Back to Journals » Patient Preference and Adherence » Volume 17
Patients’ Preferences for Adjunctive Parkinson’s Disease Treatments: A Discrete-Choice Experiment
Authors Serbin M, Marras C, Mansfield C , Leach C, Yonan C , Sheehan M, Donnelly A, Klepitskaya O
Received 23 May 2023
Accepted for publication 12 August 2023
Published 13 September 2023 Volume 2023:17 Pages 2263—2277
DOI https://doi.org/10.2147/PPA.S420051
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Michael Serbin,1 Connie Marras,2 Carol Mansfield,3 Colton Leach,3 Charles Yonan,1 Margaret Sheehan,4 Anne Donnelly,5 Olga Klepitskaya1
1Neurocrine Biosciences, Inc., San Diego, CA, USA; 2The Edmond J. Safra Program in Parkinson’s Disease, University Health Network, University of Toronto, Toronto, ON, Canada; 3RTI Health Solutions, Research Triangle Park, NC, USA; 4Ashurst, Washington, DC, USA; 5Kellogg School of Management, Northwestern University, Evanston, IL, USA
Correspondence: Carol Mansfield, RTI Health Solutions, 3040 East Cornwallis Road, PO Box 12194, Research Triangle Park, NC, 27709, USA, Tel +19195418053, Email [email protected]
Background: Several adjunctive medications are available to reduce OFF time between levodopa/carbidopa (LD/CD) doses for people with Parkinson’s disease (PD).
Objective: To explore how individuals with PD balance benefits and burdens when considering adjunctive medications.
Methods: US adults (30– 83 years) with self-reported PD, currently treated with LD/CD, who experienced OFF episodes were recruited through the Fox Insight study to complete a discrete-choice experiment survey. Respondents selected among experimentally designed profiles for hypothetical adjunctive PD treatments that varied in efficacy (additional ON time), potential adverse effects (troublesome dyskinesia, risk of diarrhea, risk of change in bodily fluid color), and dosing frequency or the option “No additional medicine”. Data were analyzed with random-parameters logit models.
Results: Respondents (N=480) would require ≥ 60 additional minutes of daily ON time to accept either a 40% risk of change in bodily fluid color or 10 additional minutes with troublesome dyskinesia daily. Respondents would require 40 additional minutes of daily ON time to accept a 10% risk of diarrhea and 22 additional minutes of daily ON time to switch from 1 additional pill each day to 1 pill with each LD/CD dose. On average, respondents preferred adjunctive PD medication over no additional medication. Results predicted that 59.1% of respondents would select a hypothetical treatment profile similar to opicapone, followed by no additional medication (27.5%) and a hypothetical treatment profile similar to entacapone (13.4%).
Limitations: The data collected were based on responses to hypothetical choice profiles in the survey questions. The attributes and levels selected for this study were intended to reflect the characteristics of opicapone and entacapone; attributes associated with other adjunctive therapies were not evaluated.
Conclusion: Patients with PD expressed interest in adjunctive treatment to increase ON time and would accept reduced ON time to avoid adverse effects.
Plain Language Summary: People with Parkinson’s disease may experience OFF time, when their symptoms return between doses of levodopa/carbidopa. Some medicines can reduce OFF time when taken in addition to levodopa/carbidopa. These additional medicines have their own benefits and side effects. In this study, researchers used a survey to understand what side effects people with Parkinson’s disease would accept to have more ON time. Respondents were presented with a series of choices between 2 hypothetical medicines that increased daily ON time or no additional medicine. The medicines were described by 5 features (benefits and side effects). Respondents’ answers to the survey questions allowed researchers to estimate which unwanted side effects people with Parkinson’s disease would accept in exchange for additional ON time. The most important features of a medicine to reduce OFF time, in order, were (1) Lower risk of a change in urine, sweat, or saliva color; (2) Fewer additional minutes with troublesome dyskinesia each day; and (3) Increase in daily ON time. Introducing a 40% risk of change in urine, sweat, or saliva color (from no risk) would require more than 60 minutes of additional daily ON time to offset. An additional 10 minutes of troublesome dyskinesia each day would require more than 60 minutes of additional daily ON time. Introducing a 10% risk of diarrhea (from no risk) would require 40 minutes of additional ON time. Individuals’ preferences for the key features of medications that reduce OFF time for people with Parkinson’s disease should be considered when making treatment decisions.
Keywords: discrete choice, Parkinson’s, stated preferences
Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders, second only to Alzheimer’s disease,1 affecting an estimated 7 to 10 million people worldwide. Symptoms of PD vary across individuals, thus requiring treatment options that are tailored to the specific person. The gold-standard pharmacological treatment for those with PD is levodopa, given with a dopamine decarboxylase inhibitor (eg, carbidopa, benserazide) to minimize breakdown in the periphery.2 Progression of disease is associated with a “wearing-off” phenomenon, characterized by the return of PD motor and nonmotor symptoms before the next scheduled dose. The time spent in the state when PD symptoms re-emerge is called “OFF time”. In some cases, OFF time is predictable, whereas in others it can be sudden and unexpected. This adds to the complexity of selecting therapeutic options, demonstrating a need for reliable, effective, and tolerable treatments to help patients optimize “ON time”, during which their PD symptoms are managed.
A number of daily and on-demand therapies are available to expand the therapeutic window of levodopa/carbidopa (LD/CD) and reduce burdensome OFF time between LD/CD doses; such therapies include monoamine oxidase B inhibitors, dopamine agonists (eg, apomorphine), LD/CD intestinal gel, and catechol-O-methyltransferase (COMT) inhibitors.3–8 COMT inhibitors are widely used first-line oral adjunctive therapies for PD.9 The COMT inhibitors opicapone and entacapone work by inhibiting the metabolism of levodopa, thereby prolonging its therapeutic effect.10 Opicapone and entacapone differ by frequency of administration and adverse event (AE) profile. Opicapone is a long-acting, once-a-day medicine, while entacapone should be taken with each dose of LD/CD and is associated with risks of bodily fluid discoloration and diarrhea.11,12 Little is known about patients’ preferences for the potential benefits and AEs of these adjunctive medications.
This study aimed to explore how individuals with PD balance treatment benefits and burdens, such as adverse effects, when considering adjunctive treatment with a COMT inhibitor. The specific study objectives were (1) to quantify patient preferences for features associated with oral adjunctive COMT inhibitors for OFF episodes, and to estimate the conditional relative importance of treatment features using a discrete-choice experiment (DCE) approach; (2) to quantify the minimum acceptable benefit in additional minutes of ON time that patients would require to accept specific increases in risk of treatment-related AEs; (3) to calculate the probability of patients selecting a medicine with one profile over another for a set of alternative treatments for PD and the option of no treatment; and (4) to test for differences in preferences for treatment attributes between patient subgroups.
Methods
Study Design
An online DCE survey to measure how individuals with PD consider tradeoffs between the benefits and burdens of adjunctive medications was developed on the basis of published data and prescribing information for 2 approved COMT inhibitors: entacapone and opicapone. DCEs have been used to explore patients’ treatment preferences in a variety of disease areas.13–15 In a DCE, respondents select between pairs of hypothetical treatment profiles, each containing various combinations of attributes with varying levels, in a series of questions. The pattern of respondents’ choices reveals their preferences, on average, for the attributes and levels included in the experiment. The DCE was conducted following good research practice guidelines.16–18 To ensure that the survey was patient centered, the study team included patient advisors and clinical experts. The RTI International Institutional Review Board reviewed the study materials and deemed the study exempt from full review.
The DCE exercise presented respondents with a series of 9 questions (see Figure 1). Each question offered a choice between 2 hypothetical adjunctive PD treatment profiles and the option “no additional medicine”. The hypothetical treatment profiles were created by an experimental design and defined by 5 attributes, each with varying levels: increase in daily ON time; additional minutes with troublesome dyskinesia each day; risk of diarrhea; risk of change in urine, sweat, or saliva color; and frequency of daily administration (Table 1). The attributes were selected on the basis of the published data and input from the study team, and the levels were determined on the basis of product labels and head-to-head clinical data for opicapone and entacapone.8,11,12,19
 |
Table 1 Oral Adjunctive Therapy Discrete-Choice Experiment Attributes and Levels |
 |
Figure 1 Discrete-Choice Experiment Question. |
Consistent with established good research practices,16,20 we pretested the survey instrument using semistructured individual interviews via telephone/web conference, with a convenience sample of 15 participants who were living in the United States, were aged 30 to 83 years, had a self-reported physician diagnosis of PD, were being treated with LD/CD, had experience with OFF episodes, and were not taking prescription medication for cognitive concerns. Patients were recruited through the Michael J. Fox Foundation for Parkinson’s Research (MJFF) online observational research study Fox Insight. Initially, interviewees also had to experience at least 90 minutes of OFF time each day to be eligible. However, because PD is such a heterogenous condition, some participants indicated that this was difficult to estimate and that their OFF time can vary greatly week to week. A new series of more direct screening questions was tested in place of the original question, and the amount of OFF time per day was removed from the eligibility criteria. During the pretest interviews, the wording and descriptions in the surveys were tested to ensure comprehension by study participants, minimal cognitive bias, and patient centricity. An important insight from the pretest interviews was that the participants found the concept of a gain in ON time to be more understandable than a decrease in OFF time. Thus, although the adjunctive treatments of interest are for management of OFF time, the treatment benefit attribute in the survey was defined as an increase in ON time. After the pretest interviews, the survey was finalized and programmed for online administration.
Study Population
Patients were recruited through Fox Insight, an ongoing study of people with PD. The Fox Insight initiative is focused on building a cohort of individuals living with PD who are interested in contributing patient-reported data, including medical and medication history, symptoms, and lifestyle habits, to better understand the patient experience. Participants in Fox Insight are also eligible to take part in ancillary research studies such as this one. Email invitations to participate in the survey were distributed by the MJFF. Eligible participants met the following criteria: were living in the United States; were aged 30 to 83 years, to approximate the age range of participants in clinical trials of adjunctive PD treatments; had a self-reported physician diagnosis of PD; were currently treated with LD/CD; had experience with OFF episodes; had experienced an OFF episode in the past week; and were not taking a medicine for cognitive concerns. The invitation to complete the survey was sent to Fox Insight panel members on 5 August 2021, and the survey was closed on 24 August 2021.
Statistical Analyses
The DCE data were analyzed with the use of a random-parameters logit (RPL) model that relates the choices respondents make to the differences in the attribute levels across the alternatives in each choice question.18 The RPL model mitigates potential estimation bias from unobserved preference heterogeneity among respondents; it does this by estimating a distribution of preferences for each mean preference parameter.21,22 The analyses were performed in STATA 16 (StataCorp; College Station, TX).
The RPL results, which produce a relative preference weight for each attribute level, were used to calculate the conditional relative importance of the attributes. The conditional relative importance provides a measure of the overall relative importance of the attributes, given the range of levels selected, and is calculated as the difference between the preference weights for the most preferred and least preferred levels of each attribute.
Minimum acceptable benefits, which are the level of benefit that offsets exactly the burden attributable to an increase in risk or a worsening in the levels of another attribute, were also calculated. Estimates of minimum acceptable benefit were calculated as the increase in minutes of daily ON time (from a baseline of 60 minutes) provided by the additional treatment required for respondents to accept a given worsening in the levels of treatment-related risks (more time with troublesome dyskinesia, greater risk of diarrhea, and greater risk of discoloration of bodily fluids) and a greater frequency of treatment administration. These calculations hold constant the levels of all attributes other than the one attribute that is changing.
The results of a DCE survey can be used to assess predicted choice probabilities, or the probability that respondents would select medicines with different profiles on the basis of preferences expressed in the DCE data. To provide a real-world context, predicted choice probabilities using attribute levels resembling those of opicapone and entacapone, as well as the “No additional medicine” option, were calculated from the RPL models. These probabilities were based on the differences in preference weights associated with the levels of the attributes for each treatment alternative. Individual-level preference weights for each respondent were estimated using the sequence of choices from the DCE question and the estimated sample preference weights; for the full sample, predicted choice probabilities were calculated as the average of the individual-level probabilities.
Finally, subgroup models were estimated to test for systematic differences in attribute preferences for 5 subgroups, defined by the following self-reported characteristics: prior experience with discoloration of bodily fluids (vs no experience), prior experience with troublesome dyskinesia (vs no experience), less than 2 hours of OFF time in a typical day (vs ≥ 2 hours of OFF time in a typical day), gender (male vs female), and age (respondents who were younger than the median sample age of 68 years vs those aged 68 years and older).
Results
Respondent Characteristics
The survey was administered online between 2 August 2021 and 25 August 2021, and a total of 480 adults completed the survey. The survey respondents had an average age of 67 years, and 69% had received a diagnosis of PD at least 5 years before completing the survey (Table 2). Among the respondents, 27% reported experiencing ≥ 2 hours of OFF time daily, 34% reported having experience with bodily fluid discoloration, and 44% reported having experience with troublesome dyskinesia.
 |
Table 2 Respondent Characteristics |
Preference Weights and Conditional Relative Importance
Figure 2 presents the normalized mean preference weight estimates for each attribute level for the full sample. Attribute levels with higher weights are preferred (ie, have higher utility) to attributes with lower weights. Preferences were ordered as expected, with respondents preferring better levels to worse levels and, on average, preferring adjunctive medications to “no additional medicine”. An increase from no risk of a change in urine, sweat, or saliva color to a 40% risk was the most important change in utility, given the levels selected for the study, yielding a utility change of 3.79. The smallest utility change, although still statistically different from zero, was from a change of 95 more minutes in ON time each day to 120 more minutes in ON time each day (0.72). The utility change associated with a 40% increase in risk of change in bodily fluid color was 5 times more than the utility change for the last 25 additional minutes in ON time (5.26 = 3.79 ÷ 0.72).
Figure S1 shows the scaled conditional relative attribute importance of changing each attribute from the least preferred level to the most preferred level, revealing the relative impact of each attribute on respondents’ treatment preferences. The conditional relative importance values for all the study attributes were statistically significantly different from one another. Over the ranges presented, the most important attribute was a 40% risk of change in urine, sweat, or saliva color, followed by 10 additional minutes with troublesome dyskinesia, a 60-minute increase in daily ON time, a 10% risk of diarrhea, and the change from taking the additional medicine once a day to an additional pill with each dose of LD/CD.
Minimum Acceptable Benefit
Minimum acceptable benefits provide another way to quantify the relative importance of changes from one level of an attribute to another: the average increase in additional minutes of ON time each day (from a baseline of 60 minutes) that would compensate for a given worsening in the levels of risks or frequency of administration. On average, respondents would require more than 60 additional minutes of daily ON time (where 60 additional minutes was the largest change in ON time presented in the survey) to accept either a change from a 0% or 10% risk to a 40% risk of change in urine/sweat/saliva color or a change from 0 additional minutes to 10 additional minutes with troublesome dyskinesia daily (Table 3). Respondents would require 40 additional minutes of daily ON time to accept a 10% risk of diarrhea and 22 additional minutes of daily ON time to switch from 1 additional pill each day to 1 pill with each LD/CD dose.
 |
Table 3 Respondent Minimum Acceptable Benefit as Increase in Additional ON Time in Minutes for a Given Change in Treatment Attributes |
Predicted Choice Probabilities
To understand how respondents considered medication profiles that reflect real-world treatment options, the attribute levels could be selected to define treatment profiles corresponding with the levels for opicapone, entacapone, and “no additional medicine”, as shown in Table 4. The DCE results predicted that a majority of respondents (59.1%) would select a treatment profile similar to opicapone, followed by “no additional medicine” (27.5%) and a treatment profile similar to entacapone (13.4%), implying that 78% of respondents who selected an additional medicine would prefer a profile similar to opicapone. For the profile similar to opicapone, the preference for 25 additional minutes of ON time, no risk of diarrhea and bodily fluid discoloration, and once-daily dosing outweighed 5 additional minutes of troublesome dyskinesia, contributing to patient preference for this profile.
 |
Table 4 Profiles for Preference Choice Prediction: Opicapone versus Entacapone |
Subgroup Analyses
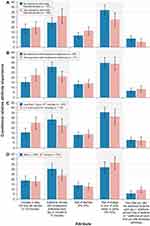
Across the subgroup analyses, prior experience with an AE (bodily fluid discoloration or troublesome dyskinesia) tended to make that AE comparatively less salient to respondents than it was to respondents who had not experienced the AE, although it was still a significant concern. Figure 3 shows the conditional relative importance estimates from the subgroup analyses. Respondents with a prior history of bodily fluid discoloration placed less importance on the risk of bodily fluid discoloration compared with respondents without a prior history of bodily fluid discoloration. Respondents with prior experience with bodily fluid discoloration ranked avoiding additional minutes with troublesome dyskinesia as the most important attribute (Figure 3A); respondents with no prior experience with bodily fluid discoloration, however, ranked avoiding the risk of bodily fluid discoloration as the most important attribute. Similarly, respondents with a prior history of troublesome dyskinesia placed less importance on additional minutes with troublesome dyskinesia compared with respondents without a prior history of troublesome dyskinesia. Respondents with troublesome dyskinesia experience ranked avoiding a 40% risk of bodily fluid discoloration as most important, followed by increasing their daily ON time by 60 minutes and avoiding 10 additional minutes of troublesome dyskinesia each day (Figure 3B). Finally, respondents with 2 or more hours of OFF time each day placed greater importance on increasing daily ON time compared with respondents who had less than 2 hours of OFF time each day (Figure 3C). For respondents with less than 2 hours of OFF time each day, all conditional relative importance values were statistically different from the next greatest conditional relative importance at the 95% confidence level, except for the conditional relative importance values between increase in daily ON time and the risk of diarrhea. These respondents ranked avoiding a 40% risk of bodily fluid discoloration as most important, followed by avoiding 10 additional minutes of troublesome dyskinesia each day and increasing their daily ON time by 60 minutes.
In the subgroup analysis defined by gender, both respondents who identified as female and those who identified as male ranked avoiding the risk of bodily fluid discoloration as the most important attribute and ranked how often you take the additional medicine each day as the least important attribute (Figure 3D). For respondents who identified as male, the relative importance of avoiding the risk of bodily fluid discoloration was not statistically different from the relative importance of additional minutes with troublesome dyskinesia each day. Although respondents’ gender identities had a statistically significant impact on preferences, this did not affect the ranking of attribute importance, and overall, preferences were similar across the subgroups and the full sample. In the subgroup analysis defined by respondents’ median age (< 68 years vs ≥ 68 years), preferences between these 2 groups of respondents were not statistically significantly different.
Discussion
This research highlights the importance of patient preference when choosing adjunctive PD medications. Our results show that most patients are willing to add adjunctive therapy to manage OFF time and that, among the attributes associated with adjunctive therapies, avoiding a 40% risk of a change in urine, sweat, and saliva color was most important to patients over the range of attributes and levels presented in the survey. While change in bodily fluid color, a risk associated with entacapone, may not be considered a major concern among physicians (as it is not of medical concern), this finding indicates that change in bodily fluid color matters to patients when starting a new therapy and may lead to a poor treatment experience. After this concern, patients most prioritized avoiding 10 additional minutes with troublesome dyskinesia each day; increasing daily ON time by 60 minutes; avoiding a 10% risk of diarrhea; and, finally, less frequent administration.
The increases in additional daily ON time a respondent would require to offset changes between levels of treatment-related attributes and risks were largest for a change from no additional minutes with troublesome dyskinesia to 10 additional minutes, and for changes from a 0% or 10% risk of bodily fluid discoloration to a 40% risk. All of these changes would require, on average, more than 60 additional minutes of ON time. We also predicted the probability that each respondent would select a medicine profile similar to opicapone over another profile similar to entacapone or “no additional medicine”. On average, an additional treatment similar to opicapone had the highest probability of being selected (approximately 59%). On average, respondents would be more likely to opt out from additional treatment (28%) than to select an additional treatment with characteristics similar to entacapone (13%). This finding indicates a preference for a treatment that provides more ON time and avoids a higher risk of diarrhea and higher risk of change in bodily fluid color, even if the treatment causes 10 more minutes of troublesome dyskinesia. Subgroup analyses revealed that respondents with a prior history of an AE placed less importance on the risk of that AE compared with respondents without a prior history. This may be helpful in patient counselling when discussing options with patients who have not had experience with a particular AE.
To our knowledge, this study is the first to explore how individuals with PD trade off the specific attributes of daily adjunctive therapy to reduce OFF time. Previous evidence has shown that individuals with PD experience significantly reduced health-related quality of life during OFF episodes and that these individuals highly value increases in ON time as well as being able to predict the onset of OFF time.23 A DCE survey to evaluate patient preferences for attributes of on-demand therapies for OFF time—including mode of administration (with associated AEs), time to and duration of ON time, and out-of-pocket cost—found that patients prioritize cost, mode of administration with associated AEs, and time to ON time more than they do duration of ON time.24 Our results are broadly consistent with prior studies in demonstrating that, while optimizing ON time is a priority for individuals with PD, AEs and risks associated with PD treatments are key drivers of preferences.
The aim of this study was to explore patients’ preferences for the attributes that differentiate opicapone and entacapone. The second-generation COMT inhibitor entacapone requires multiple doses per day and is associated with adverse effects including diarrhea and bodily fluid discoloration,11 while the third-generation COMT inhibitor opicapone is administered once daily and is not known to cause gastrointestinal adverse effects or bodily fluid discoloration.11,12,25,26 Understanding how patients value the attributes of these therapies may be useful to physicians in shared decision-making discussions with patients who are considering adjunctive therapy with a COMT inhibitor. It must be noted, however, that other oral adjunctive treatment options are available for individuals with PD but were not considered in our study, including monoamine oxidase B inhibitors and dopamine agonists.27,28 Clinical understanding of PD and the therapeutic landscape continue to evolve.29–32 Selecting the best adjunctive therapy to optimize an individual’s “ON” time with LD/CD must be a shared decision based on a physician’s clinical judgment and a patient’s individual preferences.28
Limitations of this study need to be acknowledged. First, this study recruited patients from the MJFF Fox Insight cohort, which may not reflect the United States population with PD. In particular, the sample is mostly White and highly educated. The survey was written in English, limiting the sample to English speakers. The final DCE survey instrument was administered online, further introducing the potential for selection bias into the sample. The data collected were based on responses to hypothetical choice profiles in the DCE tasks. These choices are intended to simulate possible clinical decisions but do not have the same clinical, financial, or emotional consequences of actual real-world decisions; thus, differences can arise between stated and actual choices. Finally, the attributes and levels selected for this study were intended to reflect the characteristics of opicapone and entacapone. The study did not evaluate attributes associated with other adjunctive therapies, and preferences may have been different if these had been included.
Conclusions
In conclusion, our results demonstrate that patients with PD are willing to add adjunctive therapy with a COMT inhibitor to manage OFF time and that nuances of safety, efficacy, and administration frequency in combination may result in large differences in patients’ preference for medications. As new treatments for PD become available, individual patients’ preferences when balancing the benefits and risks of adjunctive medications that reduce daily OFF time are an important consideration in shared decision-making with their physicians. Future research should explore patient preferences for attributes associated with other adjunctive treatment options.
Abbreviations
AE, adverse event; CD, carbidopa; COMT, catechol-O-methyltransferase; DCE, discrete-choice experiment; LD, levodopa; MJFF, Michael J. Fox Foundation for Parkinson’s Research; PD, Parkinson’s disease; RPL, random-parameters logit; RTI, RTI International.
Ethics Approval and Informed Consent
The RTI International Institutional Review Board reviewed the study materials and deemed the study exempt from full review. All study participants provided informed consent electronically before completing the survey. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Acknowledgments
The authors thank the individuals who participated in the study. Kimberly Moon of RTI Health Solutions provided overall project management for this study. Kate Lothman of RTI Health Solutions provided medical writing services, which were funded by Neurocrine Biosciences. The Michael J. Fox Foundation for Parkinson’s Research provided support to recruit participants through the Fox Insight study.
Interim findings and selected results from this study were presented in the following poster presentations:
Serbin M, Sutphin J, Leach C, Mansfield C, Yonan C, Klepitskaya O, et al. Evaluating patients’ preferences for Parkinson’s disease treatments. Poster presented at the MDS Virtual Congress 2021; September 17, 2021.
Serbin M, Sutphin J, Leach C, Mansfield C, Yonan C, Klepitskaya O, et al. Evaluating patients’ preferences for Parkinson’s disease treatments. Poster presented at the 146th 2021 American Neurological Association (ANA) Virtual Meeting; October 17, 2021.
Klepitskaya O, Serbin M, Sutphin J, Leach C, Mansfield C, Yonan C, et al. Development of a patient survey on preferences for adjunctive parkinson’s disease medications: results from pretest interviews. Poster presented at the AMCP Nexus 2021; October 18, 2021.
Serbin M, Mansfield C, Sheehan M, Donnelly A. Development of a patient survey on preferences for adjunctive parkinson’s disease medications: results from pretest interviews. Poster presented at the 2021 OAANP Statewide Conference; October 21, 2021. Columbus, OH.
Serbin M, Mansfield C, Leach CA, Yonan C, Klepitskaya O, Sheehan M, et al. Patients’ preferences for adjunctive Parkinson’s disease treatments: a discrete-choice experiment. Poster presented at the 2022 AAN Annual Meeting; April 2–7, 2022. Seattle, WA.
Serbin M, Mansfield C, Leach CA, Yonan C, Sheehan M, Donnelly A, et al. Comparison of patient preferences for Parkinson’s disease treatments and reductions in OFF-time. Poster presented at the 2022 IAPRD National Conference; May 1–4, 2022. Prague, Czech Republic.
Serbin M, Mansfield C, Leach CA, Yonan C, Klepitskaya O, Sheehan M, et al. Patients’ preferences for adjunctive Parkinson’s disease treatments: a discrete-choice experiment. Poster presented at the PSG 32nd Annual Meeting; June 3–5, 2022. Phoenix, AZ.
Serbin M, Mansfield C, Leach CA, Yonan C, Klepitskaya O, Sheehan M, et al. Patients’ preferences for adjunctive Parkinson’s disease treatments: a discrete-choice experiment. Poster presented at the 2022 AANP National Conference; June 22–26, 2022. Orlando, FL.
Serbin M, Mansfield C, Leach CA, Yonan C, Klepitskaya O, Sheehan M, et al. Do patients’ experiences with side effects affect their preferences for adjunctive Parkinson’s disease treatments? Poster presented at the 8th EAN Congress; June 25, 2022. Vienna, Austria.
Author Contributions
Michael Serbin, Connie Marras, Carol Mansfield, Colton Leach, Charles Yonan, Margaret Sheehan, Anne Donnelly, and Olga Klepitskaya each made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas, and participated in drafting, substantially revising, or critically reviewing the article. All authors agreed on the journal to which the article will be submitted; reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. All authors agree to take responsibility and be accountable for the contents of the article.
Funding
This study was performed under a research contract between Neurocrine Biosciences and RTI Health Solutions and was funded by Neurocrine Biosciences.
Disclosure
Michael Serbin, Charles Yonan, and Olga Klepitskaya are employees of Neurocrine Biosciences. Carol Mansfield is an employee of RTI Health Solutions, and Colton Leach was an employee of RTI Health Solutions when this research was conducted. Connie Marras is on the steering committee for the Fox Insight study. Margaret Sheehan and Anne Donnelly have nothing to disclose for this work.
References
1. Naqvi E. Parkinson’s disease statistics. Available from: https://parkinsonsnewstoday.com/parkinsons-disease-statistics/.
2. Zahoor I, Shafi A, Haq E. Pharmacological treatment of Parkinson’s disease. In: Stoker TB, Greenland JC, editors. Parkinson’s Disease: Pathogenesis and Clinical Aspects. Brisbane, Australia: Codon Publications; 2018:129–145.
3. Dezsi L, Vecsei L. Monoamine oxidase B inhibitors in Parkinson’s disease. CNS Neurol Disord Drug Targets. 2017;16(4):425–439. doi:10.2174/1871527316666170124165222
4. Swope DM. Rapid treatment of “wearing off” in Parkinson’s disease. Neurology. 2004;62(6 Suppl 4):S27–S31. doi:10.1212/wnl.62.6_suppl_4.s27
5. Olanow CW, Poewe W, Rascol O, Stocchi F. On-demand therapy for OFF episodes in Parkinson’s disease. Mov Disord. 2021;36(10):2244–2253. doi:10.1002/mds.28726
6. Standaert DG, Boyd JT, Odin P, Robieson WZ, Zamudio J, Chatamra K. Systematic evaluation of levodopa-carbidopa intestinal gel patient-responder characteristics. NPJ Parkinsons Dis. 2018;4(1):4. doi:10.1038/s41531-017-0040-2
7. Kuoppamäki M, Vahteristo M, Ellmén J, Kieburtz K. Pooled analysis of Phase III with entacapone in Parkinson’s disease. Acta Neurol Scand. 2014;130(4):239–247. doi:10.1111/ane.12278
8. Ferreira JJ, Lees A, Rocha JF, Poewe W, Rascol O, Soares-da-Silva P. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol. 2016;15(2):154–165. doi:10.1016/S1474-4422(15)00336-1
9. Fabbri M, Ferreira JJ, Rascol O. COMT inhibitors in the management of Parkinson’s disease. CNS Drugs. 2022;36(3):261–282. doi:10.1007/s40263-021-00888-9
10. Jenner P, Rocha JF, Ferreira JJ, Rascol O, Soares-da-silva P. Redefining the strategy for the use of COMT inhibitors in Parkinson’s disease: the role of opicapone. Expert Rev Neurother. 2021;21(9):1019–1033. doi:10.1080/14737175.2021.1968298
11. Entacapone [Prescribing information]. Novartis Pharmaceuticals Corporation; 2014.
12. Ongentys (Opicapone) Capsules, for Oral Use [Prescribing Information]. San Diego: Neurocrine Biosciences, Inc; 2020.
13. de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21(2):145–172. doi:10.1002/hec.1697
14. Clark M, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. Pharmacoeconomics. 2014;32:883–902. doi:10.1007/s40273-014-0170-x
15. Soekhai V, de Bekker-Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–226. doi:10.1007/s40273-018-0734-2
16. Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013
17. Johnson FR, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis discrete-choice experiment experimental design good research practices task force. Value Health. 2013;16(1):3–13. doi:10.1016/j.jval.2012.08.2223
18. Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis experimental design task force. Value Health. 2016;19(4):300–315. doi:10.1016/j.jval.2016.04.004
19. Parkinson Study Group. Entacapone improves motor fluctuations in levodopa-treated Parkinson’s disease patients. Ann Neurol. 1997;42(5):747–755. doi:10.1002/ana.410420511
20. Innovative Medicines Consortium. PREFER recommendations: why, when and how to assess patient preferences in medical product decision-making; 2022. Available from: https://zenodo.org/record/6592304#.ZEv5Ts7MJPY.2022.
21. Train K. Discrete Choice Methods with Simulation.
22. Train K, Sonnier G. Mixed logit with bounded distributions of correlated partworths. In: Scarpa R, Alberini A, editors. Application of Simulation Methods in Environmental and Resource Economics. Dordrecht, Netherlands: Springer; 2005:117–134.
23. Kerr C, Lloyd EJ, Kosmas CE, et al. Health-related quality of life in Parkinson’s: impact of ‘off’ time and stated treatment preferences. Qual Life Res. 2016;25(6):1505–1515. doi:10.1007/s11136-015-1187-0
24. Thach A, Sutphin J, Coulter J, Leach C, Pappert E, Mansfield C. Patient preferences for treating “OFF” episodes in Parkinson’s disease: a discrete choice experiment. Patient Prefer Adherence. 2021;15:1187–1196. doi:10.2147/PPA.S301644
25. Salamon A, Zádori D, Szpisjak L, Klivényi P, Vécsei L. What is the impact of catechol-O-methyltransferase (COMT) on Parkinson’s disease treatment? Expert Opin Pharmacother. 2022;23(10):1123–1128. doi:10.1080/14656566.2022.2060738
26. Jost WH. Evaluating opicapone as add-on treatment to levodopa/DDCI in patients with Parkinson’s disease. Neuropsychiatr Dis Treat. 2022;18:1603–1618. doi:10.2147/NDT.S279362
27. Regensburger M, Ip CW, Kohl Z, et al. Clinical benefit of MAO-B and COMT inhibition in Parkinson’s disease: practical considerations. J Neural Transm. 2023;130(6):847–861. doi:10.1007/s00702-023-02623-8
28. Fabbri M, Barbosa R, Rascol O. Off-time treatment options for Parkinson’s disease. Neurol Ther. 2023;12(2):391–424. doi:10.1007/s40120-022-00435-8
29. Yadav SK, Rai SN, Singh SP. Mucuna pruriens reduces inducible nitric oxide synthase expression in Parkinsonian mice model. J Chem Neuroanat. 2017;80:1–10. doi:10.1016/j.jchemneu.2016.11.009
30. Rai SN, Yadav SK, Singh D, Singh SP. Ursolic acid attenuates oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in MPTP-induced Parkinsonian mouse model. J Chem Neuroanat. 2016;71:41–49. doi:10.1016/j.jchemneu.2015.12.002
31. Prakash J, Chouhan S, Yadav SK, Westfall S, Rai SN, Singh SP. Withania somnifera alleviates parkinsonian phenotypes by inhibiting apoptotic pathways in dopaminergic neurons. Neurochem Res. 2014;39(12):2527–2536. doi:10.1007/s11064-014-1443-7
32. Rai SN, Singh P. Advancement in the modelling and therapeutics of Parkinson’s disease. J Chem Neuroanat. 2020;104:101752. doi:10.1016/j.jchemneu.2020.101752
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.