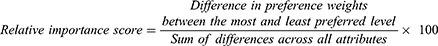Back to Journals » Patient Preference and Adherence » Volume 16
Patient Preference for Self-Injection Devices in Rheumatoid Arthritis: A Discrete Choice Experiment in China
Authors Wei Y, Zhao J , Ming J, Zhang X, Chen Y
Received 30 May 2022
Accepted for publication 19 August 2022
Published 31 August 2022 Volume 2022:16 Pages 2387—2398
DOI https://doi.org/10.2147/PPA.S375938
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Yan Wei,1,* Jin Zhao,1,* Jian Ming,1 Xuewu Zhang,2 Yingyao Chen1
1National Health Commission Key Laboratory of Health Technology Assessment, School of Public Health, Fudan University, Shanghai, People’s Republic of China; 2Department of Rheumatology, Peking University People’s Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xuewu Zhang, Department of Rheumatology, Peking University People’s Hospital, Beijing, People’s Republic of China, Tel +8613681573403, Fax +86-010-88366323, Email [email protected] Yingyao Chen, National Health Commission Key Laboratory of Health Technology Assessment, School of Public Health, Fudan University, Shanghai, 200032, People’s Republic of China, Tel +86-21-33565183, Fax +86-21-64169552, Email [email protected]
Objective: Rheumatoid arthritis (RA) is increasingly treated with biologics, which is commonly administered by injection devices. The aim of this study is to evaluate patients’ preferences for self-injection devices in RA, and to elicit their willingness to pay (WTP) for injector devices in China.
Methods: RA patients were recruited from Beijing, Shanghai, Guangzhou, Chengdu, Wuhan and Xi’an in China. A discrete choice experiment (DCE) was employed to elicit patient preferences. Patients were presented with 15 choice sets that consisted of seven attributes, which were developed based on literature review and further validated by physicians. The seven attributes were as follows: operation steps, injection pain, feedback indication, needle visibility, needle protection, size and out-of-pocket costs. A mixed logit model was used to analyze the relative importance of seven attributes and to calculate the WTP for a changed attribute level.
Results: Analyses included 114 adults with RA (mean age of 44.6 years old). When choosing the injection device, all attributes had significant influence. Among nonmonetary attributes, very slight injection pain was the most preferred for patients, followed by auto-injection method, thicker size, hidden needle, with needle protection and multiple feedback indication. Patients had a negative preference for increasing out-of-pocket costs. Patients were willing to pay CNY 45.26 for improving slight injection pain to very slight injection pain, CNY 13.26 for the auto-injection method, CNY 12.22 for the thicker size, CNY 10.06 for the hidden needle, CNY 9.82 for needle protection, and CNY 9.70 for multiple feedback indicators.
Conclusion: The results suggested that injection pain was the most important attribute for RA patients. Meanwhile, all other enhanced attributes of self-injection devices also significantly influence patients’ selection on injection devices. Findings suggested that there is significant potential value in developing self-injection devices that could further help improve treatment adherence and promote patient-centered care in autoimmune diseases.
Keywords: self-injection devices, rheumatoid arthritis, patient preferences, discrete choice experiment
Introduction
Rheumatoid arthritis (RA) is an autoimmune and inflammatory disease characterized by inflammatory arthritis and extra-articular involvement,1 which could result in loss of function, progressive disability, and increased morbidity and mortality.2 RA can occur at any age, with about 80% occurring between 35 and 50 years old.3,4 Meanwhile, RA is more prevalent in females than males, and the ratio of females to males with RA is approximately 4:1.1,4,5 Based on the data from the Chinese Registry of Rheumatoid Arthritis (CREDIT), there are more than 5 million RA patients in mainland China in 2020.4 RA not only causes physical impairment and mental stress but also leads to a huge economic burden on patients.4
Patients diagnosed with RA should start treatment with disease-modifying antirheumatic drugs (DMARDs) as early as possible to improve patient outcomes.6,7 As a newer class of treatment for RA, biologic DMARDs can decrease disease activity in a relatively short time by targeting specific steps in the inflammatory processes.8 The use of biologics in RA management has become a global trend. In the United States, the proportion of RA patients treated with biologics has increased from 32.5% in 2001–2003 to 50.7% in 2010–2012.9 The utilization of biologics in China might be lower than that in developed countries. Another study conducted by the CREDIT showed that the utilization rate of biologics in RA patients was 8.3% in 2017 in mainland China.10 However, with more and more biologics listed in the national reimbursement drug list and biosimilars approved, an increasing proportion of patients was observed to treat RA with biologics in recent years.11
Many biologics are protein molecules and could be easily broken down by the digestive system, and therefore administration is usually via injection.12,13 Compared with intravenous administration, subcutaneous administration has proven safe, effective, well-tolerated, generally preferred by both patients and healthcare providers.14,15 To reduce the impact of administration on patients and healthcare givers, biologics are usually delivered via subcutaneous self-injection, which provides several user-friendly benefits for RA patients including reduced frequency of outpatient visits, reduced costs, reduced caregivers’ burden, increased adherence rate, and increased flexibility.12,16,17 For the current situation in China, the injection devices are sold together with the biologics as a whole package. Several self-injection devices are available on the market, including prefilled syringe (PFS) and auto-injection pen (AI), etc.12 Biologics with different injection devices do not differ in prices. PFS is the most commonly used self-injection device but is associated with many challenges. Needle phobia, discomfort, fear and anxiety are often reported by patients using PFS to self-administrate biologics.12,17,18 Therefore, optimizing features of injection devices to meet patient needs is necessary to improve disease management and increase treatment adherence. AI has become a new kind of injection device with many advantages such as hidden needle, ease of grip, increased comfort and portability.18,19 However, it remains unclear which feature matters most for RA patients and how much patients are willing to pay for these enhanced features.
Discrete choice experiment (DCE) is a tool to study individual stated preferences, it can value the relative importance of various factors that may influence the choices and provide the willingness-to-pay (WTP) of these factors. DCEs are widely used to elicit patient preferences for the management of a variety of healthcare conditions in health technology assessment and patient preference studies. Study subjects will face a series of choice sets with two or more options consisting of multiple attributes and different levels of attributes that can affect the utility of the option.20,21
No previous studies were identified to report the preferences and WTP for biologic self-injection devices in China. The objective of this study is to understand and quantify patients’ preferences and elicit their WTP for self-injection devices in RA. By collecting data on RA patients from multicenter settings in China and conducting a DCE analysis, it could help us better understand the acceptability of self-injection, optimize the development of injection devices, and provide quantitative reference for healthcare providers and decision-makers.
Methods
Study Population
A multicenter survey was designed to explore RA patients’ preference in mainland China. Considering the accessibility of biologics with self-injection devices, this study focused on the first- and second-tier cities in China. To cover a wide geographical area, patients were recruited from tertiary hospitals in six cities, including Shanghai, Guangzhou, Chengdu, Beijing, Xi’an and Wuhan, to make sure at least one city was included to represent the eastern, southern, southwestern, northern, northwestern and central regions. Physicians from the rheumatology department of targeted hospitals were invited to assist in the patient recruitment. Physicians helped check whether patients met the recruitment criteria and then asked the willingness to participate in this survey for patients who met the criteria. For patients who met the recruitment criteria and were willing to participate in the study, physicians would forward their contact information to the interviewers to conduct the interview. The patient recruitment criteria were as follows: 1) patients were required to be over 18 years old with a clinical diagnosis of RA; 2) patients were receiving biologics for RA treatment; 3) patients had the condition of self-injection out of hospitals (ie, had some motor function in their hands to perform the injection themselves, or had caregivers who can help with the injection).
The rule of thumb to determine the minimum sample size in DCEs proposed by Johnson and Orme suggested that the minimum sample size can be calculated using the equation N>(500 × c)/(t × a), where c is the maximum number of attribute levels, t refers to the number of choice tasks per respondent and a is the number of alternatives within each task.22,23 Therefore, the minimum sample size N for statistical analyses can be estimated as (500 × 5)/(14 × 2) = 89. Pearmain et al suggested that sample sizes over 100 are capable of providing a basis for analyses.24 Taking into account the sample sizes used in earlier studies25 and the fact that some patients may not be able to complete the survey, we targeted to recruit 120 patients in our study.
DCE Questionnaire Development
As the first step of this study, we identified the attributes of self-injection devices and the levels of those attributes that might be important to RA patients. The attributes describing self-injection devices were developed based on the differences in existing injection devices and the review of published literature.19,26–30 Potential attributes included ease of use, safety, indicators, needle visibility, practicality, out-of-pocket costs. The out-of-pocket cost indicated the cost of the injection device per injection. After in-depth interviews with clinical experts, key attributes with different levels were identified (Table 1).
 |
Table 1 Summary of Attributes and Levels |
The combination of seven selected attributes and levels resulted in 320 hypothetical scenarios (six attributes with two levels and one attribute with five levels). To reduce patients’ cognitive burden, we used D-efficiency fractional factorial design to generate a manageable length of the questionnaire. It reduced the number of choice scenarios to a practical number of 14 representative combinations using SAS Statistics (version 9.4).
The final questionnaire mainly included three parts. The first part was the screening questions, which was designed to check if the patient met the recruitment criteria. The second part aimed to collect demographic characteristics (eg, gender, education level, and household income) and patients’ treatment experience (eg, time since diagnosis, past and current treatment). The third part included explanation of attributes and levels, qualitative importance ranking of seven attributes and DCE questions (see Figure 1 for a DCE survey example). At the end of the questionnaire, we asked patients about WTP for the AI (with all enhanced attributes) relative to the PFS by an open-ended question.
 |
Figure 1 DCE survey example. |
We conducted pilot interviews with two patients to test the acceptability, comprehensibility and validity of the survey instrument. Meanwhile, to ensure the quality of the data, a consistency test was included in the DCE questions. The fifteenth choice set was duplicated as the third choice set. Patients were considered to have failed the consistency test if they did not choose the same alternative, and their results were excluded from the analysis.
Data Collection
The survey was conducted between August 15, 2021 and November 31, 2021. Data were collected through one-on-one face-to-face interviews using the native language of the interviewees (in Chinese). Before the survey, interviewers would give all patients detailed introduction of the study purpose and the main contents of questionnaire. All patients must be clear that they were faced with selections of a series of virtual products, with no right or wrong between alternatives, and they could choose a preferred product according to their own preference. All interviewers were required not to interfere with the selection of interviewed patients.
All patients were informed to have right to refuse to participate and signed an informed consent form. The study received ethic approval from the Fudan University School of Public Health Institutional Review Board (IRB#2021-08-0914, August 12, 2021–December 31, 2021).
After finishing the patient survey, we also did a telephone revisit to verify if all patients met the inclusion criteria and the authenticity of the questionnaire results.
Data Analyses
Before conducting data analyses, we assessed the reliability of the data by checking if any patients had inconsistent answers in the third and fifteenth choice sets, or if they had always selected the same option (eg, always injector A), as indicators of non-understanding or performing the task without due attention. These unqualified data were excluded from the analysis.
Descriptive analyses were conducted to summarize the socio-demographic characteristics of the included patients. Continuous variables were summarized using mean and standard deviation (SD). Categorical variables were summarized using frequencies and percentages.
Regarding the DCE data, the attribute of out-of-pocket costs was coded for linearity, and other attributes were all coded as dummy variables. Mixed logit model was used to estimate the relative preferences of the attributes as it can take unobserved preference heterogeneity among patients into consideration by estimating a distribution for each preference parameter. The coefficients from the mixed logit model can be interpreted as relative preference weights. Each preference weight indicated the relative strength of preference for the attribute level compared with the reference level, and a higher preference weight corresponded to a more preferred level of an attribute. Then, the relative importance score was calculated based on preference weights:
The larger the score of an attribute, the greater the impact perceived with a change on the attribute’s level.
The results from the model were also used to calculate the marginal WTP for each attribute level, where patients’ valuation for obtaining an enhanced feature of the injector. Then, we calculated the value of specific combinations of enhanced attributes in a self-injection device (ie, AI) compared to the standard PFS by the compensating variation (CV).31 Finally, we simulated the selection probability changes of AI and PFS under different addition out-of-pocket costs of AI. The selection probability was calculated based on the estimated coefficients and actual combinations of nonmonetary attributes of two self-injection device. All analyses were analyzed using STATA statistical software (version 15 SE).
Results
Patient Characteristics
A total of 120 patients met the inclusion criteria and completed the survey. As six of them failed the consistency check within the DCE section, data from 114 patients were included in the final analysis. Among the 114 patients, the majority were female (74.6%). The mean age of the patients was 44.6 years old, which is consistent with the mean age at symptom onset reported in previous studies (42.2–46.2 years).32,33 The RA disease duration was less than 4 years for nearly two-thirds of patients. The most frequently used biologics was Recombinant Human TNF- α Receptor II: IgG Fc Fusion Protein, followed by adalimumab. Less than 10% of the patients were using AI for injection. More demographics and baseline characteristics of the included patients are summarized in Table 2.
 |
Table 2 Demographics and Baseline Characteristics of the Analyzed Patients |
Importance Rating
Before conducting the DCE exercises, patients were asked to rank attributes directly from the most to the least important. Based on the importance rating results, in summary, patients attached the greatest importance to injection pain, followed by operation steps and needle protection. The feedback indication and size were identified as less important. The results of importance rating are presented in Figure 2.
 |
Figure 2 Importance rating of attributes. |
DCE Preference Analysis
Table 3 shows the results of the main effects mixed logit model. Overall, the coefficients were significant for all seven attributes, which meant all these attributes had significant impact on patients’ choice of self-injection devices. More specifically, patients presented a positive preference towards the improved level over the reference level in injection pain, operation steps, size, needle visibility, needle protection and feedback indication. As the reference level of these 6 attributes represented features of PFS and AI had enhanced level of each nonmonetary attribute (very slight pain, auto-injection method, thick size, hidden needle, with needle protection and multiple feedback indications), the results could be further interpreted as patients’ greater preference of AI over PFS on these attributes. The negative coefficient of the out-of-pocket costs indicated that a cheaper injection device would be preferred by patients.
 |
Table 3 Main Effects Mixed Logit Model Results (n = 114) |
All estimated standard deviations were significant, indicating the existence of preference heterogeneity. We further investigated whether age, monthly family income, reimbursement status and injection experience contributed to preference heterogeneity. Considering all subgroups did not meet the minimum sample size requirement, the results by different subgroups may have some deviations. Detailed results for subgroup analysis were provided in Supplementary materials (Tables S1–S5).
The results of the relative importance score of nonmonetary attributes are illustrated in Figure 3. Patients placed the greatest preference on very slight injection pain, followed by auto-injection method, thicker size, hidden needle, with needle protection and multiple feedback indication.
 |
Figure 3 Relative importance score. |
Willingness-to-Pay Analysis
Patients’ marginal WTP for the improvement on each attribute was elicited by stated preference discrete choice analysis. Our analysis found that patients were willing to pay the most to improve the slight pain to very slight pain, which was estimated as 45.26 CNY (95% CI: CNY 32.01–58.51). Patients’ marginal WTP for the improved level of each attribute is listed in Table 4. The wide ranges of confidence interval showed that there were considerable differences in WTP, reflecting the existence of preference heterogeneity.
 |
Table 4 Patients’ Marginal WTP for Each Attribute Level |
When all attributes were considered together, the analysis found that patients were willing to pay CNY 100.3 additionally for AI with all enhanced attributes. While the WTP of AI was obtained via an open-ended question, it was CNY 61.1.
Selection Probability
Figure 4 shows the change in the selection probability of patients for the two self-injection devices when the additional out-of-pocket cost of AI was changed. When the out-of-pocket costs of both AI and PFS were CNY 0, the probability of selecting AI is 99%, while the probability of selecting PFS is 1%. When the additional out-of-pocket costs of AI was increased to CNY 100.3, the probability of selecting AI and PFS was the same (both 50%).
 |
Figure 4 Selection probability of two self-injection devices under different additional costs of AI. Abbreviations: CNY, Chinese Yuan; PFS, prefilled syringe; AI, auto-injection pen. |
Discussion
This study employed the DCE method to estimate RA patients’ preferences for different attributes of self-injection devices. Individual data from 114 patients were analyzed to explore how different features (operation steps, injection pain, feedback indication, needle visibility, needle protection, size, out-of-pocket costs) influence their choice of injection device. The results suggested that RA patients had strong and positive preference towards all the enhanced attributes of AI compared with PFS. Injection pain mattered most to patients and had the highest marginal WTP. To our knowledge, this is the first study to evaluate patient preferences and WTPs for self-injection device in RA patients in China. Our study provided an understanding of RA patients’ preferences and their WTP for the main attributes considered in auto-injectors and could inform clinical decision-making in China.
The findings of this study are consistent with several previous studies19,26–30 in patients with rheumatic diseases about their preferred attributes in self-injection devices. For instance, Boeri et al found that injection speed control and injection reminders were preferred features for RA patients when selecting self-injection devices.26 Shingler et al conducted a DCE and the results revealed that reducing the discomfort associated with device use, the addition of feedback indications, and improved needlestick injury prevention were highly valued features for patients.34 Scalone et al employed a DCE in Italian patients with rheumatic diseases. The results showed that patients and rheumatologists preferred subcutaneous self-injection with AI, and the reduced frequency of reactions at the site of drug administration was the most important consideration.29 Another study conducted by Rho et al in Poland enrolled RA patients who received at least one self-injection both via the PFS and the AI. They found that the AI was more preferred considering its characteristics in less pain, convenience, ease of use and safety.30
In addition to the DCE analysis, an open-ended question to ask the WTP of AI compared with PFS was also included in the questionnaire, which aimed to help estimate the differences in the monetary values of AI between the analyzed result from DCE and the open-ended question. The WTP of AI calculated in DCE was higher than that obtained via an open-ended question, which is consistent with several previous studies.35,36 Ryan et al found that the WTP value for chlamydia screening elicited using DCE approach was higher than the value obtained via an open-ended method (payment card).35 Marjor et al tested a convergent validity by comparing the WTP estimates between the DCE and an open-ended method. The results showed that the mean WTP produced by the open-ended question was substantially lower than that derived from the DCE.36 The reason could be that the DCE survey may be more likely to elicit patients’ implicit preferences and the WTP estimates were closely related to the levels that are chosen for the cost attributes.37 Besides, PFS and AI are sold with biologics as whole packages in China. For the same kind of biologics, there is no difference in patients’ out-of-pocket costs no matter using PFS or AI. The WTP of AI derived from the open-ended method might be correspondingly lower.
There are several strengths of this study. First, all attributes and levels applied in this DCE were collected from the literature review and then further validated in pilot physician interviews. To avoid misunderstandings and lack of comprehension for patients which may lead to unclear interpretations of the data, we also conducted pilot patient interviews. The results of the pilot interviews indicated that this DCE survey was easily understood by study subjects. Second, to ensure the authenticity and accuracy of patients’ answers, we reviewed each patient’s record and conducted follow-up validation with them via phone call. Besides, a consistency test was performed on two choice sets, which could help ensure the reliability of our study results. Third, we not only included DCE survey but also asked patients to rank the attributes directly. We found that the results of importance rating were directly relatively consistent with DCE result. Both results indicated patients mattered more on injection pain and injection steps.
Some limitations of this study should be noted. One limitation is that the sample size of patients might be small, which could in turn pose a challenge when conducting a subgroup analysis. Although the current sample size met the minimum sample size required for DCE statistical analyses according to the thumb rule, further researches with larger sample sizes may be helpful to reflect patients’ preference in specified subgroups.25 Second, considering biologics were currently more common in tertiary hospitals in urban areas, our patients were mainly recruited from tertiary hospitals in the first- and second-tier cities in China, where the economy is more developed than the national average. Additionally, the proportion of patients with junior college and above education in our study is much higher than the national average (76% vs 17%).38 The well-educated population with good economic level might be more likely to accept new technologies. Therefore, the results of this study may not comprehensively reflect the patient preferences in rural areas or low-income regions and may limit the generalizability of the results. With the increased use of biologics across the country, the preferences of patients in other regions can be further explored. Finally, most patients in this study had considerable experience treating with PFS, while only limited patients had experience or knowledge of AI. Our study combined pictures with texts when presenting DCE questionnaires to help improve patients’ understanding about AIs, but it may still be difficult for them to understand the differences in different attribute levels, especially the pain sensation.
Conclusion
The results indicated that all six enhanced attributes would significantly influence RA patients’ preference on self-injection devices, and patients were willing to pay CNY 100.3 additionally for the improved attributes of AI compared with PFS. The findings of this study provided useful evidence to understand patients’ preference on injection devices, which may further help promote patient-centered care in autoimmune diseases, improve treatment adherence and even drive better treatment outcomes.
Ethics Statement
Ethical approval was approved by the Medical Ethics Committee of the School of Public Health, Fudan University (No. IRB2021080914). All of the procedures in the study complied with the Declaration of Helsinki.
Acknowledgments
We are grateful to Xinran Zhao and Min Jin who are employees of IQVIA, Real World Solutions, China for their assistance in questionnaire design, data collection and analysis. We would like to thank all the patients who participated in our study.
Author Contributions
Yan Wei and Jin Zhao should be regarded as co-first authors. Xuewu Zhang and Yingyao Chen are regarded as co-correspondence.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was funded by Becton Dickinson Medical Devices Co. Ltd.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chinese Rheumatology Association. 2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis. Chin J Intern Med. 2018;57(4):242–251. doi:10.3760/cma.j.issn.0578-1426.2018.04.004
2. Baek HJ, Lim MJ, Park W, et al. Efficacy and safety of tocilizumab in Korean patients with active rheumatoid arthritis. Korean J Intern Med. 2019;34(4):917–931. doi:10.3904/kjim.2017.159
3. Wasserman AM. Diagnosis and management of rheumatoid arthritis. Am Fam Physician. 2011;84(11):1245–1252.
4. Tian X, Li M, Zeng X. The current status and challenges in the diagnosis and treatment of rheumatoid arthritis in China: an annual report of 2019. Rheumatol Immunol Res. 2021;2(1):49–56. doi:10.2478/rir-2021-0008
5. Hresko A, Lin TC, Solomon DH. Medical care costs associated with rheumatoid arthritis in the US: a systematic literature review and meta-analysis. Arthritis Care Res. 2018;70(10):1431–1438. doi:10.1002/acr.23512
6. Hyrich KL. Patients with suspected rheumatoid arthritis should be referred early to rheumatology. BMJ. 2008;336(7637):215–216. doi:10.1136/bmj.39381.597454.AE
7. Ragab OM, Zayed HS, Abdelaleem EA, Girgis AE. Effect of early treatment with disease-modifying anti-rheumatic drugs and treatment adherence on disease outcome in rheumatoid arthritis patients. Egypt Rheumatol. 2017;39(2):69–74. doi:10.1016/j.ejr.2016.11.004
8. Atzinger C, Guo B. Biologic disease-modifying antirheumatic drugs in a national, privately insured population: utilization, expenditures, and price trends. Am Health Drug Benefits. 2017;10(1):27–36.
9. Strand V, Greenberg JD, Griffith J, et al. Impact of treatment with biologic agents on the use of mechanical devices among rheumatoid arthritis patients in a large US patient registry. Arthritis Care Res. 2016;68(7):914–921. doi:10.1002/acr.22784
10. Jin S, Li M, Fang Y, et al. Chinese Registry of rheumatoid arthritis (CREDIT): II. prevalence and risk factors of major comorbidities in Chinese patients with rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):251. doi:10.1186/s13075-017-1457-z
11. Wen Y, Hu Z, Xie B, et al. The trend of targeted therapies in Chinese patients with ankylosing spondylitis: results from a real-life survey. Front Pharmacol. 2021;12:763707. doi:10.3389/fphar.2021.763707
12. van den Bemt BJF, Gettings L, Domańska B, et al. A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience. Drug Deliv. 2019;26(1):384–392. doi:10.1080/10717544.2019.1587043
13. Schiff M, Saunderson S, Mountian I, Hartley P. Chronic disease and self-injection: ethnographic investigations into the patient experience during treatment. Rheumatol Ther. 2017;4(2):445–463. doi:10.1007/s40744-017-0080-4
14. Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018;32(5):425–440. doi:10.1007/s40259-018-0295-0
15. Pivot X, Gligorov J, Müller V, et al. Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol. 2014;25(10):1979–1987. doi:10.1093/annonc/mdu364
16. Arthur A, Klinkhoff B. Safety of self-injection of gold and methotrexate. J Rheumatol. 1999;26(2):302–305.
17. Keininger D, Coteur G. Assessment of self-injection experience in patients with rheumatoid arthritis: psychometric validation of the Self-Injection Assessment Questionnaire (SIAQ). Health Qual Life Outcomes. 2011;9:2. doi:10.1186/1477-7525-9-2
18. Vermeire S, D’Heygere F, Nakad A, et al. Preference for a prefilled syringe or an auto-injection device for delivering golimumab in patients with moderate-to-severe ulcerative colitis: a randomized crossover study. Patient Prefer Adherence. 2018;12:1193–1202. doi:10.2147/PPA.S154181
19. Rekaya N, Vicik SM, Hulesch BA-O, McDonald LL. Enhancement of an auto-injector device for self-administration of etanercept in patients with rheumatoid arthritis confers emotional and functional benefits. Rheumatol Ther. 2020;7(3):537–552. doi:10.1007/s40744-020-00216-5
20. World Health Organization. How to conduct a discrete choice experiment for health workforce recruitment and retention in remote and rural areas: a user guide with case studies. World Health Organization; 2012.
21. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–677. doi:10.2165/00019053-200826080-00004
22. Johnson R, Orme B. Getting the Most from CBC. Sequim: Sawtooth Software Research Paper Series, Sawtooth Software; 2003.
23. Orme B. Sample Size Issues for Conjoint Analysis Studies. Sawtooth Software Research Paper Series Squim. WA, USA: Sawthooth Software Inc; 1998.
24. Pearmain D, Swanson J, Kroes E, Bradley M. Stated Preference Techniques: A Guide to Practice.
25. de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8(5):373–384. doi:10.1007/s40271-015-0118-z
26. Boeri M, Szegvari B, Hauber B, et al. From drug-delivery device to disease management tool: a study of preferences for enhanced features in next-generation self-injection devices. Patient Prefer Adherence. 2019;13:1093–1110. doi:10.2147/PPA.S203775
27. Tischer B, Mehl A. Patients’ and nurses’ preferences for autoinjectors for rheumatoid arthritis: results of a European survey. Patient Prefer Adherence. 2018;12:1413–1424. doi:10.2147/PPA.S169339
28. Kishimoto MA-O, Yamairi FA-O, Sato NA-O, et al. Patient preference for treatment mode of biologics in rheumatoid arthritis: a 2020 web-based survey in Japan. Rheumatol Ther. 2021;8(3):1095–1111. doi:10.1007/s40744-021-00325-9
29. Scalone L, Sarzi-Puttini P, Sinigaglia L, et al. Patients’, physicians’, nurses’, and pharmacists’ preferences on the characteristics of biologic agents used in the treatment of rheumatic diseases. Patient Prefer Adherence. 2018;12:2153–2168. doi:10.2147/PPA.S168458
30. Rho YH, Rychlewska-Hańczewska A, Śliwowska B, Kim TH. Usability of prefilled syringe and autoinjector for SB4 (An Etanercept Biosimilar) in patients with rheumatoid arthritis. Adv Ther. 2019;36(9):2287–2295. doi:10.1007/s12325-019-01027-z
31. Hanemann WM. Welfare evaluations in contingent valuation experiments with discrete responses. Am J Agric Econ. 1984;66(3):332–341. doi:10.2307/1240800
32. Yang H, Bian S, Chen H, et al. Clinical characteristics and risk factors for overlapping rheumatoid arthritis and Sjögren’s syndrome. Sci Rep. 2018;8(1):6180. doi:10.1038/s41598-018-24279-1
33. Yu C, Li M, Duan X, et al. Chinese registry of rheumatoid arthritis (CREDIT): i. Introduction and prevalence of remission in Chinese patients with rheumatoid arthritis. Clin Exp Rheumatol. 2018;36(5):836–840.
34. Shingler SL, Swinburn P, Ali S, Perard R, Lloyd AJ. A discrete choice experiment to determine patient preferences for injection devices in multiple sclerosis. J Med Econ. 2013;16(8):1036–1042. doi:10.3111/13696998.2013.811079
35. Ryan M, Watson V. Comparing welfare estimates from payment card contingent valuation and discrete choice experiments. Health Econ. 2009;18(4):389–401. doi:10.1002/hec.1364
36. van der PM, Shiell A, Au F, Johnston D, Tough S. Convergent validity between a discrete choice experiment and a direct, open-ended method: comparison of preferred attribute levels and willingness to pay estimates. Soc Sci Med. 2008;67(12):2043–2050. doi:10.1016/j.socscimed.2008.09.058
37. Ratcliffe J. The use of conjoint analysis to elicit willingness-to-pay values. Proceed with caution? Int J Technol Assess Health Care. 2000;16(1):270–275. doi:10.1017/s0266462300161227
38. Office of the Leading Group of the State Council for the Seventh National Population Census. Major Figures on 2020 Population Census of China. Press CS; 2021.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.