tong D, Casal M. Community venue exposure risk estimator for the COVID-19 pandemic. Health Place. 2020;66:102450. doi:10.1016/j.healthplace.2020.102450
45. Rowen D, Stevens K, Labeit A, et al. Using a discrete-choice experiment involving cost to value a classification system measuring the quality-of-life impact of self-management for diabetes. Value Health. 2018;21(1):69–77. doi:10.1016/j.jval.2017.06.016
46. Echazu L, Nocetti DC. Willingness to pay for morbidity and mortality risk reductions during an epidemic. Theory and preliminary evidence from COVID-19. Geneva Risk Insur Rev. 2020;45(2):114–133. doi:10.1057/s10713-020-00053-0
47. Bliemer MCJ, Rose JM. Construction of experimental designs for mixed logit models allowing for correlation across choice observations. Trans Res B Meth. 2010;44(6):720–734. doi:10.1016/j.trb.2009.12.004
48. Milte R, Ratcliffe J, Miller M, Whitehead C, Cameron ID, Crotty M. What are frail older people prepared to endure to achieve improved mobility following hip fracture? A discrete choice experiment. J Rehabil Med. 2013;45(1):81–86. doi:10.2340/16501977-1054
49. Nieboer AP, Koolman X, Stolk EA. Preferences for long-term care services: willingness to pay estimates derived from a discrete choice experiment. Soc Sci Med. 2010;70(9):1317–1325. doi:10.1016/j.socscimed.2009.12.027
50. Özdemir S, Johnson FR, Hauber AB. Hypothetical bias, cheap talk, and stated willingness to pay for health care. J Health Econ. 2009;28(4):894–901. doi:10.1016/j.jhealeco.2009.04.004
51. de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8(5):373–384. doi:10.1007/s40271-015-0118-z
52. Rose JM, Bliemer MCJ. Sample size requirements for stated choice experiments. Transportation. 2013;40(5):1021–1041. doi:10.1007/s11116-013-9451-z
53. Krinsky I, Robb AL. On approximating the statistical properties of elasticities. Rev Econ Stat. 1986;68(4):715–719. doi:10.2307/1924536
54. Çıkrıkçı Ö, Çıkrıkçı N, Griffiths M. Fear of COVID-19, stress and depression: a meta-analytic test of the mediating role of anxiety. Psychol Psychother. 2022;95(4):853–874. doi:10.1111/papt.12406
55. Heiat M, Heiat F, Halaji M, et al. Phobia and Fear of COVID-19: origins, complications and management, a narrative review. Ann Ig. 2021;33(4):360–370. doi:10.7416/ai.2021.2446
56. Wasim A, Truong J, Bakshi S, Majid U. A systematic review of fear, stigma, and mental health outcomes of pandemics. J Ment Health. 2023;32(5):920–934. doi:10.1080/09638237.2022.2091754
57. Catma S, Varol S. Willingness to pay for a hypothetical COVID-19 vaccine in the United States: a contingent valuation approach. Vaccines. 2021;9(4):4. doi:10.3390/vaccines9040318
58. Zhou L, Yan W, Li S, et al. Cost-effectiveness of interventions for the prevention and control of COVID-19: systematic review of 85 modelling studies. J Glob Health. 2022;12:05022. doi:10.7189/jogh.12.05022
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.