Back to Journals » Integrated Pharmacy Research and Practice » Volume 4
Patient views on an electronic dispensing device for prepackaged polypharmacy: a qualitative assessment in an ambulatory setting
Authors Allemann S , Hersberger KE , Arnet I
Received 22 June 2015
Accepted for publication 1 September 2015
Published 6 November 2015 Volume 2015:4 Pages 167—174
DOI https://doi.org/10.2147/IPRP.S90923
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Jonathan Ling
Video abstract presented by Samuel S Allemann
Views: 313
Samuel S Allemann, Kurt E Hersberger, Isabelle Arnet
Pharmaceutical Care Research Group, Department of Pharmaceutical Sciences, University of Basel, Basel, Switzerland
Objective: To collect opinions on medication management aids (MMAs) in general and on an electronic MMA (e-MMA) dispensing prepackaged polypharmacy in sealed pouches.
Study setting: The setting involved community-dwelling older adults in Basel, Switzerland, in 2013.
Study design: The study involved 1) a 14-day trial with the e-MMA and 2) a focus group to identify general attributes of MMAs, their applicability to the e-MMA, and possible target groups for the e-MMA.
Data collection methods: Six participants using long-term polypharmacy and willing to try new technologies completed the 14-day trial and participated in the focus group. Inductive content analysis was performed to extract data.
Principal findings: Participants rated ten of 17 general attributes as clearly applicable to the e-MMA and five as unsuitable. Attributes pertained to three interrelating themes: product design, patient support, and living conditions. Envisaged target groups were patients with time-sensitive medication regimens, patients with dementia, the visually impaired, and several patients living together to prevent accidental intake of the wrong medication.
Conclusion: The evaluated e-MMA for prepackaged polypharmacy met the majority of the requirements set for an MMA. Patients' living conditions, such as mobility, remain the key determinants for acceptance of an e-MMA.
Keywords: pharmaceutical care, medication adherence, medication management aids, automated drug dispensing
Introduction
Health care professionals not only have to provide patients with the correct diagnosis and appropriate therapy. They must also enable patients to “take their medication as prescribed”, a seemingly simple behavior which is known as medication adherence.1
A review of 50 years of adherence research estimates a mean adherence rate of 75.2%, ranging from 65.5% for sleep disorders to 88.3% for HIV.2 Nonadherence, or the failure to take medication as prescribed, strongly relates to negative outcomes.3 The development of effective interventions to improve adherence is a quest many researchers and practitioners have been pursuing for decades. Due to its inherent complexity, there is no one-size-fits-all solution to combat nonadherence.4 A simple method is the use of a device that holds a predefined number of medications organized by day and time according to a patient’s individual therapy plan. Such medication management aids (MMAs) exist in various forms and they are widely used for presumably nonadherent patients, especially older adults.5
Annotation of relevant literature
Between 62% and 75% of older adults report at least part-time use of MMAs.6,7 MMAs can be managed by the patient or are prefilled at a pharmacy or by another caregiver.8 Despite their widespread use, the authors of a review of the effects of MMA concluded that the design and targeting of these devices need further research.5 MMAs generally target therapy-related factors, condition-related factors, and social factors of nonadherence, aiming to improve unintentional nonadherence during the implementation phase.1 Given the fact that all doses need to be prepared in advance for each intake time, patients are able to see whether they have already taken their medication or not. Hence, MMAs classify as “feedback and monitoring” interventions according to the behavior change technique taxonomy.9 Until now, the measurement of adherence with MMAs was restricted to indirect or subjective measures like pill counts, timeliness of refills, or patients’ self-report.10 Electronic measurement is considered a “gold standard”, but with polypharmacy, this method is in its early stages.10 Recently, electronic MMAs (e-MMAs) emerged, reminding patients with acoustic or visual alerts to take their medication, dispensing the right medication at the right time, and tracking each event. These developments allow for the objective measurement of adherence. We could identify only very few studies about e-MMAs, either focusing on measuring adherence only11,12 or on the technical specifications.13–15 In a study assessing the satisfaction of 96 older adults with an electronic medication-dispensing device in home care, participants accepted the device as “very easy to use, very reliable and helpful in the management of their medications”.16 Although a high rejection rate was reported, the study report did not address participants’ motivation to use or reject the device in the first place. The final report on a project with e-MMAs aiming at improving self-management among nonadherent patients concluded that “anyone who has difficulty remembering to take their medication” may benefit from such an intervention.17 Of 380 participants of this project, more than 30% were in the early stages of dementia and approximately 20% had physical disabilities such as dexterity issues or visual impairment. Approximately 10% left the study because they did not like the dispenser and approximately 7.5% because they were nonadherent. Approximately half of all patients approached to participate declined for various reasons, eg, they did not like the look or the sound of the dispenser, they felt the dispenser was taking control of their medication management, or they did not want to take the device out to social events.
Thus, we hypothesized that programs using e-MMAs often missed targeting the optimal users. The goal of this study was to gather information regarding the use of an e-MMA by community-dwelling older adults using chronic polypharmacy. The aims were:
- to collect and evaluate attributes of MMAs important to patients;
- to evaluate the use of a specific e-MMA with polypharmacy prepackaged in pouches in relation to these attributes; and
- to identify the target group that could benefit most from the e-MMA.
Materials and methods
Participant selection
The investigators (IA and KEH) recruited a convenience sample of community-dwelling older adults with self-disclosed long-term use of polypharmacy and willingness to try innovative technologies from a community pharmacy in Basel, Switzerland. This study did not require ethics approval according to Swiss law. Informed consent was obtained from all participants.
MMA
An automatic tablet-dispensing and packaging system (Desk Type JV-30DE; HD-Medi, Düsseldorf, Germany) was used to repack all solid oral prescription medications for each participant into unit-of-dose pouches. Each pouch was imprinted with the patient’s name, date of birth, and date and time for intake, as well as the number, name, color, and shape of the medication contained (Figure 1). Every participant received a roll with pouches for 14 days loaded into a dispenser installed at their homes. The dispenser (Medido®; Innospense BV, Den Haag, the Netherlands) was a remote-controlled e-MMA reminding the patients with acoustic alerts to take their medication (Figure 2). Pushing the “OK” button stops the alarm and delivers the pouches with prepackaged medication. Date and time of delivery are simultaneously recorded with general packet radio service technology. Delivery of doses ahead of schedule is possible by pushing the OK button for 5 seconds. This important feature, named pocket-doses, enables patients to go out of the house during intake times. Time of dispensing was individually set according to participants’ preferences. One investigator (SSA) demonstrated the use of the dispenser during the installation and provided written instructions with telephone numbers to call an investigator for assistance in case of technical problems.
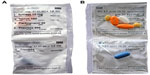  | Figure 1 Unit-of-dose pouches with prepacked oral solid medication from front (A) and back (B). |
Data collection
Participants were asked to write down any dispensing of pocket-doses, malfunctions, or noteworthy events during the 14 days of use. Upon returning the dispenser, they were interviewed based on a short interview guide with the following questions to collect spontaneous reactions:
- How was the operation of the device?
- How did you integrate the device into your daily life?
- What additional benefits can the device provide for daily medication intake?
- What monthly fee would you be willing to pay for the device?
Additionally, we invited all participants to attend a focus group. Focus groups provide concentrated interactions in a short time frame and allow the generation of data based on the synergy of the members in a group.18 For this exploratory study, only one focus group was carried out using a semi-structured approach. Based on the answers from the short interviews and literature, a preliminary list of attributes was compiled by the investigators. A focus group script was developed and pilot-tested with regard to comprehension and timeline with an 83-year-old female using chronic polypharmacy who was not enrolled in the study.
The focus group took place in a conference room of the University of Basel (Basel, Switzerland) and lasted 2 hours. First, participants filled out a short form including demographics and data about their medication therapy. After a brief introduction, participants were guided through the following four steps:
- write down attributes of MMAs in general judged as important (every participant individually);
- clarify the meaning of the attributes (plenary discussion);
- vote on the applicability of the attributes to the electronic dispenser, inclusive of additional attributes from the preliminary list; and
- define target groups for the dispenser based on one’s 14-day experience.
SSA moderated the focus group, while IA took notes and compared the proposed attributes with the preliminary list. Participants used playing cards to vote on the attributes: one color (red hearts) for “yes I agree”; another color (black clubs) for “no I disagree”; and the joker to initiate a discussion. Whenever a joker was raised, participants discussed issues and repeated their voting afterward, until no joker was displayed. The focus group was held in Swiss German and audiotaped. One researcher (SSA) orthographically transcribed the recording in German, preserving dialect expressions. Attributes and quotes mentioned in this article were translated into English by SSA and IA.
Content analysis
Inductive content analysis was used as theoretical framework based on Krueger’s approach19 as outlined by Rabiee.18 In brief, this method uses categories, which are derived directly from the data, as opposed to deductive content analysis that is based on earlier work.20 Krueger’s approach includes five interrelating stages: familiarization; identifying a thematic framework; indexing; charting; and mapping and interpretation.18 The data were coded by SSA, reviewed by IA, and discussed by both for validation. Inconsistencies were resolved by consensus. Attributes were grouped in sets to form major themes. No attributes were excluded.
Qualitative data were entered into the software MAXQDA 11 (VERBI GmbH, Berlin, Germany) to support the analyses.
The study data are reported according to the COREQ guideline, a checklist with consolidated criteria for reporting qualitative research.21
Results
Seven persons were contacted between February and May 2013. All agreed to participate and completed the full 14-day assessment period. One participant refrained from participating in the focus group due to conflicting dates and was excluded from the analyses. Six participants (four women, two men) aged 55 to 76 years (mean 65.3 years) attended the focus group (Table 1). All but two women were retired and all declared they conducted, on average, 38% of their daytime activities (excluding weekends) at home. Three women lived alone (50%), and the other participants shared a household with a partner. Participants were taking, daily, two to five (mean 3.3) solid oral medicines with a dosing schedule of one to three intake times and at least one intake in the morning. All 168 scheduled doses were delivered (100% reliability). All patients retrieved, in total, 28 doses (17%) as pocket-doses for intake outside of the home.
During step 1, participants wrote down 13 individual attributes of MMAs judged as important. Two further attributes emerged from the discussion (step 2), and another two were proposed from the preliminary list, summing to a total of 17 attributes (Table 2).
Participants rated ten attributes as clearly applicable to the electronic dispenser (Table 2). Five attributes were rated as unsuitable, including the consumption of power and production of waste (ecological aspect), lack of mobility, insufficient information about the prepackaged medication, and perceived inflexibility of the intake times. Participants also expressed the desire to receive reminders of upcoming refill events and appointments with the physician. The votes on two attributes (good-looking and place-saving) were equally distributed.
The themes that emerged during step 2 (discussion) were interrelated and concerned product design, patient support, and living conditions.
Product design
This theme relates to tangible attributes of the dispenser, which can be directly modified by changing its hardware or software (eg, the size, ease of use, and reliability). Participants controversially discussed the size and appearance of the dispenser, which was compared to a “monster” and a “toaster” by participant P5. The same participant found it difficult to find a place for the dispenser, wanting to hide it in “a corner of the home” (“eine Ecke in der Wohnung”). Conversely, P3 described the dispenser as “more beautiful than expected” (“schöner als erwartet”). Participants agreed that the appearance was subject to individual likings, as was the location where it was placed. All participants found it easy to use the dispenser as instructed. P3 found the OK button quite “rough-running”, but had no problems operating the device. P2 stated that the dispenser should allow narrower intervals than the 10-minute intervals to set reminders. P1, P2, and P6 mentioned the light emitted from the device was relatively bright, and the sound quite loud. P5 stated that the design became less important when she started experiencing benefits from the dispenser:
If I had to rely on it [the dispenser], I would have looked for some corner in the home, where it wouldn’t be very dominant [laughs]. However, the look didn’t play such a big role anymore. That has taken a back seat. (Ja wenn ich jetzt darauf angewiesen wäre, hätte ich irgend eine Ecke in der Wohnung gesucht, wo es nicht gerade dominant ist [lacht]. Aber es hat ja das Aussehen hat dann keine so grosse Rolle mehr gespielt. Das ist in den Hintergrund getreten).
The prepackaged medication in pouches was perceived as extremely reassuring and convenient. Simultaneous recording of the dispensing time did not worry the participants.
Patient support
This theme relates to the impact of the dispenser on patients’ abilities to adhere to their therapy (eg, the effectiveness of the dispenser in assuring the regular and timely intake of the correct dose). P1 mentioned that the dispenser acted as an alarm clock in the morning and that she took her medications on time, while she would otherwise just take them “any time before going to bed” (“irgendwann vor dem ins Bett gehen”). Participants also discussed the complexity of medication regimens, stating that the dispenser seemingly reduced the burden of taking multiple medications:
Because I only had one pouch, I only took one. It was like, less than before, when I have to take three drugs. Because it was like the three were on their own. (Weil ich nur eine Tüte hatte, habe ich nur eins genommen. Es war wie, weniger als vorher, wenn ich drei Medikamente nehmen muss. Weil es wie von alleine die drei gewesen ist). [P4]
However, participants voiced concerns about the handling of medication changes when there were still pouches in the dispenser:
I find it difficult, when I have to go to a doctor and I receive a new drug, it’s not in the pouches. How does one do it, do I take it myself until the roll is finished, the additional drug? Or when something needs to go out, does one empty out all pouches into a pill box, so it’s not wasted? (Ich finde es noch schwierig, wenn ich jetzt zum Arzt muss und ein neues Medikament erhalte, ist das nicht in den Beuteln. Wie macht man das, nehme ich es dann einfach selber bis die Rolle fertig ist, das zusätzliche Medikament. Oder wenn etwas raus muss, leert man alle Beutel in ein Dosett, damit es keine Verschwendung ist?) [P1]
Similarly, participants had the feeling of losing knowledge about their medication:
It is a danger; one simply takes what comes out and doesn’t think about how they [the drugs] act and how it plays together. (Es ist schon die Gefahr, man nimmt einfach was da hinaus kommt und überlegt sich gar nicht, wie die wirken und wie es zusammenspielt). [P1]
Two participants felt relieved by the device and mentioned that they had not to think about taking their medication because the dispenser took care of everything:
He [the dispenser] thinks for me and he beeps and then he spits it [the medication] out, everything ready, found it wonderful actually. Well, it is a luxury for me, you see, I don’t need it but it is, er, would be a great luxury. (Der denkt für mich und er piepst und dann spickt er es hinaus, alles parat, habe es wunderbar gefunden eigentlich. Also ist Luxus für mich oder brauche es nicht aber ist äh wär ist ein toller Luxus gewesen). [P5]
Living conditions
This theme covers the attributes flexibility of intake times and patient mobility. Three participants felt under pressure and other-directed because they had to be at home at specific times. P4 described a feeling of resistance to “take commands” from the dispenser. P2 reacted by switching the device off when leaving the house:
Well, for me it was stress, especially in the evening. When I knew I wasn’t there I just switched it [the dispenser] off. And then I switched it on again in the morning and it started up and that was no problem. (Gut für mich war auch Stress, vor allem am Abend. Wenn ich gewusst habe dass ich nicht da bin habe ich ihn einfach abgestellt. Und dann habe ich ihn am Morgen halt wieder eingeschaltet dann hat es wieder aufgestartet und das ist kein Problem gewesen).
P6 acknowledged the usefulness for retrieving pocket-doses for planned absences, but not for unplanned belatedness.
The impressions and expectations at first sight changed for most participants over time, sometimes dramatically. P3 and P5 declared initial negative attitudes toward the product design, which changed to a positive attitude after using the dispenser for 2 weeks. Conversely, P2, P4, and P6, with initially neutral or positive expectations, developed strong negative feelings over time, describing aggression and stress. P6 was expecting no problems but felt enslaved and had the feeling of “not taking the medication out of free will” (“es war nicht mein freier Wille”). The participant (P5) with the most positive experiences after the 14-day use was also the only one to report prior difficulties with taking the medication.
Target groups for the dispenser
All participants agreed that the dispenser could be beneficial to some patients. The envisaged target groups were patients with time-sensitive medication regimens (transplant patients, HIV patients), patients with dementia, the visually impaired, or generally patients requiring assistance with their medication. P4 mentioned that the dispenser could help distinguish the medication of several patients living together and prevent accidental intake of someone else’s medication. P4 mentioned the possibility of using the dispenser for feedback purposes to discuss irregularities in a patient’s medication-taking behavior. Participants had contradictory opinions about the appropriate age to start using the dispenser. On one hand, they stated that the dispenser would be appropriate only when patients could not cope without external assistance. On the other hand, they favored an early inception when patients were still capable of adopting to new technologies. Participants agreed that the device would be appropriate only for patients spending most of their time at home, or when only taking medication in the morning. All participants emphasized the importance of individually assessing patients’ motivation and need of such a device.
When asked for the monthly fee participants would be willing to pay, the answers ranged from 0 to 25 Swiss francs (SFr) per month (0 to 28 USD). P6 noted that 25 SFr would be appropriate when the dispenser could show a clear beneficial effect. In contrary, P2 stated that the dispenser should be free of charge when someone is in need of it. However, if someone is able to cope without the dispenser but wishes to use it, a monthly fee of 10 to 20 SFr seems appropriate, according to this participant. The same participant pointed out that one might treat the device carelessly when provided at no charge.
Discussion
The concept of an e-MMA, or “smart pillbox”, is not new, and an increasing number of devices combine the functionality of an MMA with electronic monitoring.22 e-MMAs with prepackaged pouches mostly target patients living at home who receive support from home care services for their medication therapy. Instead of the daily visit(s) to prepare the doses and supervise correct intake, the caregiver only has to refill the dispenser at predefined intervals while maintaining supervision of correct dosing.
Our results show that the assessed e-MMA meets most of the general requirements set for an MMA in the areas of patient support and implications on patient habits. The participants reported no technical problems with the e-MMA, probably due to careful oral and written instructions before use and their interest in electronic technologies. The major limitation voiced by our participants concerned the restricted mobility inherent to a bulky device that needs continuous power supply. This aspect may restrict the applicability of the e-MMA to patients with limited mobility. Similarly, a report published by the University of Birmingham reviewing electronic dispensers also stated that “people who regularly leave the home may also find it less practical”.23 Retrieving pouches before intake times (pocket-dose) to overcome this limitation was not often put into practice by our mobile participants. Anticipating an absence that will collide with an intake time requires cognitive abilities known as prospective memory.24 A lack of prospective memory is associated with nonadherence.24 Therefore, the patients who could benefit from an e-MMA may be those who are unlikely to anticipate an absence during a later intake time. Alternately, the mobile patients who could most benefit from the dispenser may be those with only one intake time in the morning, since they do not need to be at home at specific times in the afternoons or evenings. Obtrusiveness was not an issue; however, the participants only judged the physical dimension of the term (ie, technology is not perceived as undesirable and physically prominent,25 giving the German word “unauffällig”). The psychological dimension of the term (ie, the tendency to intrude, especially upon privacy)25 was not mentioned as a drawback of the e-MMA, even though all participants were aware of the electronic real-time monitoring. Two participants declared a certain reluctance to “follow” a machine, which, however, is not an objection to the e-MMA per se, but much more refers to their personal relationship to the aspect of dependence, and its loss of functions and abilities.25 Thus, according to the model of obtrusiveness in telehealth,26 our e-MMA possesses an adequate size (physical dimension), is user-friendly (usability dimension), does not violate the personal sphere (privacy dimension), and has optimal performance (function dimension).
The design of an MMA is important, as acknowledged by other authors.5 Our results suggest that design might be an initial barrier but is likely to fade after the patient experiences concrete benefits from the device. Thus, health care professionals should point out more the potential benefits of the device on the regulated intake and less the external appearance. The fact that the voting for the two attributes “looks good” and “is space-saving” were distributed equally demonstrates the mixed feelings of the participants. However, since appearance and size are subject to personal liking, those attributes should not be emphasized by the health professional. Further, participants of our study became accustomed to the e-MMA in only 14 days and were likely to change their preconceived opinions about the device during this short period. Therefore, it may be appropriate to propose an evaluation period of 2 to 4 weeks to reluctant patients and to offer the device at no cost for this accommodation time.
Patients’ characteristics represent only one of the five dimensions of nonadherence (socioeconomic, condition, therapy, patient, and health care system).27 An adherence intervention like an e-MMA can have a significant influence on clinical outcomes, as long as it targets patients with the need for and the motivation to use an e-MMA. Thus, each case needs individual assessment and, eg, intentionally nonadherent patients should be ruled out, as stated in the University of Birmingham report.23
We could not find any publication concerning the appropriate age to propose an e-MMA to a patient taking chronic medication. Our study participants recognized that the main condition for adopting and integrating an e-MMA into daily routine remains that it fits patients’ habits. This favors an early inception of the device, since mental flexibility may decrease with advancing age. As a consequence, cognitive dysfunction or dementia may be incompatible with an e-MMA, although some authors suggest that those patients represent the target group for the provision of an e-MMA to combat nonadherence.28 In the Automated Pill Dispenser Project, more than 70% of participants were 75 years of age and older, and almost half of them were older than 85 years.17 The same study also advised against the use of such aids in patients with moderate-to-severe dementia. Further studies should investigate these contradictory suggestions.
We acknowledge some limitations to this study. Our sample was not representative for the general population. This may limit the external validity and generalizability of our findings, since other participants could have judged different attributes important. This could be overcome by conduction of additional focus groups in different populations. The literature suggests conducting at least three to four focus groups to reach theoretical data saturation.18 We chose to conduct only one focus group since the topic of electronic medication devices is not new, and a preliminary list of attributes could be generated from the literature. As a consequence, we considered the literature as the reflection of several experts’ opinions and our focus group as the final opinions-gathering group.
Our study shows some strengths. Consensus on the most attributes of the e-MMA was obtained unanimously. Because participants voted by raising their cards simultaneously and individually without seeing the others’ choices, this consensus cannot mirror the desire to vote in accordance with the group.
Our results have theoretical and practical implications, such as the need to improve the design and targeting of MMAs. Not only the appearance of the MMA, but also its functionality and the whole medication supply process should be considered during the design process. Further prospective, randomized, and controlled intervention trials should aim at quantitatively evaluating the validity of our findings in larger populations of patients with time-sensitive medication regimens, patients with dementia, the visually impaired, and several patients living together.
Acknowledgments
We would like to thank the staff of the pharmacy Apotheke Hersberger am Spalebärg for their support with recruitment and the staff of the Notfall Apotheke Basel for housing our equipment to produce the unit-of-dose pouches. This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
Vrijens B, De Geest S, Hughes DA, et al; ABC Project Team. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. | |
DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. | |
DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002; 40(9):794–811. | |
Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008(2):CD000011. | |
Mahtani KR, Heneghan CJ, Glasziou PP, Perera R. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Database Syst Rev. 2011(9):CD005025. | |
Gould ON, Todd L, Irvine-Meek J. Adherence devices in a community sample: how are pillboxes used? Can Pharm J (Ott). 2009; 142(1):28–35. | |
Lakey SL, Gray SL, Borson S. Assessment of older adults’ knowledge of and preferences for medication management tools and support systems. Ann Pharmacother. 2009;43(6):1011–1019. | |
Hersberger KE, Boeni F, Arnet I. Dose-dispensing service as an intervention to improve adherence to polymedication. Expert Rev Clin Pharmacol. 2013;6(4):413–421. | |
Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. | |
Arnet I, Walter PN, Hersberger KE. Polymedication Electronic Monitoring System (POEMS) – a new technology for measuring adherence. Front Pharmacol. 2013;4:26. | |
Stip E, Vincent PD, Sablier J, Guevremont C, Zhornitsky S, Tranulis C. A randomized controlled trial with a Canadian electronic pill dispenser used to measure and improve medication adherence in patients with schizophrenia. Front Pharmacol. 2013;4:100. | |
Ingerski LM, Hente EA, Modi AC, Hommel KA. Electronic measurement of medication adherence in pediatric chronic illness: a review of measures. J Pediatr. 2011;159(4):528–534. | |
Ho VQ, Gale TJ, Stack CR. Medication dispenser for narcotic rehabilitation patients. Conf Proc IEEE Eng Med Biol Soc. 2009; 2009:1742–1745. | |
Takacs B, Hanak D. A prototype home robot with an ambient facial interface to improve drug compliance. J Telemed Telecare. 2008;14(7):393–395. | |
Waeber B, Vetter W, Darioli R, Keller U, Brunner HR. Improved blood pressure control by monitoring compliance with antihypertensive therapy. Int J Clin Pract. 1999;53(1):37–38. | |
Reeder B, Demiris G, Marek KD. Older adults’ satisfaction with a medication dispensing device in home care. Inform Health Soc Care. 2013;38(3):211–222. | |
Bowsher M. Improvement and Efficiency West Midlands, NHS Midlands and East; 2012. | |
Rabiee F. Focus-group interview and data analysis. Proc Nutr Soc. 2004;63(4):655–660. | |
Krueger RA, Casey MA. Focus Groups: A Practical Guide for Applied Research. 4th ed. Thousand Oaks: SAGE Publications, Inc.; 2009. | |
Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107–115. | |
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. | |
Naditz A. Medication compliance – helping patients through technology: modern “smart” pillboxes keep memory-short patients on their medical regimen. Telemed J E Health. 2008;14(9):875–880. | |
McArthur M. Automatic Medicine Dispensers, A Review of Evidence and Current Practice. Birmingham: University of Birmingham Health Services Management Centre; 2008. | |
Zogg JB, Woods SP, Sauceda JA, Wiebe JS, Simoni JM. The role of prospective memory in medication adherence: a review of an emerging literature. J Behav Med. 2012;35(1):47–62. | |
Hensel BK, Demiris G, Courtney KL. Defining obtrusiveness in home telehealth technologies: a conceptual framework. J Am Med Inform Assoc. 2006;13(4):428–431. | |
Courtney KL, Demiris G, Hensel BK. Obtrusiveness of information-based assistive technologies as perceived by older adults in residential care facilities: a secondary analysis. Med Inform Internet Med. 2007; 32(3):241–249. | |
Sabaté E, editor. Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003. Available from: http://apps.who.int/medicinedocs/en/d/Js4883e/. Accessed September 28, 2015. | |
Stilley CS, Bender CM, Dunbar-Jacob J, Sereika S, Ryan CM. The impact of cognitive function on medication management: three studies. Health Psychol. 2010;29(1):50–55. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.