Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Patient Selection for Bronchoscopic Lung Volume Reduction
Authors Welling JBA , Hartman JE , Augustijn SWS, Kerstjens HAM , Vanfleteren LEGW , Klooster K, Slebos DJ
Received 2 December 2019
Accepted for publication 10 March 2020
Published 23 April 2020 Volume 2020:15 Pages 871—881
DOI https://doi.org/10.2147/COPD.S240848
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Jorrit BA Welling, 1, 2 Jorine E Hartman, 1, 2 Sonja WS Augustijn, 1 Huib AM Kerstjens, 1, 2 Lowie EGW Vanfleteren, 3 Karin Klooster, 1, 2 Dirk-Jan Slebos 1, 2
1University of Groningen, University Medical Center Groningen, Department of Pulmonary Diseases, Groningen, the Netherlands; 2Groningen Research Institute for Asthma and COPD, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands; 3COPD Center, Sahlgrenska University Medical Hospital and Institute of Medicine, Gothenburg University, Gothenburg, Sweden
Correspondence: Jorrit BA Welling
Department of Pulmonary Diseases, University Medical Center Groningen, Groningen, the Netherlands
Email [email protected]
Purpose: Bronchoscopic lung volume reduction (BLVR) is a valuable treatment option for carefully selected patients with severe COPD. There is limited knowledge about the characteristics and outcomes of patients referred to a specialized center for BLVR. The study objectives were to investigate the selection rate for BLVR treatment in patients referred for this treatment and to investigate the differences between patients that were selected for BLVR and patients that were not.
Patients and Methods: We performed a retrospective analysis of patients with severe COPD who were referred to our hospital to assess eligibility for BLVR treatment. Our parameters included demographics, comorbidity, chest computed tomography characteristics, reasons for rejection from BLVR treatment and patient survival.
Results: In total, 1500 patients were included (mean age 62 years, 50% female and forced expiratory volume in 1 s 33% of predicted). Out of this group, 282 (19%) patients were selected for BLVR treatment. The absence of a suitable target lobe for treatment, an unsuitable disease phenotype and insufficient lung hyperinflation were the most important factors for not being selected. Patients that were selected for any BLVR option lived significantly longer than the group of patients that were not selected for BLVR (median 3060 versus 2079 days, P< 0.001).
Conclusion: We found that only a small proportion of patients that are referred for BLVR treatment is eligible for a BLVR treatment, indicating a need for both better referral tools and for the development of new therapies for this group of patients. Furthermore, our data suggest that selection for BLVR is associated with a significant survival benefit.
Keywords: bronchoscopic lung volume reduction, patient selection, endobronchial valves, lung volume reduction coils
Corrigendum for this paper has been published
Introduction
Bronchoscopic lung volume reduction (BLVR) is a valuable treatment option for patients with severe COPD and emphysema, aimed at reducing hyperinflation of the lung.1 BLVR using endobronchial valves (EBV) and lung volume reduction coils (LVRC) have been studied most extensively and demonstrated to be effective, with an acceptable safety profile.2
Dedicated patient selection for BLVR is essential in achieving clinically meaningful results after treatment. For example, for the EBV treatment, the absence of interlobar collateral ventilation is necessary to achieve successful outcomes and for the LVRC treatment superior outcomes are observed in patients with very severe static hyperinflation and absence of significant airway disease.3–7
Several questions on patient selection for BLVR remain unanswered. For example, it is unknown what proportion of patients referred for BLVR is potentially eligible for any form of BLVR treatment and to our knowledge, this group of patients has not been well characterized in the literature. Furthermore, the development of new insights in BLVR treatment during this period led to changes in the inclusion and exclusion criteria for these treatments which potentially could influence the proportion of selected patients.
Therefore, we aimed to investigate 1) which proportion of patients that were referred to our hospital were actually selected for BLVR treatment; 2) the differences in characteristics and survival between patients that were and were not selected for BLVR; 3) to what extent applying updated criteria for eligibility would have affected the selection rate.
Patients and Methods
Study Design and Patient Population
We performed a retrospective analysis of the first 1500 patients who were consecutively referred to assess eligibility for BLVR treatment between March 2007 and October 2014, from 62 different hospitals in the Netherlands to our hospital. Given the retrospective and anonymous nature of the analyses, this research did not fall within the scope of the WMO (Dutch Medical Research with Human Subjects Law) and therefore review by a medical ethical committee was not required.
Evaluation of Eligibility
Patient selection for BLVR in our hospital starts with the referral of a patient by their pulmonary physician. Referring physicians are requested to include recent lung function results (spirometry and body plethysmography), chest computed tomography (HRCT) scan, and a complete medical history in their referrals. During a multidisciplinary team meeting, a first selection is made. Potential BLVR candidates are invited to our hospital for a consultation with an interventional pulmonologist.
Treatment
Patients that were eligible for BLVR treatment were included in clinical trials investigating EBV,3,8–11 LVRC,12–15 polymeric lung volume reduction,16 pneumostoma17–19 and airway bypass stents20 or in our regular EBV treatment program (BREATH-NL: NCT02815683).
Outcomes
The primary outcome of this study was the selection rate for BLVR treatment. Secondary outcomes were derived from the referral documentation and included demographics, lung function (spirometry and body plethysmography), smoking status, oxygen therapy use and maintenance anticoagulant use. Furthermore, the medical history of all patients was screened for a selection of comorbidities. All available CT scans were visually reviewed and assessed by JBAW for the presence of specific characteristics, these assessments were supervised by DJS.
The degree of emphysema destruction was scored on a 0 to 4 qualitative Likert scale with higher scores indicating more emphysematous destruction (Figure 1).21,22 In case of ineligibility for BLVR, we reported the reasons why patients were found not to be eligible for treatment. The survival status of the referred patients was verified with the Dutch government (Personal Records Database) on June 16, 2019.
 |
Figure 1 Qualitative scale of emphysematous destruction, scored on a 0 to 4 scale with higher scores indicating more emphysematous destruction. |
Theoretical Model
We applied some of the most recent inclusion and exclusion criteria for EBV and LVRC, according to the guidelines,1 on our cohort to assess the proportion of patients eligible for these treatments and whether this proportion was different from the proportion of patients actually selected for these treatments. The criteria applied for EBV treatment included forced expiratory volume in 1 s (FEV1) between 20% and 50% of predicted, residual volume (RV) ≥175% of predicted, RV/total lung capacity (TLC) ratio of ≥0.58, visually intact major fissure (left or right) and emphysema destruction ≥2 on destruction scale (Figure 1).
The criteria applied for LVRC included FEV1 between 20% and 50% of predicted, RV ≥200% of predicted, RV/TLC ratio of ≥0.58 and emphysema destruction ≥2 on the destruction scale (Figure 1).
Statistical Analysis
Differences in patient characteristics between the group that was selected for treatment and the group that was not were analyzed using an independent-samples T-test in case of normal distribution of data and a Mann–Whitney-U test in case of non-normal distribution. A Chi-squared test was used in the case of categorical data. Due to the explorative nature of the CT data, only demographic data are presented and no statistical analysis was performed. Survival time was defined as the time after the date of discussion in the multidisciplinary team meeting until the date of verification with the Dutch government. Survival was analyzed using the Kaplan–Meier method. Comparison in survival between the groups selected or not selected for treatment was performed using the Mantel–Cox log-rank test and comparison in survival between EBV and LVRC treatment was performed using Breslow’s test. All statistical analyses were performed using SPSS version 23 (IBM, New York, NY, USA). P-values <0.05 were considered statistically significant.
Results
In total, 1500 patients (50% female) were included in our analysis, with a mean age of 62 years and FEV1 of 33±14% of predicted (additional patient characteristics are shown in Table 1). From this group, 651 patients (43%) were invited for a consultation in our hospital. Of the total referred population 282 (19%) patients were selected for a clinical trial or regular treatment program and therefore a total of 1218 (81%) patients were considered not eligible for BLVR (see Figure 2 for patient flowchart).
 |
Table 1 Patient Characteristics |
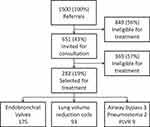 |
Figure 2 Study flowchart. Abbreviation: PLVR, polymeric lung volume reduction. |
Out of the group of 282 patients that were selected for a bronchoscopic treatment, 175 patients (62%) were selected for EBV, 93 patients (33%) for LVRC, 3 patients (0.2%) for airway bypass stents, 9 patients (3%) for polymeric lung volume reduction and 2 patients (0.1%) for a pneumostoma.
Patients selected for BLVR were significantly younger (59 versus 63 years), had a lower FEV1 (28% versus 34% of predicted) and a higher RV (237% versus 215% of predicted) compared to the group of patients not selected for BLVR (all P<0.001).
The most frequently encountered reasons for ineligibility for BLVR treatment were: absence of a suitable target lobe for treatment (18%), unsuitable disease phenotype for treatment (chronic bronchitis, frequent exacerbations, asthma) (18%) and insufficient hyperinflation of the lungs (16%). See Table 2 for the complete list of contra-indications.
 |
Table 2 Contraindications in Patients Not Selected for BLVR |
The CT scans of 1211 patients (81%) could be assessed, for 289 patients assessment was not possible because of scan unavailability or insufficient image quality for assessment. The proportion of patients with a homogeneous and heterogeneous distribution of emphysema was similar (52% versus 48%). Upper lobe predominant emphysema was observed more often than lower lobe predominant emphysema (71% versus 29%). The left major fissure was found to be visually intact in 44% of patients, the right major in 25% of patients and the right minor fissure in 12% of patients (see Table 3).
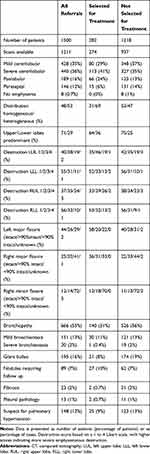 |
Table 3 CT Characteristics |
Table 4 displays the reported comorbidities. Patients referred for BLVR had an average of 1.4 comorbidities and the most frequently encountered comorbidities were hypertension (22%), confirmed or suspected asthma (18%) and coronary artery disease (10%). Patients selected for BLVR had significantly less comorbidities compared to the group of patients not selected for BLVR (1.1 versus 1.4, P<0.01).
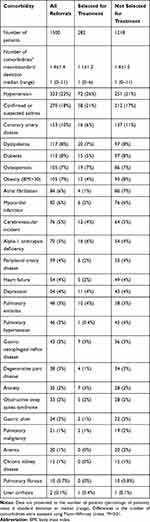 |
Table 4 Comorbidities Reported in the Referral Documentation |
The survival status of 1272 patients (85%) could be verified. The overall median survival was 2316 days (95% CI: 2146–2485 days). The median follow-up was 2351 days (95% CI: 2451–2514 days). Patients that were referred to our hospital but were not invited for consultation had a median survival of 1808 days (95% CI: 1622–1994) and patients who were invited for consultation but who were not selected for treatment had a median survival of 2524 days (95% CI: 2234–2814). Patients that were selected for BLVR lived significantly longer than the group of patients that was not selected for BLVR (median 3060 versus 2079 days, P<0.001), see Figure 3. No significant survival difference was observed between patients who were selected for EBV treatment and those who were selected for LVRC (P=0.45).
Theoretical Model
When applying some of the currently established inclusion and exclusion criteria for endobronchial valve treatment and lung volume reduction treatment, we identified 283 patients eligible for EBV treatment (19%) while 175 patients (12%) were actually selected for EBV in this cohort and 144 patients (10%) would currently be eligible for LVRC while 93 patients (6%) were actually selected for LVRC (Figure 4).
Discussion
Only one out of five patients who were referred for BLVR treatment to our hospital were selected for BLVR treatment. Ineligibility for BLVR treatment was most often caused by the absence of a suitable target lobe for treatment, an unsuitable disease phenotype for treatment and insufficient lung hyperinflation. Overall survival in the group of patients referred for BLVR was poor with a median survival of approximately 6 years.
To our knowledge, this is the largest study investigating patients referred for BLVR eligibility assessment. In a recent study by Polke et al, who studied patients that were referred to a BLVR expert center in Heidelberg (Germany), a higher proportion of patients were found to be eligible for BLVR treatment, possibly caused by a more strict preselection of patients for referral.23 The same study also found the absence of a suitable target lobe to be the most frequent contra-indication for BLVR, which is in line with the results of our study.23
Only a small proportion of the already preselected group of patients that were considered to be eligible for BLVR by the referring physician is selected for BLVR treatment. This highlights both the need for improved referral strategies on the one hand and the important need for additional therapeutic options for patients with severe COPD on the other hand. Alternative interventions for BLVR include lung volume reduction surgery or lung transplantation; however, both treatments suffer from huge limitations related to the invasiveness of the procedure, scarce availability and strict selection procedures. Patients with a severe chronic bronchitis phenotype of COPD are a common example of an unsuitable disease phenotype for BLVR. Both endobronchial treatment with liquid nitrogen cryospray and targeted lung denervation are currently under development for this phenotype. Liquid nitrogen cryospray is a treatment aimed at inducing an airway tissue healing effect by destroying the hyperplastic goblet cells and excess submucous glands.24 Target lung denervation is a treatment designed to decrease airway resistance and mucus hyper section, by inhibiting parasympathetic pulmonary nerves, using radiofrequency ablation therapy.25
New insights into BLVR treatment caused inclusion and exclusion criteria for these treatments to change over time, which might have affected the proportion of patients considered eligible for BVLR. For example, a previous contra-indication for EBV trials included the presence of alpha-1 antitrypsin deficiency, but these patients are now considered eligible for treatment.3,26 When we applied the most recent inclusion and exclusion criteria on our cohort, we observed a discrepancy between the number of patients that were eligible for treatment and those who were actually selected for treatment. This could be the result of the fact that not all treatments were available at all times during the time frame of this study, the clinical trial context with strict in and exclusion criteria or because we applied only a selection of the most stringent criteria in our model.
A significant survival benefit was observed for the group of patients that was selected for BLVR treatment, when compared to the group that was not selected for treatment. This survival benefit was already observed in several previous studies which demonstrated that when successful lobar atelectasis is achieved after EBV treatment, patients have a substantial, persisting survival benefit.27–29 Structural survival data for the LVRC treatment are not yet available. We acknowledge that the survival benefit observed in the group of patients that were selected for treatment might have not only been due to a direct result of the actual intervention but also caused by the exclusion of patients that were too frail, due to any cause, for treatment. On the other hand, both the degree of hyperinflation and airway obstruction were higher in the group selected for treatment, suggesting the selection of patients with severe disease for treatment. In addition, given that most treatments in this cohort took place in the early phase of the development of these treatments, the current data might actually underestimate the survival benefit of these treatments.
Patients selected for BLVR had significantly less comorbidities than patients who were not selected for BLVR. On average, the referred patients had more than one comorbidity. However, this was still lower than in a study by Putcha et al, possibly caused by the underreporting of comorbidities by the referring physicians in our cohort or because of the fact that the referring physicians already referred a preselected population due to study selection criteria on comorbidity.30,31
We assessed the CT characteristics of the referred patients and found the left major fissure to be most often intact on the CT scans of the referred patients, followed by the right major fissure and the right minor fissure. The proportion of visually intact fissures was in line with previously published data on this topic, and also in agreement with the latest clinical trials investigating EBV and intrabronchial valves, in which the left upper and left lower lobe were selected for treatment in more than 75% of cases.11,32,33
This study has several limitations: first of all, our population is representative of the group of patients referred to a BLVR center but not of the total population of patients with severe emphysema, and can therefore not serve to accurately assess the proportion of eligible patients for BLVR in the total population of patients with emphysema. Second, inherent to the retrospective nature of this study, we had to rely on the quality of the referral documentation from other hospitals. Incomplete or incorrect referral documentation might have especially affected the data presented on comorbidity, which was based on the medical history included in the referral documentation, probably leading to an underestimation of comorbidity.31 Third, the CT scans were of very different quality and settings, because referral material was used, making a preferred quantitative assessment not possible.34 These scans were assessed by one reviewer only (JBAW), under supervision of one of the authors (DJS), a task that in an ideal setting would have been performed by a panel of reviewers. Fourth, since these were the first 1500 BLVR referrals sent to our hospital, most patients were treated in a clinical trial context, which probably led to a more strict selection compared to treatment outside the clinical trial context, underestimating the number of patients eligible for BLVR treatment. Fifth, it would have been of additional value to include a survival prediction index like BODE, but we did not have the necessary data available to perform this.35
A strength of our study is the large number of patients that were included in this retrospective study. Another strength of our study is the fact that we were able to verify the survival status of our patients with the Dutch government, which increased the reliability of our survival data.
Future research might include the development of a model that is able to predict the à priori chances of BLVR eligibility. Such a model could assist both physicians and patients in deciding whether referral to a BLVR center is indicated. Indeed, the right patient should be referred for the right treatment, to improve efficiency and avoid the burden for the patient. Future research is needed to identify the size of the potential pool of patients eligible for BLVR treatment as a previous study by Pietzsch et al suggested that BLVR currently is only used in a small proportion of patients with severe emphysema.36
In conclusion, we found that only a small proportion of patients that are referred for BLVR treatment is eligible for a BLVR treatment, indicating a need for the development of new therapies for this group of patients and better referral tools. Furthermore, our data suggest that selection for BLVR is associated with a significant survival benefit.
Abbreviations
BLVR, bronchoscopic lung volume reduction; COPD, chronic obstructive pulmonary disease; CT, computed tomography; EBV, endobronchial valves; FEV1, forced expiratory volume in 1 s; LVRC, lung volume reduction coil; RV, residual volume; TLC, total lung capacity.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics and Consent Statement
Given the retrospective and anonymous nature of the analyses, this research did not fall within the scope of the WMO (Dutch Medical Research with Human Subjects Law) and therefore review by a medical ethical committee was not required.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The University of Groningen, Junior Scientific Masterclass provided financial support for the research position of JW. This analysis was part of the SOLVE project, funded by The Dutch Lung Foundation (Longfonds) (no. 5.1.17.171).
Disclosure
LEGWV reports grants and personal fees from AstraZeneca, personal fees from Novartis, personal fees from GSK, personal fees from Chiesi, personal fees from Menarini, personal fees from Pulmonx, grants from Fisher & Paykel, grants from Philips, personal fees from Boehringer, all outside the submitted work. HAMK reports grants from GSK, grants from Novartis, grants from Boehringer Ingelheim, outside the submitted work. DJS reports grants, personal fees, and non-financial support from PulmonX Inc., CA, USA, grants, personal fees, and non-financial support from PneumRx/BTG, CA, USA, grants and non-financial support from Nuvaira, MN, USA, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Herth FJF, Slebos D-J, Criner GJ, Valipour A, Sciurba F, Shah PL. Endoscopic lung volume reduction: an expert panel recommendation - update 2019. Respiration. 2019;1–10. doi:10.1159/000496122
2. van Geffen WH, Slebos D-J, Herth FJ, S V K, Weder W, Shah PL. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med. 2019;7(4):313–324. doi:10.1016/S2213-2600(18)30431-4
3. Klooster K, Ten Hacken NHT, Hartman JE, Kerstjens HAM, van Rikxoort EM, Slebos D-J. endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373(24):2325–2335. doi:10.1056/NEJMoa1507807
4. Valipour A, Herth FJF, Burghuber OC, et al. Target lobe volume reduction and COPD outcome measures after endobronchial valve therapy. Eur Respir J. 2014;43(2):387–396. doi:10.1183/09031936.00133012
5. Koster TD, van Rikxoort EM, Huebner R-H, et al. Predicting lung volume reduction after endobronchial valve therapy is maximized using a combination of diagnostic tools. Respiration. 2016;92(3):150–157. doi:10.1159/000448849
6. Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet. 2015;386(9998):1066–1073. doi:10.1016/S0140-6736(15)60001-0
7. Slebos D-J, Cicenia J, Sciurba FC, et al. Predictors of response to endobronchial coil therapy in patients with advanced emphysema. Chest. 2019;155(5):928–937. doi:10.1016/j.chest.2019.02.012
8. Herth FJF, Eberhardt R, Gompelmann D, et al. Radiological and clinical outcomes of using ChartisTM to plan endobronchial valve treatment. Eur Respir J. 2013;41(2):302–308. doi:10.1183/09031936.00015312
9. Valipour A, Slebos D-J, Herth F, et al. Endobronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med. 2016;194(9):1073–1082. doi:10.1164/rccm.201607-1383OC
10. Kemp SV, Slebos D-J, Kirk A, et al. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (TRANSFORM). Am J Respir Crit Care Med. 2017;196(12):1535–1543. doi:10.1164/rccm.201707-1327OC
11. Criner GJ, Sue R, Wright S, et al. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE). Am J Respir Crit Care Med. 2018;198(9):1151–1164. doi:10.1164/rccm.201803-0590OC
12. Sciurba FC, Criner GJ, Strange C, et al. Effect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: the RENEW randomized clinical trial. JAMA. 2016;315(20):2178–2189. doi:10.1001/jama.2016.6261
13. Slebos D-J, Klooster K, Ernst A, Herth FJF, Kerstjens HAM. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest. 2012;142(3):574–582. doi:10.1378/chest.11-0730
14. Deslee G, Klooster K, Hetzel M, et al. Lung volume reduction coil treatment for patients with severe emphysema: a European multicentre trial. Thorax. 2014;69(11):980–986. doi:10.1136/thoraxjnl-2014-205221
15. Klooster K, Ten Hacken NHT, Franz I, Kerstjens HAM, van Rikxoort EM, Slebos D-J. Lung volume reduction coil treatment in chronic obstructive pulmonary disease patients with homogeneous emphysema: a prospective feasibility trial. Respiration. 2014;88(2):116–125. doi:10.1159/000362522
16. Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J. 2015;46(3):651–662. doi:10.1183/09031936.00205614
17. Slebos D-J, Klooster K, Erasmus M. Emphysema! Am J Respir Crit Care Med. 2012;186(2):197. doi:10.1164/rccm.201201-0067IM
18. Snell GI, Holsworth L, Khorramnia S, et al. Feasibility and safety of a transthoracic pneumostoma airway bypass in severe emphysema patients. Respiration. 2017;93(4):236–246. doi:10.1159/000455878
19. Slebos D-J, Shah PL. Collateral ventilation: friend or foe in patients with severe emphysema. Respiration. 2017;93(4):232–233. doi:10.1159/000456672
20. Shah P, Slebos D-J, Cardoso P, et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet. 2011;378(9795):997–1005. doi:10.1016/S0140-6736(11)61050-7
21. National Emphysema Treatment Trial Research Group, Fishman A, Fessler H, et al. Patients at high risk of death after lung-volume–reduction surgery. N Engl J Med. 2001;345(15):1075–1083. doi:10.1056/NEJMoa11798.
22. Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;22(140):55.
23. Polke M, Rötting M, Sarmand N, et al. Interventional therapy in patients with severe emphysema: evaluation of contraindications and their incidence. Ther Adv Respir Dis. 2019;13:175346661983549. doi:10.1177/1753466619835494
24. Slebos D-J, Breen D, Coad J, et al. safety and histological effect of liquid nitrogen metered spray cryotherapy in the lung. Am J Respir Crit Care Med. 2017;196(10):1351–1352. doi:10.1164/rccm.201611-2220LE
25. Slebos D-J, Shah PL, Herth FJ, et al. Safety and adverse events after targeted lung denervation for symptomatic moderate to severe COPD (AIRFLOW): a multicenter randomized controlled trial. Am J Respir Crit Care Med. 2019:
26. Tuohy MM, Remund KF, Hilfiker R, Murphy DT, Murray JG, Egan JJ. Endobronchial valve deployment in severe α-1 antitrypsin deficiency emphysema: a case series. Clin Respir J. 2013;7(1):45–52. doi:10.1111/j.1752-699X.2012.00280.x
27. Hopkinson NS, S V K, Toma TP, et al. Atelectasis and survival after bronchoscopic lung volume reduction for COPD. Eur Respir J. 2011;37(6):1346–1351. doi:10.1183/09031936.00100110
28. Garner J, S V K, Toma TP, et al. Survival after endobronchial valve placement for emphysema: a 10-year follow-up study. Am J Respir Crit Care Med. 2016;194(4):519–521. doi:10.1164/rccm.201604-0852LE
29. Gompelmann D, Benjamin N, Bischoff E, et al. Survival after endoscopic valve therapy in patients with severe emphysema. Respiration. 2019;97(2):145–152. doi:10.1159/000492274
30. Putcha N, Han MK, Martinez CH, et al. Comorbidities of COPD have a major impact on clinical outcomes, particularly in African Americans. Chronic Obstr Pulm Dis J COPD Found. 2014;1(1):105. doi:10.15326/JCOPDF.1.1.2014.0112
31. Triest FJJ, Franssen FME, Spruit MA, Groenen MTJ, Wouters EFM, Vanfleteren LEGW. Poor agreement between chart-based and objectively identified comorbidities of COPD. Eur Respir J. 2015;46(5):1492–1495. doi:10.1183/13993003.00667-2015
32. Koster TD, Slebos D-J. The fissure: interlobar collateral ventilation and implications for endoscopic therapy in emphysema. Int J Chron Obstruct Pulmon Dis. 2016;11:765–773. doi:10.2147/COPD.S103807
33. Criner GJ, Delage A, Voelker K, et al. Improving lung function in severe heterogenous emphysema with the spiration® valve system (EMPROVE): a multicenter, open-label, randomized, controlled trial. Am J Respir Crit Care Med. 2019:
34. Tenda ED, Ridge CA, Shen M, Yang G-Z, Shah PL. Role of quantitative computed tomographic scan analysis in lung volume reduction for emphysema. Respiration. 2019;98(1):1–9. doi:10.1159/000498949
35. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa021322
36. Pietzsch JB, Busca R, Rott C, et al. Adoption patterns of bronchoscopic lung volume reduction procedures in germany and predicted procedure volumes for other European countries. Respiration. 2019;97(1):34–41. doi:10.1159/000491677
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


