Back to Journals » Patient Preference and Adherence » Volume 16
Patient Satisfaction with Once-Daily Single-Tablet Darunavir, Cobicistat, Emtricitabine, and Tenofovir Alafenamide (DRV/c/FTC/TAF): A Real-World Study of Patient Self-Reported Outcomes in HIV-1–Diagnosed Adults
Authors LaMori J, Seignez A, Radoszycki L
Received 4 August 2021
Accepted for publication 10 December 2021
Published 13 January 2022 Volume 2022:16 Pages 83—94
DOI https://doi.org/10.2147/PPA.S332555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Joyce LaMori,1 Antoine Seignez,2 Lise Radoszycki2
1Janssen Scientific Affairs, LLC, Titusville, NJ, USA; 2Carenity, Paris, France
Correspondence: Joyce LaMori
Janssen Scientific Affairs, 1125 Trenton Harbourton Road, Titusville, NJ, 08560, USA
Tel +1 310 378 2876
Fax +1 609 730 3003
Email [email protected]
Purpose: Human immunodeficiency virus (HIV)-1 infection remains a concern. As patient adherence to antiretroviral therapy is essential to avoid drug resistance and virologic failure, greater understanding of patient treatment satisfaction may help facilitate ongoing medication use.
Patients and Methods: An online survey was conducted through the Carenity US HIV platform (04/07/2020– 05/26/2020). Eligible respondents were adults with HIV-1 registered on the platform who were receiving darunavir/cobicistat/emtricitabine/tenofovir alafenamide (DRV/c/FTC/TAF) and living in the United States. This descriptive study assessed patient satisfaction with DRV/c/FTC/TAF and HIV-related symptoms at baseline and follow-up (4– 6 weeks). Two HIV patient-reported outcomes tools were completed at both time points: the HIV Treatment Satisfaction Questionnaire (HIVTSQs; range: 0– 60 points [higher score indicates greater satisfaction]) and the HIV Symptom Distress Module (HIV-SDM; range: 0– 80 points [lower score indicates lower distress]).
Results: Of 100 respondents from across the United States who completed the survey at baseline, mean age was 39 years, 69 were male, 48 were Caucasian, 76 were HIV treatment-experienced, and 24 were HIV treatment-naïve. Of baseline respondents, 46 completed the follow-up survey. In the overall population, treatment discontinuation between baseline and follow-up was low (6.5%: 3/46 respondents at follow-up). Mean total HIVTSQs score at baseline was 50.2 with the highest proportion of respondents satisfied regarding their willingness to continue DRV/c/FTC/TAF (79%) and to recommend DRV/c/FTC/TAF to other patients (76%). Among all baseline respondents, mean total HIV-SDM score was 23.5. On average, respondents experienced 10.7 overall symptoms (grades 1– 4) and 3.8 bothersome symptoms (grades 3– 4). Both satisfaction rate and occurrence of symptoms with DRV/c/FTC/TAF were stable between baseline and follow-up.
Conclusion: DRV/c/FTC/TAF therapy was associated with high patient satisfaction and patients taking DRV/c/FTC/TAF had a moderate HIV symptom burden. Patient experience and health-related quality of life during HIV therapy are important metrics that may help healthcare providers increase patient adherence.
Keywords: HIV-1, ART, real-life data, PROs (HIVTSQs and HIV-SDM)
Introduction
In 2018, more than 1.2 million adults and adolescents (aged ≥13 years) were living with human immunodeficiency virus (HIV)-1 in the United States.1 Despite remaining a major public health crisis, HIV-1 has become a manageable chronic condition since the advent of combination antiretroviral therapy (ART). The US Department of Health and Human Services recommends patients with HIV-1 initiate ART immediately after diagnosis to improve ART uptake and linkage to care, reduce time to viral suppression, and increase rates of virologic suppression among people living with HIV (PLWH).2 Guidelines recommend an initial ART regimen to generally consist of 1 or 2 nucleoside reverse transcriptase inhibitors (NRTIs) administered in combination with a third active antiretroviral agent from one of the following drug classes: non-nucleoside reverse transcriptase inhibitor, integrase strand transfer inhibitor, or boosted protease inhibitor (PI).2 If one NRTI is used the second active drug should have a high genetic barrier to resistance. To improve treatment adherence and optimize clinical outcomes, healthcare providers (HCPs) may consider switching ART regimens for numerous reasons, such as adverse events (AEs), pill burden, treatment failure, or drug resistance.2
In addition to ART-related factors, PLWH may face challenges with treatment adherence due to clinical, behavioral, and social barriers.3,4 A previous cross-sectional study revealed that there is an inverse association between psychological morbidity and quality of life (QoL). People living with diagnosed HIV infection for a longer time reported poorer psychological and physical health across several outcome measures, including anxiety, symptoms of distress, depression, and health-related functional problems.4 Patient-reported outcomes (PROs) are valuable tools to assess the impact of therapy on a patient’s health-related quality of life (HR-QoL), experience with care, symptom burden, and health behaviors, without any interpretation or influence from HCPs.5,6 As the number of available therapies for HIV-1 increases, there is a need to collect real-world data regarding patient satisfaction and experiences with treatment to better understand factors of poor adherence.7
Darunavir/cobicistat/emtricitabine/tenofovir alafenamide (DRV/c/FTC/TAF) is a once-daily, single-tablet regimen (STR) containing a PI (DRV), a boosting agent (c), and 2 NRTIs (FTC and TAF). DRV/c/FTC/TAF is approved by the US Food and Drug Administration (FDA) for the treatment of HIV-1 infection in treatment-naïve and treatment-experienced, virologically suppressed patients (the initial approval was in 2018). The efficacy and safety of DRV/c/FTC/TAF were demonstrated in the Phase 3 AMBER and EMERALD studies, in which high proportions of patients achieved virologic suppression, with a low incidence of AEs.8,9 Furthermore, DRV/c/FTC/TAF was found to be associated with high levels of patient satisfaction when evaluated in a rapid initiation model of care among adults in the United States diagnosed with HIV-1 infection within 14 days prior to enrollment in the DIAMOND clinical trial (2017–2019).10 The current study is a real-world evaluation of self-reported patient satisfaction with DRV/c/FTC/TAF therapy among US adults using validated, self-administered, HIV-related PRO questionnaires.
Materials and Methods
Study Design and Population
This observational, longitudinal study was conducted through the Carenity platform from April to June 2020. Carenity is an online community and social network for patients and caregivers affected by chronic disease to share experiences, find health-related information, and contribute to medical research by participating in online studies. At the time of the study, approximately 19,000 individuals were registered on the US Carenity platform. Such online platforms can help identify and better understand patient needs through PROs.11,12 Recruitment on Carenity is primarily via search engine optimization, cooperation with other health websites, online campaigns on Google and Facebook, and through partnerships and visibility exchanges with patient organizations.
The source population consisted of all adult HIV-1 patients living in the United States and registered in the Carenity US HIV platform (698 persons at the time of the study). Eligible respondents were adults (≥18 years old) with a self-reported HIV-1 infection diagnosed by an HCP and who self-identified as currently receiving DRV/c/FTC/TAF. Additionally, all respondents were required to provide electronic informed consent via the web platform prior to participation. Respondents were stratified into subgroups by sociodemographics (ie, age, sex, race, ethnicity, comorbidities) and HIV disease characteristics (ie, age at diagnosis, time since diagnosis, HIV-1 stage, treatment status). HIV treatment status was defined as treatment naïve (ie, no ART exposure prior to DRV/c/FTC/TAF) or treatment-experienced (ie, switching from another ART to DRV/c/FTC/TAF). Treatment-experienced respondents were further categorized by virologic control into stable (virally suppressed, defined by an undetectable viral load <50 copies/mL)2 or unstable switchers (detectable viral load ≥50 copies/mL).
Ethical Considerations
This study was conducted in accordance with the principles of Good Clinical Practice and following the Declaration of Helsinki. The study protocol was approved by the New England Institutional Review Board. Prior to data collection, all participants gave their informed consent, which allowed for data collection and publication. Participants’ privacy and confidentiality were guaranteed following US applicable regulation.
Data Collection
All eligible adults were invited to complete the online survey between April 7, 2020 and May 26, 2020. Respondents of the baseline survey were recontacted to complete a follow-up survey 4 to 6 weeks later (May 5, 2020–June 26, 2020). The follow-up period was selected to validate the reliability of the PRO questionnaires and confirm the stability of the results. The surveys collected self-reported demographic, geographic, clinical, and treatment-related information, as well as 2 validated HIV-specific PRO instruments: the HIV Treatment Satisfaction Questionnaire status version (HIVTSQs) and the HIV Symptom Distress Module (HIV-SDM).
The HIVTSQs is a 10-item validated questionnaire specifically developed to measure treatment satisfaction among patients with HIV.13 The 10 items are summarized in 2 distinct subscales: general satisfaction/clinical (current treatment, control, side effects, recommendation to others, continue) and lifestyle/ease (demands, convenience, flexibility, understanding, lifestyle). The HIVTSQs uses a 7-point scale with each response ranging from 0 to 6, with higher scores indicating greater treatment satisfaction. The 10 items are summated to calculate a total treatment satisfaction score, ranging from 0 to 60. Individual item scores were dichotomized to indicate the percentage of satisfied patients (score >5) and dissatisfied patients (score ≤5).
The HIV-SDM is a validated 20-item questionnaire specifically developed to measure the overall HIV symptom distress experienced by the patient in the preceding 4 weeks.14,15 On this 5-point scale, each symptom is rated from 0 to 4 (0 = “I do not have this symptom,” 1 = “I have this symptom and it doesn’t bother me,” 2 = “It bothers me a little,” 3 = “It bothers me,” 4 = “It bothers me a lot”), with higher scores indicating greater symptoms and life disturbance. The total score (ranging from 0–80) is the sum of all 20 items. Individual items are dichotomized into overall HIV symptoms (score 1–4) or bothersome HIV symptoms (score 3– 4).
Statistical Analysis
Respondents who completed all elements of the baseline questionnaire were included in the current analyses. Statistical analyses were performed using R statistical software, version 3.6 (R Core Team, Vienna, Austria). Descriptive statistics were used to summarize demographic and clinical characteristics at baseline and follow-up. Continuous variables are shown as number, mean (standard deviation [SD]). For categorical variables, numbers and percentages of respondents are reported. Estimates are presented with 95% confidence intervals.
PRO questionnaire scores were calculated according to specific algorithms validated by their authors. Mean HIVTSQs total and subscale scores as well as mean total HIV-SDM scores were compared between treatment-experienced and treatment-naïve respondents, between stable and unstable switchers, or between subgroups stratified by baseline characteristics, using Wilcoxon’s rank sum test. Frequencies were compared using a chi-square test. Among the subset of respondents who completed surveys at baseline and follow-up, PRO scores were compared between the 2 time points using Wilcoxon’s signed rank test for subgroup analyses and paired t test for individual items.
Results
Sociodemographic and Disease Characteristics
A total of 100 eligible adults from 30 states (Supplemental Table S1) completed the online survey between April and May 2020. Of these, 46 completed the follow-up questionnaire but only those still receiving DRV/c/FTC/TAF (n=43) were included in the follow-up analysis (Figure 1). Among baseline respondents, the mean age was 39 years (SD, 11), 69% were male, and 48% were Caucasian (Table 1). Among respondents, chronic comorbidities were reported by 22% (mean 3.2 per respondent), of which depression (15%), chronic anxiety (13%), emphysema/bronchitis (8%), and hypertension (6%) were the most common. Overall, 31%, 35%, and 28% of respondents reported consulting an HCP at least once a month, between once a month and every 3 months, or every 3 to 6 months, respectively. Baseline demographic and disease characteristics were relatively similar for the follow-up group; however, among the follow-up group, respondents were more likely to be African American (56%) than Caucasian (33%), have more comorbidities (28%), and have a longer elapsed time since diagnosis (mean, 10 vs 8 years).
 |  | 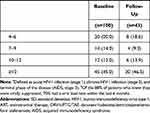 |
Table 1 Sociodemographic and Disease Characteristics for Respondents at Baseline and Follow-Up |
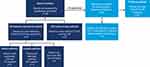 |
Figure 1 Study population flowchart. Abbreviations: DRV/c/FTC/TAF, darunavir/cobicistat/emtricitabine/tenofovir alafenamide; ART, antiretroviral therapy; HIV, human immunodeficiency virus. |
Most (76/100) respondents were HIV treatment-experienced, including 57 who were virally suppressed (stable switchers), 13 with detectable viral load (unstable switchers), and 6 with unknown viral load; 24 were HIV treatment-naïve. Compared to treatment-naïve respondents, treatment-experienced respondents were older (mean, 40 vs 34 years) and had a longer elapsed time since diagnosis (mean, 10 vs 2 years). Among treatment-experienced respondents, the primary reasons reported for switching to DRV/c/FTC/TAF were physician’s decision that another treatment would be more appropriate (46%), the desire to take less medication (36%), or side effects (25%). Baseline sociodemographic and clinical characteristics were similar between stable and unstable switchers (data not shown).
HIV Satisfaction (HIVTSQs)
High levels of treatment satisfaction were expressed by most respondents at baseline (Table 2). A mean total satisfaction of 50.2 (SD, 7.8; out of 60 maximum points) was achieved, including a mean overall satisfaction/clinical subscale score of 25.0 (SD, 4.5; out of 30 maximum points), and a mean lifestyle/ease subscale score of 25.3 (SD, 4.0; out of 30 maximum points). Treatment-experienced respondents were significantly more satisfied than treatment-naïve respondents with regards to lifestyle/ease (mean score, 25.4 vs 24.0; p=0.047). No statistically significant differences were observed between treatment-naïve and treatment-experienced respondents in total HIVTSQs score or general satisfaction/clinical subscale scores. Similarly, no statistically significant difference in treatment satisfaction was observed between stable and unstable switchers.
 |
Table 2 HIVTSQs Total and Individual Item Scores at Baseline and Follow-Up |
Total satisfaction scores were high among all subgroups (range, 48.7–53.2; Supplemental Figure S1A). Mean total HIVTSQs scores differed significantly between younger (≤40 years old) and older respondents (>40 years; 48.7 vs 52.7, respectively; p=0.007), as well as between respondents diagnosed ≤5 years and >5 years (48.9 vs 52.0, respectively; p=0.01).
Based on the HIVTSQs responses, a high percentage of respondents were satisfied with their current treatment at baseline (75%; n=100), with 79% willing to continue their ongoing therapy and 76% willing to recommend it to others (Figure 2). Treatment-experienced respondents were slightly more satisfied overall with their current treatment (76%) than treatment-naïve respondents (71%; data not shown). Among treatment-experienced respondents, the area of side effects was where the lowest level of satisfaction (61%) was reported. For treatment-naïve respondents, the lowest level of satisfaction was reported in the lifestyle area (50%), and this differed significantly from the treatment-experienced subgroup, where 75% were satisfied (p=0.04). Unstable switchers were slightly more satisfied with their current treatment (77%) than stable switchers (75%); the area of side effects was where stable switchers reported the lowest level of satisfaction (61%), while reports on treatment convenience had the lowest level (54%) among unstable switchers.
 |
Figure 2 Percentage of respondents satisfied (HIVTSQs) at baseline.a Abbreviation: HIVTSQs, HIV Treatment Satisfaction Questionnaire status version. Note: aHIVTSQs individual item names abbreviated as in prior studies.13. |
For the 43 respondents included in the follow-up analysis, HIVTSQs total and item scores were similar to those reported at baseline (Table 2). Most respondents expressed a high level of satisfaction, while no meaningful differences were observed for any of the item scores. Additionally, no relevant differences were observed either among all subgroups analyzed (Supplemental Figure S1B) or among satisfied respondents in any item or subscale analyzed between baseline and follow-up (Figure 3). The item with the lowest percentage of satisfaction among follow-up respondents was side effects (44% not satisfied).
 |
Figure 3 Percentage of respondents satisfied (HIVTSQs) among those who completed questionnaires at both baseline and follow-up.a Abbreviation: HIVTSQs, HIV Treatment Satisfaction Questionnaire status version. Note: aHIVTSQs individual item names abbreviated as in prior studies.13. |
HIV Symptoms (HIV-SDM)
Among all respondents, mean total HIV-SDM scores reached 23.5 (SD, 18.7; out of 80 maximal points). Mean total HIV-SDM scores reached 24.1 versus 21.6 in treatment-naïve versus experienced respondents, respectively, and 23.8 versus 24.5 in stable versus unstable switchers, respectively. Significant differences in mean total HIV-SDM scores were observed between respondents diagnosed ≤5 years ago and those diagnosed >5 years ago (27.3 vs 18.2; p=0.02) as well as between respondents with and without comorbidities (35.6 vs 20.1; p=0.001; Supplemental Figure S2A).
On average, respondents experienced 10.7 (SD, 6.7) overall HIV-related symptoms (grades 1–4), with the most common being fatigue (74%), muscle/joint pain (64%), dizziness (60%), body image (60%), as well as psychological symptoms such as anxiety (60%) and trouble sleeping (60%; Figure 4). No significant differences were observed in the percentage of respondents experiencing overall HIV-related symptoms (grades 1–4), either between treatment-experienced and treatment-naïve respondents, or between stable and unstable switchers. The median number of bothersome HIV-related symptoms (grades 3–4) experienced was 3.8 (SD, 4.8), with the most common being anxiety (31%), headache, and sexual problems (25% each; Figure 4). The total number of bothersome HIV-related symptoms reported per patient was not significantly different between treatment-naïve and treatment-experienced respondents (mean, 4.2 vs 2.7, respectively; p=0.55). Furthermore, there were no major differences in the percentage of respondents experiencing bothersome HIV-related symptoms between these subgroups. Across HIV-SDM items, there were no relevant differences between stable and unstable switchers; however, bothersome sexual problems were more frequent among stable switchers (32% vs 0%; p=0.016).
 |
Figure 4 Percentage of respondents with overall symptoms (grades 1–4) or bothersome symptoms (grades 3–4) on HIV-SDM at baseline.a Abbreviation: HIV-SDM, HIV Symptom Distress Module. Note: aHIV-SDM item names abbreviated as in prior studies.14. |
HIV-SDM total scores did not differ statistically between baseline and follow-up (23.5 vs 26.4; p=0.07; Supplemental Figure S2B). At baseline, respondents reported an average of 10.3 (SD, 6.4) overall HIV-related symptoms versus 11.1 (SD, 5.8) at follow-up. The percentage of respondents who experienced HIV-related symptoms ranged from 42% (for memory loss, cough, appetite loss, weight loss) to 81% (for fatigue; Supplemental Figure S3A). Regardless of time point, the most common overall HIV-related symptoms (grades 1–4) were fatigue, sleep trouble, muscle/joint pain, and body image.
At follow-up, respondents reported on average 5.0 bothersome HIV-related symptoms (grades 3–4) versus 4.1 at baseline. The most bothersome HIV-related symptoms at follow-up compared to baseline were sadness (40% vs 28%), body image (40% vs 33%), sleep trouble (35% vs 23%), and anxiety (33% vs 35%). Although these varied from baseline, the differences were not significant (Supplemental Figure S3B).
Association Between Patient Satisfaction and Symptoms Experienced
At baseline, respondents without HIV-related symptoms (all HIV-SDM scores equal to 0) had significantly higher total satisfaction scores than patients with ≥1 HIV symptom (mean, 58 vs 50, respectively; p=0.013). Similarly, a statistically meaningful difference was observed between respondents with no bothersome HIV-related symptoms and those with ≥1 bothersome HIV-related symptom (mean, 54 vs 48, respectively; p=0.0003; Supplemental Figure S4).
Discussion
This survey-based study showed high levels of patient satisfaction among US adults with HIV-1 receiving DRV/c/FTC/TAF treatment. Notably, treatment-experienced respondents were more satisfied overall with their current treatment than treatment-naïve respondents, which may be due, in part, to AEs that present during ART initiation and tend to be transient in nature.2 Previous studies suggest an inverse relationship between patient satisfaction and AEs.16,17 In one study of treatment-naïve patients, overall treatment satisfaction was significantly worse among those with AEs than those without AEs.16 Conversely, improvements in attitudes towards ART and self-perception of health were associated with higher mean HIVTSQ scores.16 Furthermore, studies have demonstrated a correlation between HIVTSQ scores and adherence.16,18 Adherence has also been associated with low pill burden, as STRs have been shown to have higher adherence rates than multi-tablet regimens.19,20 In this real-world study, patients receiving DRV/c/FTC/TAF had high treatment satisfaction scores and low rates of discontinuation (6.5%), which is consistent with the findings observed in the DIAMOND clinical trial, a phase 3 prospective study of the efficacy/safety of DRV/c/FTC/TAF in a rapid-initiation model of care.10 Moreover, in the current study, compared with baseline, no significant differences were seen across the HIVTSQs items at follow-up, indicating stable satisfaction with DRV/c/FTC/TAF over time among both treatment-naïve and treatment-experienced PLWH. The total number of HIV symptoms reported per patient was moderate according to HIV-SDM scores, and remained stable over time.
In addition to patient satisfaction, tolerability is an important consideration when selecting an ART regimen; however, symptoms of comorbidities, HIV itself, and AEs from concomitant medications can be difficult to distinguish from AEs caused by ART.2 Notably, in this study the presence of comorbidities was significantly associated with a higher burden of HIV symptoms. Overall, the HIV symptoms reported in this study were similar to those reported through PROs in previous studies.21–28 For example, fatigue, anxiety, and body image were also among the most common symptoms reported in patients who switched from a ritonavir-boosted PI to a cobicistat-boosted PI in an Italian cohort study.21
PROs, such as HIVTSQs and HIV-SDM, are becoming more prevalent in use as demand is increasing from regulatory authorities to provide real-world data that includes patient perspectives with new drug filings. In 2019, the US FDA released a framework publication on real-world studies.29 Although HIVTSQs and HIV-SDM are primarily used in ART switch studies, they can also serve as a valuable tool for evaluating established or novel therapies.21,26,27,30 ART regimens associated with high rates of patient satisfaction and few AEs may promote treatment adherence, which is key to achieving the targets established by the Global Health Sector Strategy on HIV. The World Health Organization adopted these targets, including 90-90-90 in 2016.31,32 Further PRO studies may help to achieve these goals, as they provide HCPs with relevant information to select ART regimens, optimize counseling, increase trust with their patients, and sustain treatment continuity.
A strength of this study is that the population was representative of PLWH in the United States33 and included higher proportions of women, younger patients, non-Caucasians, and those with an unsuppressed viral load than are typically seen in HIV studies.10,21,27,34,35 Limitations of this study include the observational design and use of self-reported patient surveys. The high rate of loss to follow-up seen in this study may contribute to selection bias, particularly if respondents are missing due to a nonrandom effect; this may limit conclusions regarding changes over time. Additionally, patient symptoms and experiences may be subject to perception bias and recall bias. Moreover, the use of the Carenity platform may have introduced selection bias as older patients (>60 years) and those without online access are underrepresented, while HIV-1 patients who are more health-conscious may be overrepresented. Notably, this online survey was conducted from April to May 2020, the time that the COVID-19 pandemic was spreading in the United States. As an estimated 41% to 42% of US adults reported a delay or avoidance in seeking care during this time,36,37 the pandemic could have had direct or indirect consequences on their healthcare and HR-QoL.
Conclusion
In this real-world study of PLWH and receiving DRV/c/FTC/TAF, high baseline rates of patient satisfaction and a moderate HIV symptom burden were seen. These findings may inform ART regimen selection as well as enhance the relationship between patients and their physicians. Moreover, overall satisfaction with treatment and regimen simplification may improve patient adherence.
Abbreviations
AE, adverse event; AIDS, Acquired Immunodeficiency Syndrome; ART, antiretroviral therapy; FDA, Food and Drug Administration; HCP, healthcare professional; HIV-1, Human Immunodeficiency Virus Type 1; HIV-SDM, HIV Symptom Distress Module; HIVTSQs, HIV Treatment Satisfaction Questionnaire (status version); HR-QoL, health-related quality of life; NRTI, nucleoside reverse transcriptase inhibitor; PLWH, people living with HIV; PRO, patient-reported outcome; PI, protease inhibitor; QoL, quality of life; RCT, randomized clinical trials; RWE, real-world evidence; SD, standard deviation; STR, single-tablet regimen; US, United States.
Data Sharing Statement
The datasets generated and analyzed during the current study are not publicly available due to the presence of identifiable data, but some parts may be available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study protocol was approved by the New England Institutional Review Board.
Consent for Publication
Prior to data collection, all patients have given their informed consent, allowing data collection and publication.
Acknowledgments
The authors would like to acknowledge Wing Chow (former employee of Janssen Scientific Affairs, LLC) for her contributions to the analysis of the results reported here. Medical writing assistance was provided by Florence Boulmé on behalf of ELSECARE, Inc. Editorial assistance was provided by Kurt Kunz, MD, MPH, and Caryn Gordon, PharmD (Cello Health Communications/MedErgy), and was funded by Janssen Scientific Affairs, LLC.
Author Contributions
All authors have made significant contribution to this work; contributed to data analysis, drafting, or revising the article; gave final approval of the version to be published; agreed to the submitted journal; and agree to be accountable for all aspects of the work.
Funding
This work was supported and funded by Janssen Scientific Affairs, LLC. Janssen Scientific Affairs participated in the conception of the study, the design of the questionnaire, the interpretation of the results and the writing of the manuscript.
Disclosure
JL is a current employee of Janssen Scientific Affairs, LLC and stockholder in Johnson & Johnson; AS and LS are current employees of Carenity. DRV/c/FTC/TAF is manufactured by Janssen Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
1. Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas, 2018 (Updated). HIV Surveillance Report 2018 (Updated). Vol. 31; 2018.
2. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV; 2021. Available from: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf.
3. Al-Dakkak I, Patel S, McCann E, Gadkari A, Prajapati G, Maiese EM. The impact of specific HIV treatment-related adverse events on adherence to antiretroviral therapy: a systematic review and meta-analysis. AIDS Care. 2013;25(4):400–414. doi:10.1080/09540121.2012.712667
4. McGowan JA, Sherr L, Rodger AJ, et al. Age, time living with diagnosed HIV infection, and self-rated health. HIV Med. 2017;18(2):89–103. doi:10.1111/hiv.12398
5. Snyder CF, Jensen RE, Segal JB, Wu AW. Patient-reported outcomes (PROs): putting the patient perspective in patient-centered outcomes research. Med Care. 2013;51(8 Suppl 3):S73–79. doi:10.1097/MLR.0b013e31829b1d84
6. Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68. doi:10.4137/HSI.S11093
7. Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med. 2016;13(11):e1002183. doi:10.1371/journal.pmed.1002183
8. Orkin C, Eron JJ, Rockstroh J, et al. Week 96 results of a phase 3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS. 2020;34(5):707–718. doi:10.1097/QAD.0000000000002463
9. Eron JJ, Orkin C, Cunningham D, et al. Week 96 efficacy and safety results of the phase 3, randomized EMERALD trial to evaluate switching from boosted-protease inhibitors plus emtricitabine/tenofovir disoproxil fumarate regimens to the once daily, single-tablet regimen of darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) in treatment-experienced, virologically-suppressed adults living with HIV-1. Antiviral Res. 2019;170:104543. doi:10.1016/j.antiviral.2019.104543
10. Huhn GD, Crofoot G, Ramgopal M, et al. Darunavir/cobicistat/emtricitabine/tenofovir alafenamide in a rapid initiation model of care for HIV-1 infection: primary analysis of the DIAMOND study. Clin Infect Dis. 2020;71(12):3110–3117. doi:10.1093/cid/ciz1213
11. Jacinto J, Varriale P, Pain E, Lysandropoulos A, Esquenazi A. Patient perspectives on the therapeutic profile of botulinum neurotoxin type a in spasticity. Front Neurol. 2020;11:388. doi:10.3389/fneur.2020.00388
12. Lambert J, Chekroun M, Gilet H, Acquadro C, Arnould B. Assessing patients’ acceptance of their medication to reveal unmet needs: results from a large multi-diseases study using a patient online community. Health Qual Life Outcomes. 2018;16(1):134. doi:10.1186/s12955-018-0962-3
13. Woodcock A, Bradley C. Validation of the HIV treatment satisfaction questionnaire (HIVTSQ). Qual Life Res. 2001;10(6):517–531. doi:10.1023/A:1013050904635
14. Justice AC, Holmes W, Gifford AL, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54(Suppl 1):S77–90. doi:10.1016/S0895-4356(01)00449-8
15. Marc LG, Wang MM, Testa MA. Psychometric evaluation of the HIV symptom distress scale. AIDS Care. 2012;24(11):1432–1441. doi:10.1080/09540121.2012.656567
16. Casado JL, Marin A, Romero V, et al. The influence of patient beliefs and treatment satisfaction on the discontinuation of current first-line antiretroviral regimens. HIV Med. 2016;17(1):46–55. doi:10.1111/hiv.12280
17. Mtambo A, Harris M, Toy J, et al. Evaluation of treatment tolerability, satisfaction and laboratory parameters in HIV+ patients switching from ritonavir capsule to tablet formulation. Curr HIV Res. 2013;11(3):231–237. doi:10.2174/1570162X11311030008
18. Jordan J, Cahn P, Goebel F, Matheron S, Bradley C, Woodcock A. Abacavir compared to protease inhibitors as part of HAART regimens for treatment of HIV infection: patient satisfaction and implications for adherence. AIDS Patient Care STDS. 2005;19(1):9–18. doi:10.1089/apc.2005.19.9
19. Cohen CJ, Meyers JL, Davis KL. Association between daily antiretroviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US medicaid population with HIV. BMJ Open. 2013;3(8):e003028. doi:10.1136/bmjopen-2013-003028
20. Clay PG, Yuet WC, Moecklinghoff CH, et al. A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Res Ther. 2018;15(1):17. doi:10.1186/s12981-018-0204-0
21. Antinori A, Cossu MV, Menzaghi B, et al. Patient-reported outcomes in an observational cohort of HIV-1-infected adults on darunavir/cobicistat-based regimens: beyond viral suppression. Patient. 2020;13(3):375–387. doi:10.1007/s40271-020-00413-y
22. Palella F, Fisher M, Tebas P, et al. SPIRIT: switching to rilpivirine/emtricitabine/tenofovir DF single-tablet regimen from boosted protease inhibitor maintains HIV-1 suppression through week 48.
23. Mills A, Garner W, Pozniak A, et al. Patient-reported symptoms over 48 weeks in a randomized, open-label, phase IIIb non-inferiority trial of adults with HIV switching to co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir DF versus continuation of non-nucleoside reverse transcriptase inhibitor with emtricitabine and tenofovir DF. Patient. 2015;8(4):359–371. doi:10.1007/s40271-015-0129-9
24. Gathe J, Arribas JR, Van Lunzen J, et al. Patient-reported symptoms over 48 weeks in a randomized, open-label, phase 3b non-inferiority trial of adults with HIV switching to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir DF versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir DF. Patient. 2015;8(5):445–454. doi:10.1007/s40271-015-0137-9
25. van Lunzen J, Antinori A, Cohen CJ, et al. Rilpivirine vs. efavirenz-based single-tablet regimens in treatment-naive adults: week 96 efficacy and safety from a randomized phase 3b study. AIDS. 2016;30(2):251–259. doi:10.1097/QAD.0000000000000911
26. Brunetta J, Moreno Guillen S, Antinori A, et al. Patient-reported outcomes after a switch to a single-tablet regimen of rilpivirine, emtricitabine, and tenofovir DF in HIV-1-positive, virologically suppressed individuals: additional findings from a randomized, open-label, 48-week trial. Patient. 2015;8(3):257–267. doi:10.1007/s40271-015-0123-2
27. Wohl D, Clarke A, Maggiolo F, et al. Patient-reported symptoms over 48 weeks among participants in randomized, double-blind, Phase III non-inferiority trials of adults with HIV on co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus co-formulated Abacavir, dolutegravir, and lamivudine. Patient. 2018;11(5):561–573. doi:10.1007/s40271-018-0322-8
28. Quatremere G, Guiguet M, Girardi P, et al. How are women living with HIV in France coping with their perceived side effects of antiretroviral therapy? Results from the EVE study. PLoS One. 2017;12(3):e0173338. doi:10.1371/journal.pone.0173338
29. ElZarrad MK, Corrigan-Curay J. The US Food and Drug Administration’s real-world evidence framework: a commitment for engagement and transparency on real-world evidence. Clin Pharmacol Ther. 2019;106(1):33–35. doi:10.1002/cpt.1389
30. Huhn GD, Tebas P, Gallant J, et al. A randomized, open-label trial to evaluate switching to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide plus darunavir in treatment-experienced HIV-1-infected adults. J Acquir Immune Defic Syndr. 2017;74(2):193–200. doi:10.1097/QAI.0000000000001193
31. Lazarus JV, Safreed-Harmon K, Barton SE, et al. Beyond viral suppression of HIV - the new quality of life frontier. BMC Med. 2016;14(1):94. doi:10.1186/s12916-016-0640-4
32. Joint United National Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic; 2014. Available from: https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf.
33. Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas 2019: national profile. HIV Surveillance Report 2019. Vol. 32; 2021.
34. Murray M, Antela A, Mills A, et al. Patient-reported outcomes in ATLAS and FLAIR participants on long-acting regimens of cabotegravir and rilpivirine over 48 weeks. AIDS Behav. 2020;24(12):3533–3544. doi:10.1007/s10461-020-02929-8
35. Murray M, Pulido F, Mills A, et al. Patient-reported tolerability and acceptability of cabotegravir + rilpivirine long-acting injections for the treatment of HIV-1 infection: 96-week results from the randomized LATTE-2 study. HIV Res Clin Pract. 2019;20(4–5):111–122. doi:10.1080/25787489.2019.1661696
36. Czeisler ME, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19-related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250–1257. doi:10.15585/mmwr.mm6936a4
37. Hartnett KP, Kite-Powell A, DeVies J, et al. Impact of the COVID-19 pandemic on emergency department visits - United States, January 1, 2019–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):699–704. doi:10.15585/mmwr.mm6923e1
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
