Back to Journals » Patient Related Outcome Measures » Volume 13
Patient-Reported Outcomes in Rheumatoid Arthritis: A Key Consideration for Evaluating Biosimilar Uptake?
Authors Horta-Baas G
Received 9 January 2022
Accepted for publication 16 March 2022
Published 30 March 2022 Volume 2022:13 Pages 79—95
DOI https://doi.org/10.2147/PROM.S256715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lynne Nemeth
Gabriel Horta-Baas
Rheumatology Department, Hospital General Regional # 1, Instituto Mexicano del Seguro Social, Merida, Yucatan, Mexico
Correspondence: Gabriel Horta-Baas, Rheumatology Department, Hospital General Regional # 1, Instituto Mexicano del Seguro Social, 41 Street # 439, Colonia Industrial, Merida, 97150, Yucatan, Mexico, Tel +52 999 386 0846, Email [email protected]
Purpose: This review aims to provide an overview of the impact of TNFis biosimilars, with marketing authorization, in patient-reported outcome measures (PROMs) scores and explore how PROMs endpoints might add value in biosimilars uptake in RA patients.
Patients and Methods: A comprehensive search of Medline, Scopus, Lilacs, and CINAHL databases was performed for papers published between January 2012 and December 2021. For inclusion, studies had to be prospective, published in a peer-reviewed journal, published in English or Spanish language; studies using PROMs as an outcome measure. After screening title and abstracts and assessing the remaining full texts fulfilling the inclusion criteria, 31 papers were used in this narrative review.
Results: PROMs were used as secondary outcomes in included studies. The most frequently employed domains to assess biosimilar efficacy include physical function, patient global assessment (PtGA), health-related quality of life (HRQoL), and fatigue. The results of randomized clinical trials uniformly showed that mean change in PROMs scores is comparable between biosimilar and reference biologic treatment groups. However, open-label and real-world studies revealed high rates of discontinuation of therapy, mainly for subjective worsening of disease activity or non-specific adverse events. Even without objective clinical evidence of inflammation, patients who are considered to have active disease (higher scores on PtGA) have higher discontinuation rates of biosimilars. The available information suggests that the nocebo effect is the most likely cause for the discontinuation of biosimilars.
Conclusion: There is scarce literature surrounding the impact of biosimilars in PROMs, especially in open-label studies. In real-life studies, biosimilars have a higher discontinuation rate than reference products. TNFis biosimilars treatment efficacy in RA depends on disease activity and other factors such as PtGA and fatigue. The nocebo effect is the best explanation for biosimilar’s discontinuation.
Keywords: patient-reported outcome measures, biologics, biosimilars, disease-modifying antirheumatic drug, drug therapy
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that affects between 0.5% and 1% of the general population.1,2 Pharmacological treatment for RA includes using non-steroidal anti-inflammatory drugs, glucocorticoids, and synthetic or biologics disease-modifying anti-rheumatic drugs (DMARDs). The standard of care for RA patients who respond inadequately to synthetic DMARDs is to add a biological DMARD (biologics). However, biologics are not curative. To maintain disease remission, patients require long-term treatment. Withdrawal of biologics may eventually lead to arthritis flares.3,4
RA patients represent one of the largest populations receiving the broadest spectrum of currently available biologics.5 Earlier treatment with biologics can reduce symptom severity and articular damage.1,6 Unfortunately, biologics are high-cost medications, predominantly due to manufacturing costs. Biologics contain an active substance from a biological source and are manufactured by complex processes using living systems.7,8 Increasing the use of biologics has contributed to escalating significantly to the economic burden of disease.9–12 Therefore, biologics’ cost limited their accessibility and delayed their early use. Usually, biological therapy is indicated when synthetic DMARDs have failed in most countries.11
Tumor necrosis factor-alpha (TNF-α) plays an essential role in the inflammatory processes of RA. Biologics directed against TNF-α (TNFis) therapy down-regulates inflammatory cytokines stimulated by this cytokine.13 TNFis are often considered first-line biologic therapy due to efficacy, safety, and the availability of long-term data from clinical trials and extensive real-world experience.14 TNFis are highly effective medicines with rates of clinical control in over 60% of patients.15,16 Although there are differences in the sites of action and molecular structure of TNFis, all are similarly effective. Nevertheless, approximately 50% of patients discontinue their TNFi over the first five years due to inefficacy or adverse events (drug-related toxicity, infusion reactions, infections, and development of comorbidity).17
To date, there is a strong interest in the pharmaceutical industry to develop TNFis biosimilars. Biosimilar TNFis manufacturing has been motivated by increased health-care costs, and patent protection for some TNFis has expired. Infliximab, adalimumab, and etanercept (including biosimilar forms) are the most frequently used TNFis.14
Biosimilars production aims to create a product highly similar in terms of structure (identical amino acid sequence).18,19 However, biosimilars are not manufactured precisely as the reference product. Minor differences in clinically inactive components are inevitable.20,21 These differences in manufacture are one of the main barriers in doctors’ acceptance of biosimilars, given the possibility that differences may induce immunogenicity-related side effects and loss of efficacy.21,22 Nevertheless, the structural complexity of monoclonal antibodies combined with the sensitivity of their manufacturing process to environmental conditions means that biologics cannot be replicated exactly, so there is variability between batch to batch, regardless of whether the product is a reference product or a biosimilar.12,20,21
Effectiveness, safety, and bioequivalence are essential factors contributing to the uptake of biosimilars drugs. Unlike clinical trials for approving new drugs, biosimilar clinical trials are designed to establish bioequivalence to the brand-name product and demonstrate that any differences are not clinically significant.7,23–26 Regulatory authorities such as the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) have established guidelines for the approval of biosimilar products. TNFis for the treatment of RA, including etanercept, infliximab, and adalimumab biosimilars, have received regulatory approval. A biosimilar with marketing authorization has demonstrated bioequivalence (in terms of efficacy, safety, and immunogenicity) to its reference product.27
Over the past few years, several studies have shown that TNFis biosimilars drugs are equally effective as brand-name products and reduce the cost of treatment.28,29 However, it is important to recognize that biosimilars do not provide any clinical advantage to reference biologics. The key driver for the uptake of biosimilars is cost reduction.21,30 Biosimilar competition has already reduced average list prices in European countries. Although it is uncertain to what degree biosimilars will lower costs, discounts vary by market and product, early entrants have validated the estimates of a price 15–60% lower than the originator biologic.27,30,31
Biosimilars uptake may improve access and quality of care in RA.6,7 The lower price of biosimilars may allow more patients to be treated earlier in their disease course.1,6,11,32 Biosimilars are increasingly used in routine care of patients with RA.33 However, the uptake of biosimilars has varied considerably between countries.34 Randomized clinical trials (RCTs) and real-world data are crucial to improving physician and patient confidence in these medicines.22
Preferences of physicians or patients can impact biosimilar uptake. Low uptake of biosimilars can partly be explained by a lack of confidence among physicians, mainly driven by efficacy and safety concerns and the need for more evidence, particularly in the real-world setting.35 Biosimilar uptake can be influenced by patient-related factors, including refusal to change or negative perceptions.6,36,37 Patients satisfied with RA control or confident with their current treatment did not perceive a need to change their treatment, which may risk losing the currently acceptable health state or fearing new side effects.38,39
A significant key to advances in the assessment of DMARDs efficacy has been the development of patient-reported outcome measures (PROMs).1 PROMs are used as secondary outcomes in most clinical trials. They are recognized as measures of treatment efficacy by the FDA and EMA.40 PROMs quantity the impact of treatment from the patient’s perspective in a standardized way and provide complementary information, based on different viewpoints, to physician-derived measures (eg, joint counts) or laboratory data (eg, C-reactive protein). Combining PROMs with objective measures is crucial to insight into treatment effects and patients’ well-being.41,42
PROMs are instruments (usually questionnaires) that allow patients to report their symptoms directly, but their role in assessing inflammation and articular damage is imprecise.43 Symptom severity (eg, pain and fatigue), global patient assessment, physical function, satisfaction with treatments, and quality of life (QoL) are essential outcomes in RA that need to be measured with PROMs.40,42,44 Reduction in pain and improvement in physical function and fatigue were consistently identified as important treatment outcomes.45 The results obtained from PROMs in RCTs can inform realistic expectations for patients and promote shared decision-making with their physicians. Furthermore, medicines with a similar efficacy may produce different PROMs scores.46
With the increasing interest in biosimilar use, understanding patient perspectives and changes in PROMs after the onset of these medicines is indispensable. Considering patients’ views and preferences regarding the use of biosimilars is essential. However, they are often not considered thoroughly. Currently, the literature lacks an adequate summary of the evidence of the effect of biosimilars in PROMs. This review aims to provide an overview of the impact of TNFis biosimilars, with marketing authorization, in PROMs scores and explore how PROMs endpoints might add value in biosimilars uptake in RA patients.
Literature Search
A comprehensive search of Medline (PubMed and Ovid), Lilacs, CINAHL (EBSCO host), and Scopus databases was performed for papers published between January 2012 and December 2021 (Table 1). The search for relevant studies using Medical Subject Headings (MeSH) terms and keywords “arthritis, rheumatoid,” “patient-reported outcome measures,” “biosimilars,” “biocomparables,” “biosimilar,” were used in various combinations using Boolean operators like “AND” and “OR.” For inclusion, studies had to be: a) prospective (clinical trials and observational studies); b) published in a peer-reviewed journal; c) published in English or Spanish language; d) studies using PROMs as an outcome measure (primary or secondary); e) studies conducted in human subjects. Letters, reviews, meeting abstracts, cost-efficacy studies, and studies with participants aged less than 18 years old were excluded. A total of 543 citations were retrieved from the four databases. The PRISMA 2020 flow diagram of selection studies is shown in Figure 1.47 Duplicates were removed using EndNote’s duplicate identification strategy (n = 29) and then manually (n = 75). After screening titles and abstracts and assessing the remaining full texts fulfilling the inclusion criteria, 31 papers were used in this narrative review.
 |
Table 1 Search Strategy |
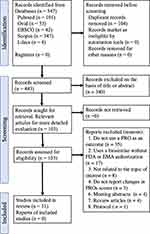 |
Figure 1 PRISMA 2020 flow diagram for selection of studies. Note: Adapted from: Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.48 Copyright © Author(s) (or their employer(s)) 2019. Creative Commons Attribution (CC BY 4.0) license (https://creativecommons.org/licenses/by/4.0/legalcode). |
Results
This review focuses on three topics related to the use of PROMs in the assessment efficacy of biosimilars: PROMs in RCTs, PROMs in open-label and real-world studies, and the nocebo effect in biosimilar discontinuation.
Patient-Reported Outcome Measures in Clinical Trials
Several TNFis biosimilars have been approved in Europe and the US, based on results from RCTs comparing the bioequivalence of the biosimilar with originator drug (Table 2). Most of the studies were funded by the pharmaceutical industry. Therefore, it is logical that the primary outcomes of the studies consider the regulatory perspective to obtain approval by the regulatory authorities.42 Efficacy, safety, or immunogenicity endpoints were incorporated into most studies, but only a limited number of studies included PROMs, especially in real-world registries. Usually, PROMs were used as secondary or exploratory endpoints in RCTs. In general, changes in PROMs scores were comparable between patients maintained on biosimilars or reference products and those who switched to biosimilars from reference products.
 |
Table 2 Lists the Biologics Directed Against TNF-α (TNFis) Biosimilars That Have Been Approved by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) |
The most frequently employed domains or constructs to assess biosimilar efficacy include physical function, patient global assessment (PtGA), health-related quality of life (HRQoL), and less commonly fatigue. However, other important domains in RA patients, such as sleep, psychological well-being, or ability to cope were not assessed. The most frequent PROMs instruments reported were the Health Assessment Questionnaire Disability Index (HAQ-DI), patient global visual analog scale, and the Short-Form 36 (SF-36) physical and mental components. Two studies used the European Quality of Life-5 Dimensions (EQ-5D) index; two studies used the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-fatigue) to assess fatigue, and one study employed the Rheumatoid Arthritis Impact of Disease (RAID).
The HAQ-DI questionnaire is considered the gold standard for assessing functional limitations in RA patients.48 The results of RCTs uniformly exhibit that the mean change in HAQ-DI scores is comparable between biosimilar and reference biologic treatment groups (Tables 3 and 4). Improvements in HAQ-DI scores perceived in patients with RA treated with biosimilars demonstrate that these medicines improve functional capacity and reduce disability of patients in the short- and long term.29,49–52 In most instances, mean values and changes from baseline in HAQ-DI were similar between the two treatment groups (biosimilar vs reference product).53
 |
Table 3 Mean Changes of PROMs Score Between Week 24 to 30 from Baseline |
 |
Table 4 Mean Changes of PROMs Score Between Week 48 to 54 from Baseline |
The EQUIRA study50,54 aimed demonstrated similar efficacy and comparable safety and immunogenicity profile of GP2015 (Erelzi®; Sandoz GmbH, Kundl, Austria) to etanercept (Enbrel®; Immunex Corporation, Thousand Oaks, CA, USA) at week 24 in patients with moderate-to-severe RA who had an inadequate response to either synthetic or biologic DMARDs. Improvements from baseline in HAQ-DI and scores were comparable between GP2015 and etanercept groups. At week 24, the mean change from baseline in HAQ-DI score was −0.57 in the GP2015 group and −0.67 in the etanercept group.54 At week 48, the mean change from baseline in HAQ-DI score was −0.62 in the “continued GP2015” group and −0.66 in the “switched to GP2015” group. Furthermore, the proportion of patients achieving HAQ-DI ≤0.5 up to week 48 was comparable between “continued GP2015” group and “switched to GP2015” group (34.7% vs 39.5%).50
The mean change of HAQ-DI score at week 78 in patients treated with the infliximab biosimilar PF06438179/GP1111 (Ixifi®; Pfizer Ireland Pharmaceuticals Ringaskiddy, Co. Cork, Ireland) after switching from infliximab (Remicade®; Janssen Pharmaceutica, Beerse, Belgium) or continuing PF06438179/GP1111 was −0.8.42 The ADMYRA study51 aimed to demonstrate similar efficacy and safety of GP2017 (Hyrimoz®; Sandoz, Holzkirchen, Germany) and adalimumab (Humira®; AbbVie Inc., Chicago, IL, USA) in patients with moderate to severe RA with inadequate response to DMARDs. In this study, the proportion of patients achieving a HAQ-DI score ≤0.5 at week 24 was similar between treatment groups (GP2017 37.8% vs adalimumab 36.3%).51 Furthermore, after switching, mean HAQ-DI scores remained stable, with no clinically meaningful differences between the treatment groups.51
The Patient’s Global Assessment of Disease Activity (PtGA) was reported in 3 studies, making one the most frequently reported PRO after the physical function. The PtGA is a single item that measures disease impact. PtGA scores can range from 0 to 100 mm; higher scores represent a higher level of disease activity.55 PtGA plays a major role in determining fulfillment of remission criteria in RA.55 Parallel reductions in PtGA accompanied disease improvement. The mean change of PtGA among patients treated with biosimilars was −30.3 to −35.1, while for patients treated with the reference product was −26.6 to −39.4 (Table 3).51,56
HRQoL measures a patient’s value on their current health state.45 To date, limited HRQoL data are available on biosimilars studies. Some studies used generic questionnaires to assess the HRQoL. Three studies did not demonstrate any differences between the biosimilar and the product reference in changes in SF-36 scores in the short- and long term (Tables 3 and 4).56–58 These results support TNFis biosimilars treatment in RA patients improved patients’ HRQoL.
The PLANETRA study56 was an RCT Phase 3 trial comparing safety and efficacy of infliximab and biosimilar CT-P13 (Inflectra®; Hospira, Lake Forest, IL, USA; and Remsima®; Celltrion, Incheon, South Korea) in 606 patients with active RA who had inadequate responses to methotrexate. This study showed that CT-P13 and infliximab have equivalent pharmacokinetic profiles, comparable efficacy and safety, and no clinically important differences in PROMs. Mean scores on pain, PtGA, and HAQ-DI decreased at week 14 and remained stable after that up to week 54 in both treatment groups. Contrarily, both groups’ mean SF-36 score increased (better QoL) from baseline to week 54. In the extension study, no notable differences in HAQ-DI scores were noted between treatment groups (CT-P13 vs infliximab) at weeks 14, 30, 54, 78, or 102.52
In a RCT, Takeuchi et al59 showed that the mean changes from baseline scores in HAQ-DI were similar for the CT-P13 (n = 50) and infliximab (n = 51) groups in Japanese patients with RA at week 14 (−0.36 vs −0.33, p = 0.74) and week 30 (−0.47 vs −0.36, p = 0.25). However, at week 54, the mean changes from baseline scores were slightly higher in the group treated with CT-P13 (−0.54 vs −0.25, p < 0.01). Subsequently, Tanaka et al60 reported the results of the open phase of this study. This study evaluated the efficacy of CT-P13 in patients with RA during long-term treatment (n = 37) or after switching from infliximab (n = 32). Patients’ assessments of HAQ-DI score in the switch group were slightly higher than those in the maintenance group (0.56 vs 0.36). The mean changes from baseline scores in HAQ-DI were slightly higher in the long-term treatment group at week 62 (−0.70 vs −0.37; p = 0.01), week 110 (−0.66 vs −0.38; p = 0.04), and week 134 (−0.67 vs −0.40, P = 0.06).
The non-medical switch of CT-P13 in patients with several stable inflammatory conditions (RA, spondyloarthritis, Psoriatic arthritis, ulcerative colitis, Crohn's disease, and Psoriasis) was evaluated in the NOR-SWITCH study.58 This study shows that switching from infliximab to biosimilar CT-P13 is not inferior to continued treatment with the originator drug. Authors found no significant differences between those who had switched and those who remained on infliximab in disease worsening, PtGA, SF-36, and EQ-5D scores. The extension phases of the NOR-SWITCH study61 reported that the biosimilar CT-P13 produces comparable changes in PROMs as the original drug. In another study, EQ-5D index values and EQ-5D-5L VAS scores were similar for biosimilar MSB11022 (Idacio®; Fresenius Kabi Deutschland GmbH, Bad Homburg v.d.Höhe, Germany) versus adalimumab at weeks 24 and 52.57 Both treatment groups showed a similar increase in the SF-36 physical and mental component scores.62
In the ADMYRA study, the biosimilar GP2017 (Hyrimoz®; Sandoz, Holzkirchen, Germany) demonstrated comparable improvements in FACIT-fatigue scores to adalimumab. At week 24, the mean percent change from baseline in the FACIT-fatigue score was 75.4 in GP2017 and 73.0 in adalimumab groups. The improvement with GP2017 was maintained after switching at week 48.50 In the EQUIRA study, the mean change from baseline in the FACIT-fatigue score at week 24 was 9.45 in the biosimilar GP2015 group and 11.82 in the etanercept group. Furthermore, no difference in FACIT-fatigue score was reported after switching to GP2015. The mean change from baseline in FACIT-fatigue score was 11.6 in the “continued GP2015” group and 10.6 in the “switched etanercept to GP2015” group.50 The data of these studies showed changes in fatigue levels were comparable between treatment groups.
The RAID questionnaire is a patient-derived composite measure of the impact of RA.63 The total RAID score ranges from 0 to 10, with 10 representing worst health. The RAID scores were similar with CT-P13 compared to infliximab in the NOR-SWITCH study.58 The mean RAID score in the group of patients treated with the biosimilar CT-P13 was 2.2 at baseline and 0.6 at week 52. In the infliximab-treated group, the mean RAID score was 2.0 at baseline and 0.2 at week 52. The difference between groups at week 52 was not significant (0.47, 95% CI −0.14 to 1.08). Changes in the RAID from the extension phase baseline (week 52) to the end of follow-up (week 78) were generally similar in both groups.61
Patient-Reported Outcomes in Open-Label and Real-World Studies
Patients in RCTs differ from patients treated in routine clinical practice.64 It is debated whether the biosimilars are bioequivalent to the originator when patients are switched to biosimilar in real-word. Patient registry studies and observational studies conducted in larger, more heterogeneous cohorts (eg, older patients, more comorbidities, or atypical disease) and longer follow-up time can provide valuable additional information.65 The knowledge of real-world clinical use of approved biosimilars would help assess the effectiveness, safety, and QoL of biosimilars in the treatment of RA.50 However, lack of randomization makes observational effect estimates vulnerable to confounding.66
In 2016, Denmark mandated a switch to the biosimilar from the reference product among patients with rheumatic diseases, providing an opportunity to compare treatment outcomes of those who switched and those who did not. The DANBIO registry includes rheumatologic patients treated in routine care with biological DMARDs. The analyses of the DANBIO registry reported that disease activity three months before and after the switch was essentially unchanged in most patients, demonstrating that switching to CT-P13 had no negative impact on disease activity, and 81% of RA patients remained on therapy at one year.67 The adjusted retention rate with CT-P13 after 54 weeks was slightly lower than for infliximab in a historic cohort. The median HAQ-DI score three months before switch, at switch, and three months post-switch was 0.6. Interestingly, higher PtGA scores at baseline and monotherapy were associated with poorer retention in RA patients. The authors considered that the lower retention of the biosimilar might not necessarily be attributable to the lack of efficacy of CT-P13. Other explanations such as negative expectations towards the biosimilar (nocebo effect) or some other confounding factor should be considered.
In a small cohort of patients with inflammatory arthritis (n = 34), PROs were compared before (the last six months of infliximab treatment) and after switching to CPT-13 (mean follow-up was 5.8 months). Before switching, patients were on infliximab treatment for a median duration of 57 months. In this study, there was no significant difference in ptGA (28.3 vs 35.2, p = 0.11) and HAQ-DI scores (0.38 vs 0.69, p = 0.11) before and after switching. However, the pain score increased significantly following switching (28.8 vs 38.1, p = 0.04).68
In patients switching from etanercept to SB4 (Benepali® or Brenzys®; Samsung Bioepis, Seoul, South Korea) in the DANBIO registry, pre- and post-switch changes over three months were not clinically different for a range of disease activity measures in patients with rheumatic diseases and no major safety concerns were observed. During follow-up, 299 switchers (18%) and 145 non-switchers (33%) withdrew from treatment with SB4 and etanercept, respectively. A subgroup of SB4-treated patients switched back to etanercept; the main reason for SB4 withdrawal was lack of effect.64 However, changes in disease activity at the etanercept restart were mainly subjective (PtGA) rather than objective (C-reactive protein and swollen joint counts were close to zero).64
The BIO-SWITCH study aimed to assess the effect of switching treatment from infliximab to biosimilar CT-P13 on drug survival, effectiveness, safety, and immunogenicity in patients with rheumatic diseases (RA, spondyloarthritis, and psoriatic arthritis) in daily clinical care.69 At month six, 47 of 192 patients (24%) discontinued CT-P13 due to a perceived lack of effect (n = 26), adverse events (n = 11), or both. Most of the adverse events reported (78%) were subjective (eg, mood disorders and fatigue). Subjective complaints (arthralgia and fatigue) were the main reason for biosimilar discontinuation. Furthermore, among those who discontinued CT-P13 due to a perceived lack of efficacy, tender joint count and PtGA scores worsened relative to baseline. In contrast, swollen joint count and C-reactive protein were stable. Interestingly, most patients who discontinued CT-P13 (79%) restarted infliximab, decreasing the number of tender joints and the PtGA and lowering disease activity scores. Authors suggested that the discontinuation of CT-P13 due to subjective health complaints might be explained by nocebo or incorrect causal attribution effects.69
Kay et al70 demonstrated the equivalence efficacy to CT-P17 (Yuflyma®; Celltrion Healthcare Hungary, Budapest, Hungary) at week 24 to reference adalimumab in 648 patients with moderate-to-severe active RA despite methotrexate treatment. Overall safety profiles were similar between groups. SF-36 scores increased from baseline to week 24 in both groups. At week 24, mean change from baseline in the SF-36 physical component score was 7.86 for CT-P17 and 8.21 for adalimumab. Similarly, mean change from baseline in the SF-36 mental component score was 5.87 for CT-P17 and 6.58 for adalimumab. The extension study71 (week 52) demonstrates comparable efficacy, PROMs scores changes, pharmacokinetics, safety, and immunogenicity among subjects with RA who continued treatment with CT-P17, or adalimumab, or who switched from adalimumab to CT-P17. Mean changes from baseline in SF-36 physical component scores were 9.63, 10.70, and 9.74 in the continued CT-P17, continued adalimumab, and switched to CT-P17 groups, respectively. Analogous mean changes from baseline in SF-36 mental component scores were 5.90, 7.73, and 7.19.71
Codreanu et al62 compared the efficacy and safety of SB4 to etanercept in a real-life national cohort. The study included 242 RA patients from the Romanian Registry of Rheumatic Diseases. Results showed that etanercept and SB4 have equivalent efficacy and safety at six months of treatment. Although patients on SB4 tended to have higher PtGA (35.7 mm vs 30.7 mm; p = 0.077) and lower mean CRP levels (3.6 mg/L vs 5.6 mg/L, p = 0.071), these differences were not statistically significant. The authors could not assess PROMs (eg, HAQ-DI, SF-36, or EQ-5D) because of the lack of data in the registry.
In Germany, Kiltz et al72 retrospectively evaluated the effectiveness of non-medical switching from etanercept to SB4 in adult patients with rheumatic diseases. HAQ-DI mean scores remain stable in RA and Psoriatic arthritis patients from baseline (1.2 SD 0.7) to 12 and 24 weeks (1.3 SD 0.7). Interestingly, in this study, there was no difference in any outcome at 24 weeks related to the fact that patients were or were not informed about switching to a biosimilar. In another study, Maucksch et al73 reported that most patients (426/492; 86.5%) with AR and Spondyloarthritis treated with SB4 were satisfied or very satisfied with the SB4 pre-filled pen (based on a 5-point Likert scale).
Bruni et al74 described the efficacy of SB5 (Imraldi®; Biogen, Hillerød, Denmark) after switching from adalimumab in 19 patients with RA. At three months of switching, there was no significant difference in ptGA (2.85 vs 2.96), VAS for pain (2.86 vs 2.5), and HAQ-DI (0.32 vs 0.35) compared to baseline scores. Similar results were observed at six months, except for the HAQ-DI. Compared to the baseline, there was a statistically significant decrease in HAQ-DI scores (0.32 vs 0.11, p = 0.02).
Nocebo Effect in Biosimilar Discontinuation
Although data from RCTs were positive, open-label and real-world studies revealed high discontinuation rates of therapy, mainly for subjective worsening of disease activity or non-specific adverse events.75 In a systematic review, the median discontinuation rates for any reason were 14.3% in observational studies compared with 6.95% in RCTs.76 Because biosimilars withdrawal was closely associated with non-specific complaints, several authors suggest that the nocebo effect is probably the best explanation for biosimilar’s discontinuation.12,37,77,78 The nocebo effect is the worsening of symptom induction by pharmacological or non-pharmacological treatments.20,79 The nocebo responses may result from patients’ negative expectations and not the pharmacologic action of the biosimilar itself.75
A significant barrier to the uptake of biosimilar medicines is misinformation and mistrust from patients and health-care professionals.32 Patients’ preferences regarding switching remain important but are often not considered fully. The available data indicate that some RA patients may be more susceptible to nocebo effects, especially in patients with non-medical switching, suggesting that knowledge of a switch to a biosimilar product may have affected treatment efficacy.31,76,80 Switching between a reference biologic and a biosimilar for non-medical reasons may generate negative sentiment in those unwilling or reluctant to change.
Nocebo effects may be influenced by the patient’s perception of the medicines; some patients hold negative perceptions and might feel reluctant to accept or refuse to change if they consider biosimilar as second-choice drugs or drugs of lower quality.38,81 On the other hand, some patients may consider the lower price of biosimilars as an indication of lower quality.81,82
In addition, health-care workers’ perception of biosimilars may influence patients to develop the nocebo effect. Physician’s and nursing staff communications might contain unintentional negative suggestions that may trigger a nocebo response.79 Earlier studies suggest some physicians mistrust biosimilars or are not fully confident in their use.12
Patients’ perspectives and the variability in patient preferences highlight the need to individualize treatment choices in RA.5,45 Even without objective clinical evidence of inflammation, patients who are considered to have an active disease (higher scores on PtGA) have higher discontinuation rates of biosimilars. PROMs provide unique information on the impact of biosimilar treatment from the patient’s perspective. Shared decision-making processes between RA patients and their clinicians are associated with good disease response, increased biosimilars’ acceptance, and reducing the nocebo effect.32,39,75,80
There are several limitations of these analyses, which can influence the validity of the reported results. Despite its importance, there is scarce literature surrounding the impact of biosimilars in PROMs, especially in open-label studies. Several studies that evaluated the efficacy and safety of biosimilars were excluded from this review because they did not include PROMs as outcome measures. The total number of open-label studies, including PROMs as outcome measures, was lower than that of RCTs. Most of the studies included in this review only present data descriptively. No hypothesis testing was performed to determine whether the results of PROMs scores were significantly different between patients treated with biosimilars and patients treated with reference products. Studies included in this review were not powered to detect differences in PROMs scores between treatment groups. Actually, no study has considered the effect of biosimilars on PROMs as a primary outcome. The available information suggests that the nocebo effect is the most likely cause for the withdrawal of biosimilars. However, there is not yet a study designed to evaluate this association.
PROMs data may help in the selection of optimal treatment, patient’s symptom experience, and treatment satisfaction. The information provided by PROMs can help physicians, and future patients evaluate the efficacy, safety, and patient’s experience with the treatment and thus select the best treatment promoting shared decision-making. Future studies are necessary aimed to demonstrate differences in PROMs scores among biosimilars and reference products. Similarly, further studies are needed to evaluate the association between the nocebo effect and continuation rates of biosimilars DMARDs.
Conclusions
Biosimilars expand therapeutic options for RA patients, and their use is increasing worldwide, especially in European countries. Growing evidence from clinical trials and real-world registries demonstrate that TNFis biosimilars are effective and safe as reference biologics. Similarly, available data suggest that switching from the originator drug to a biosimilar tends to be effective.
There is scarce literature surrounding the impact of biosimilars in PROMs, especially in open-label studies. In real-life studies, biosimilars have a higher discontinuation rate than reference products. TNFis biosimilars treatment efficacy in RA depends on disease activity and other factors such as PtGA and fatigue. The nocebo effect is the best explanation for biosimilar’s discontinuation.
Funding
The author received no financial support for the research, authorship, and/or publication of this article.
Disclosure
The author declared no conflicts of interest for this work and no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Uhlig T, Moe RH, Kvien TK. The burden of disease in rheumatoid arthritis. Pharmacoeconomics. 2014;32:841–851. doi:10.1007/s40273-014-0174-6
2. Codreanu C, Popescu CC, Mogosan C, et al. Efficacy and safety of original and biosimilar etanercept (SB4) in active rheumatoid arthritis - A comparison in a real-world national cohort. Biologicals. 2019;62:27–32. doi:10.1016/j.biologicals.2019.10.009
3. Elliott MJ, Feldmann M, Maini RN. TNF alpha blockade in rheumatoid arthritis: rationale, clinical outcomes and mechanisms of action. Int J Immunopharmacol. 1995;17:141–145. doi:10.1016/0192-0561(94)00092-3
4. Tanaka Y, Hirata S, Saleem B, Emery P. Discontinuation of biologics in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2013;31:S22–27.
5. Edwards CJ, Galeazzi M, Bellinvia S, Ringer A, Dimitroulas T, Kitas G. Can we wean patients with inflammatory arthritis from biological therapies? Autoimmun Rev. 2019;18:102399. doi:10.1016/j.autrev.2019.102399
6. Boccia R, Jacobs I, Popovian R, de Lima Lopes G. Can biosimilars help achieve the goals of US health care reform? Cancer Manag Res. 2017;9:197–205. doi:10.2147/CMAR.S133442
7. Stebbing J, Mainwaring PN, Curigliano G, et al. Understanding the role of comparative clinical studies in the development of oncology biosimilars. J Clin Oncol. 2020;38:1070–1080. doi:10.1200/JCO.19.02953
8. Cohen HP, Blauvelt A, Rifkin RM, Danese S, Gokhale SB, Woollett G. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78:463–478. doi:10.1007/s40265-018-0881-y
9. Mallam N, Byalakere-Rudraiah CS, Rudraswamy S. Generic drugs: current status and future potential. J Gene Med. 2017;13:1–6.
10. Choy EH, Kingsley GH, Panayi GS. Monoclonal antibody therapy in rheumatoid arthritis. Br J Rheumatol. 1998;37:484–490. doi:10.1093/Rheumatology/37.5.484
11. Rezk MF, Pieper B. Unlocking the value of anti-TNF biosimilars: reducing disease burden and improving outcomes in chronic immune-mediated inflammatory diseases: a narrative review. Adv Ther. 2020;37:3732–3745. doi:10.1007/s12325-020-01437-4
12. O’Callaghan J, Barry SP, Bermingham M, Morris JM, Griffin BT. Regulation of biosimilar medicines and current perspectives on interchangeability and policy. Eur J Clin Pharmacol. 2019;75:1–11. doi:10.1007/s00228-018-2542-1
13. Jin J, Chang Y, Wei W. Clinical application and evaluation of anti-TNF-alpha agents for the treatment of rheumatoid arthritis. Acta Pharmacol Sin. 2010;31:1133–1140. doi:10.1038/aps.2010.134
14. Mehta P, Manson JJ. What is the clinical relevance of TNF inhibitor immunogenicity in the management of patients with rheumatoid arthritis? Front Immunol. 2020;11:589. doi:10.3389/fimmu.2020.00589
15. Ramirez-Herraiz E, Escudero-Vilaplana V, Alanon-Plaza E, et al. Efficiency of Adalimumab, etanercept and infliximab in rheumatoid arthritis patients: dosing patterns and effectiveness in daily clinical practice. Clin Exp Rheumatol. 2013;31:559–565.
16. Kievit W, Fransen J, Adang EM, et al. Long-term effectiveness and safety of TNF-blocking agents in daily clinical practice: results from the Dutch Rheumatoid Arthritis Monitoring register. Rheumatology. 2011;50:196–203. doi:10.1093/rheumatology/keq325
17. Arora A, Mahajan A, Spurden D, Boyd H, Porter D. Long-term drug survival of TNF inhibitor therapy in RA patients: a systematic review of European National Drug Registers. Int J Rheumatol. 2013;2013:764518. doi:10.1155/2013/764518
18. Araujo F, Goncalves J, Fonseca JE. Biosimilar DMARDs: what does the future hold? Drugs. 2016;76:629–637. doi:10.1007/s40265-016-0556-5
19. Moorkens E, Simoens S, Troein P, Declerck P, Vulto AG, Huys I. Different policy measures and practices between Swedish counties influence market dynamics: part 2-biosimilar and originator etanercept in the outpatient setting. BioDrugs. 2019;33:299–306. doi:10.1007/s40259-019-00346-5
20. Chew C, Aguiar M, Bansback N, Law MR, Harrison M. Patient perspectives on the British Columbia Biosimilars Initiative: a qualitative descriptive study. Rheumatol Int. 2021. doi:10.1007/s00296-021-04874-8
21. Castaneda-Hernandez G, Szekanecz Z, Mysler E, et al. Biopharmaceuticals for rheumatic diseases in Latin America, Europe, Russia, and India: innovators, biosimilars, and intended copies. Joint Bone Spine. 2014;81:471–477. doi:10.1016/j.jbspin.2014.03.019
22. Kim TH, Lee SS, Park W, et al. A 5-year retrospective analysis of drug survival, safety, and effectiveness of the infliximab biosimilar CT-P13 in patients with rheumatoid arthritis and ankylosing spondylitis. Clin Drug Investig. 2020;40:541–553. doi:10.1007/s40261-020-00907-5
23. Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469–478.
24. Jensen TB, Bartels D, Saedder EA, et al. The Danish model for the quick and safe implementation of infliximab and etanercept biosimilars. Eur J Clin Pharmacol. 2020;76:35–40. doi:10.1007/s00228-019-02765-3
25. Farhat F, Othman A, El Karak F, Kattan J. Review and results of a survey about biosimilars prescription and challenges in the Middle East and North Africa region. Springerplus. 2016;5:2113. doi:10.1186/s40064-016-3779-8
26. Kim H, Alten R, Avedano L, et al. The future of biosimilars: maximizing benefits across immune-mediated inflammatory diseases. Drugs. 2020;80:99–113. doi:10.1007/s40265-020-01256-5
27. Nabhan C, Valley A, Feinberg BA. Barriers to oncology biosimilars uptake in the United States. Oncologist. 2018;23:1261–1265. doi:10.1634/theoncologist.2018-0066
28. Bellinvia S, Cummings JRF, Ardern-Jones MR, Edwards CJ. Adalimumab biosimilars in Europe: an overview of the clinical evidence. BioDrugs. 2019;33:241–253. doi:10.1007/s40259-019-00355-4
29. Alten R, Batko B, Hala T, et al. Randomised, double-blind, Phase III study comparing the infliximab biosimilar, PF-06438179/GP1111, with reference infliximab: efficacy, safety and immunogenicity from week 30 to week 54. RMD Open. 2019;5:e000876. doi:10.1136/rmdopen-2018-000876
30. Dutta B, Huys I, Vulto AG, Simoens S. Identifying key benefits in European off-patent biologics and biosimilar markets: it is not only about price! BioDrugs. 2020;34:159–170. doi:10.1007/s40259-019-00395-w
31. Afzali A, Furtner D, Melsheimer R, Molloy PJ. The automatic substitution of biosimilars: definitions of interchangeability are not interchangeable. Adv Ther. 2021;38:2077–2093. doi:10.1007/s12325-021-01688-9
32. Lobo F, Rio-Alvarez I. Barriers to biosimilar prescribing incentives in the context of clinical governance in Spain. Pharmaceuticals. 2021;14:283. doi:10.3390/ph14030283
33. Nabi H, Georgiadis S, Loft AG, et al. Comparative effectiveness of two Adalimumab biosimilars in 1318 real-world patients with inflammatory rheumatic disease mandated to switch from originator Adalimumab: nationwide observational study emulating a randomised clinical trial. Ann Rheum Dis. 2021;80:1400–1409. doi:10.1136/annrheumdis-2021-219951
34. Allocati E, Bertele V, Gerardi C, Garattini S, Banzi R. Clinical evidence supporting the marketing authorization of biosimilars in Europe. Eur J Clin Pharmacol. 2020;76:557–566. doi:10.1007/s00228-019-02805-y
35. El Zorkany B, Al Ani N, Al Emadi S, et al. Biosimilars in rheumatology: recommendations for regulation and use in Middle Eastern countries. Clin Rheumatol. 2018;37:1143–1152. doi:10.1007/s10067-018-3982-9
36. Chau J, Delate T, Ota T, Bhardwaja B. Patient perspectives on switching from infliximab to infliximab-dyyb in patients with rheumatologic diseases in the United States. ACR Open Rheumatol. 2019;1:52–57. doi:10.1002/acr2.1007
37. Bhat S, Limdi JK, Cross RK, Farraye FA. Does similarity breed contempt? A review of the use of biosimilars in inflammatory bowel disease. Dig Dis Sci. 2021;66:2513–2532. doi:10.1007/s10620-021-07114-y
38. Gasteiger C, Lobo M, Dalbeth N, Petrie KJ. Patients’ beliefs and behaviours are associated with perceptions of safety and concerns in a hypothetical biosimilar switch. Rheumatol Int. 2021;41:163–171. doi:10.1007/s00296-020-04576-7
39. Chan SJ, Yeo HY, Stamp LK, Treharne GJ, Marra CA. What are the preferences of patients with rheumatoid arthritis for treatment modification? A scoping review. Patient. 2021;14:505–532. doi:10.1007/s40271-020-00488-7
40. Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353–367. doi:10.2147/PROM.S156279
41. Kluzek S, Dean B, Wartolowska KA. Patient-reported outcome measures (PROMs) as proof of treatment efficacy. BMJ Evid Based Med. 2021;
42. Gossec L, Dougados M, Dixon W. Patient-reported outcomes as end points in clinical trials in rheumatoid arthritis. RMD Open. 2015;1:e000019. doi:10.1136/rmdopen-2014-000019
43. LeBlanc TW, Abernethy AP. Patient-reported outcomes in cancer care - hearing the patient voice at greater volume. Nat Rev Clin Oncol. 2017;14:763–772. doi:10.1038/nrclinonc.2017.153
44. Crossnohere NL, Brundage M, Calvert MJ, et al. International guidance on the selection of patient-reported outcome measures in clinical trials: a review. Qual Life Res. 2021;30:21–40. doi:10.1007/s11136-020-02625-z
45. Durand C, Eldoma M, Marshall DA, Bansback N, Hazlewood GS. Patient preferences for disease-modifying antirheumatic drug treatment in rheumatoid arthritis: a systematic review. J Rheumatol. 2020;47:176–187. doi:10.3899/jrheum.181165
46. Bansal D, Bhagat A, Schifano F, Gudala K. Role of patient-reported outcomes and other efficacy endpoints in the drug approval process in Europe (2008–2012). J Epidemiol Glob Health. 2015;5:385–395. doi:10.1016/j.jegh.2015.04.006
47. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71
48. Horta-Baas G. Validation of a Spanish version of the Health Assessment Questionnaire-II to assess Mexican patients’ physical function with rheumatoid arthritis. Reumatol Clin. 2021. doi:10.1016/j.reumae.2020.11.002
49. Emery P, Vencovsky J, Sylwestrzak A, et al. 52-week results of the phase 3 randomized study comparing SB4 with reference etanercept in patients with active rheumatoid arthritis. Rheumatology. 2017;56:2093–2101. doi:10.1093/rheumatology/kex269
50. Jaworski J, Matucci-Cerinic M, Schulze-Koops H, et al. Switch from reference etanercept to SDZ ETN, an etanercept biosimilar, does not impact efficacy, safety, and immunogenicity of etanercept in patients with moderate-to-severe rheumatoid arthritis: 48-week results from the phase III, randomized, double-blind EQUIRA study. Arthritis Res Ther. 2019;21:130. doi:10.1186/s13075-019-1907-x
51. Blauvelt A, Leonardi CL, Gaylis N, et al. Treatment with SDZ-ADL, an adalimumab biosimilar, in patients with rheumatoid arthritis, psoriasis, or psoriatic arthritis: results of patient-reported outcome measures from two phase III studies (ADMYRA and ADACCESS). BioDrugs. 2021;35:229–238. doi:10.1007/s40259-021-00470-1
52. Yoo DH, Prodanovic N, Jaworski J, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2017;76:355–363. doi:10.1136/annrheumdis-2015-208786
53. Fleischmann RM, Alten R, Pileckyte M, et al. A comparative clinical study of PF-06410293, a candidate Adalimumab biosimilar, and Adalimumab reference product (Humira(R)) in the treatment of active rheumatoid arthritis. Arthritis Res Ther. 2018;20:178. doi:10.1186/s13075-018-1676-y
54. Matucci-Cerinic M, Allanore Y, Kavanaugh A, et al. Efficacy, safety and immunogenicity of GP2015, an etanercept biosimilar, compared with the reference etanercept in patients with moderate-to-severe rheumatoid arthritis: 24-week results from the comparative phase III, randomised, double-blind EQUIRA study. RMD Open. 2018;4:e000757. doi:10.1136/rmdopen-2018-000757
55. Nikiphorou E, Radner H, Chatzidionysiou K, et al. Patient global assessment in measuring disease activity in rheumatoid arthritis: a review of the literature. Arthritis Res Ther. 2016;18:251. doi:10.1186/s13075-016-1151-6
56. Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613–1620. doi:10.1136/annrheumdis-2012-203090
57. Edwards CJ, Monnet J, Ullmann M, Vlachos P, Chyrok V, Ghori V. Safety of Adalimumab biosimilar MSB11022 (acetate-buffered formulation) in patients with moderately-to-severely active rheumatoid arthritis. Clin Rheumatol. 2019;38:3381–3390. doi:10.1007/s10067-019-04679-y
58. Jorgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389:2304–2316. doi:10.1016/S0140-6736(17)30068-5
59. Takeuchi T, Yamanaka H, Tanaka Y, et al. Evaluation of the pharmacokinetic equivalence and 54-week efficacy and safety of CT-P13 and innovator infliximab in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2015;25:817–824. doi:10.3109/14397595.2015.1022297
60. Tanaka Y, Yamanaka H, Takeuchi T, et al. Safety and efficacy of CT-P13 in Japanese patients with rheumatoid arthritis in an extension phase or after switching from infliximab. Mod Rheumatol. 2017;27:237–245. doi:10.1080/14397595.2016.1206244
61. Goll GL, Jorgensen KK, Sexton J, et al. Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: open-label extension of the NOR-SWITCH trial. J Intern Med. 2019;285:653–669. doi:10.1111/joim.12880
62. Codreanu C, Sirova K, Jarosova K, Batalov A. Assessment of effectiveness and safety of biosimilar infliximab (CT-P13) in a real-life setting for treatment of patients with active rheumatoid arthritis or ankylosing spondylitis. Curr Med Res Opin. 2018;34:1763–1769. doi:10.1080/03007995.2018.1441144
63. Gossec L, Paternotte S, Aanerud GJ, et al. Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: a EULAR initiative. Ann Rheum Dis. 2011;70:935–942. doi:10.1136/ard.2010.142901
64. Glintborg B, Loft AG, Omerovic E, et al. To switch or not to switch: results of a nationwide guideline of mandatory switching from originator to biosimilar etanercept. One-year treatment outcomes in 2061 patients with inflammatory arthritis from the DANBIO registry. Ann Rheum Dis. 2019;78:192–200. doi:10.1136/annrheumdis-2018-213474
65. Cheon JH, Nah S, Kang HW, et al. Infliximab biosimilar CT-P13 observational studies for rheumatoid arthritis, inflammatory bowel diseases, and ankylosing spondylitis: pooled analysis of long-term safety and effectiveness. Adv Ther. 2021;38:4366–4387. doi:10.1007/s12325-021-01834-3
66. Gron KL, Glintborg B, Norgaard M, et al. Comparative effectiveness of certolizumab pegol, abatacept, and biosimilar infliximab in patients with rheumatoid arthritis treated in routine care: observational data from the Danish DANBIO registry emulating a randomized trial. Arthritis Rheumatol. 2019;71:1997–2004. doi:10.1002/art.41031
67. Glintborg B, Sorensen IJ, Loft AG, et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis. 2017;76:1426–1431. doi:10.1136/annrheumdis-2016-210742
68. Abdalla A, Byrne N, Conway R, et al. Long-term safety and efficacy of biosimilar infliximab among patients with inflammatory arthritis switched from reference product. Open Access Rheumatol. 2017;9:29–35. doi:10.2147/OARRR.S124975
69. Tweehuysen L, van den Bemt BJF, van Ingen IL, et al. Subjective complaints as the main reason for biosimilar discontinuation after open-label transition from reference infliximab to biosimilar infliximab. Arthritis Rheumatol. 2018;70:60–68. doi:10.1002/art.40324
70. Kay J, Jaworski J, Wojciechowski R, et al. Efficacy and safety of biosimilar CT-P17 versus reference Adalimumab in subjects with rheumatoid arthritis: 24-week results from a randomized study. Arthritis Res Ther. 2021;23:51. doi:10.1186/s13075-020-02394-7
71. Furst DE, Jaworski J, Wojciechowski R, et al. Efficacy and safety of switching from reference Adalimumab to CT-P17 (100 mg/mL): 52-week randomised study in rheumatoid arthritis. Rheumatology. 2021. doi:10.1093/rheumatology/keab460
72. Kiltz U, Pudelko JC, Tsiami S, Baraliakos X, Braun J. Non-medical switching from reference to biosimilar etanercept - no evidence for nocebo effect: a retrospective analysis of real-life data. Clin Exp Rheumatol. 2021;39:1345–1351.
73. Maucksch C, Aries PM, Zinke S, Müller-Ladner U. Patient satisfaction with the etanercept biosimilar SB4 device, among rheumatoid arthritis and spondyloarthropathy patients - a German observational study. Open Rheumatol J. 2020;14:
74. Bruni C, Bitti R, Nacci F, et al. Efficacy and safety of switching from reference Adalimumab to SB5 in a real-life cohort of inflammatory rheumatic joint diseases. Clin Rheumatol. 2021;40:85–91. doi:10.1007/s10067-020-05199-w
75. Colloca L, Panaccione R, Murphy TK. The clinical implications of nocebo effects for biosimilar therapy. Front Pharmacol. 2019;10:1372. doi:10.3389/fphar.2019.01372
76. Odinet JS, Day CE, Cruz JL, Heindel GA. The biosimilar nocebo effect? A systematic review of double-blinded versus open-label studies. J Manag Care Spec Pharm. 2018;24:952–959. doi:10.18553/jmcp.2018.24.10.952
77. Al Tabaa O, Etcheto A, Dumas S, et al. Doctor’s aptitude for switching from innovator etanercept to biosimilar etanercept in inflammatory rheumatic diseases: experience from a single French rheumatology tertiary care center. Eur J Clin Pharmacol. 2021;77:25–33. doi:10.1007/s00228-020-02957-2
78. Ebbers HC, Pieper B, Issa A, Addison J, Freudensprung U, Rezk MF. Real-world evidence on etanercept biosimilar SB4 in etanercept-naive or switching patients: a systematic review. Rheumatol Ther. 2019;6:317–338. doi:10.1007/s40744-019-00169-4
79. Planes S, Villier C, Mallaret M. The nocebo effect of drugs. Pharmacol Res Perspect. 2016;4:e00208. doi:10.1002/prp2.208
80. Petit J, Antignac M, Poilverd RM, et al. Multidisciplinary team intervention to reduce the nocebo effect when switching from the originator infliximab to a biosimilar. RMD Open. 2021;7:e001396. doi:10.1136/rmdopen-2020-001396
81. Piguet V, D’Incau S, Besson M, Desmeules J, Cedraschi C. Prescribing generic medication in chronic musculoskeletal pain patients: an issue of representations, trust, and experience in a Swiss cohort. PLoS One. 2015;10:e0134661. doi:10.1371/journal.pone.0134661
82. Guttier MC, Silveira MPT, Luiza VL, Bertoldi AD. Factors influencing the preference for purchasing generic drugs in a Southern Brazilian city. Rev Saude Publica. 2017;51:59. doi:10.1590/s1518-8787.2017051006786
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
