Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Patient Perceptions of Switching to a Generic Dry Powder Inhaler – Increased Understanding Through Journey Mapping
Authors Ray SE , Boudewyns V, Davis C, Tzeng JP, Srivastava I, Oguntimein O, Conti DS, Feibus KB
Received 16 February 2022
Accepted for publication 23 June 2022
Published 6 August 2022 Volume 2022:17 Pages 1751—1768
DOI https://doi.org/10.2147/COPD.S362696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Min Zhang
Sarah E Ray,1 Vanessa Boudewyns,1 Christine Davis,1 Janice P Tzeng,1 Ila Srivastava,2 Oluwamurewa Oguntimein,3 Denise S Conti,4 Karen B Feibus2
1Center for Communication Science, RTI International, Research Triangle Park, NC, USA; 2Office of Research and Standards, Office of Generic Drugs, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, MD, USA; 3Office of Medication Error Prevention and Risk Management, Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, MD, USA; 4Office of Safety and Clinical Evaluation, Office of Generic Drugs, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, MD, USA
Correspondence: Sarah E Ray, Center for Communication Science, RTI International, Research Triangle Park, NC, USA, Tel +1 770-407-4934, Email [email protected]
Purpose: This qualitative study explored patients’ attitudes about and perceptions of generic dry powder inhaler (DPI) substitution for the brand product and patients’ views of generic product quality, efficacy, design, and usability.
Methods: Forty COPD and asthma patients (36 adults, four adolescents), who were actively using a brand DPI product, participated in one of six focus groups. Participants completed a journey mapping exercise to assess attitudes and opinions about a scenario where they refill their prescription and unexpectedly receive a generic DPI instead of their brand DPI. The focus groups were audio recorded, transcribed, and analyzed thematically.
Results: The hypothetical scenario of unexpectedly receiving a generic DPI elicited mixed feelings including: happiness and relief about potential cost savings, confusion, disappointment, anger, and/or frustration with the unexpected switch. Participants in most groups anticipated anxiety or hesitation in using the generic DPI due to concerns about potential differences in usability, uncertainty about correct use, and questions about efficacy. Participants across all groups said they would ask a pharmacist or healthcare provider for information or answers to their questions, and some participants said they would use online resources. When participants held the brand and generic DPI devices, most preferred the brand DPI device and found it easier, less cumbersome, or more convenient to use (due to size and weight). However, many participants reiterated that the potential reduced cost of the generic DPI would be a primary factor in their decision-making related to generic DPI substitution for their brand DPI.
Conclusion: Patients experienced a mixture of positive and negative feelings when faced with an unexpected generic DPI substitution. Some patients have doubts about their ability to successfully navigate differences in generic device design, and most expressed the desire to participate in discussions and decision-making with their HCP about generic DPI sameness and substitution.
Keywords: COPD, asthma, medical devices, generic drugs, drug-device combination products, focus groups
Corrigendum for this paper has been published.
Introduction
Misconceptions and perceptions about generic drugs and their substitution for name brand (brand) drugs remain inconsistent among American consumers and patients despite extensive usage and savings attributed to generic drugs.1–5 (The terms “name brand” or “brand” are often applied to the innovator drug. A generic drug is “the same as” a particular innovator drug in defined ways. See Table 1. However, some generic drugs also have brand names. Wixela Inhub is the brand name for the first generic for Advair Diskus (the innovator product). Both Advair Diskus and Wixela Inhub are part of this study. For this article, the term “brand” refers to the innovator drug). Surveys conducted between 1996 and 2011 found that patients perceived that generic drugs are less effective than brand drugs, not the “real thing”, not as safe, and (iv) of low quality because they are less expensive.6–9 Studies also identified differences in patient perceptions of generic drugs based on certain demographics such as ethnicity, education, income, insurance coverage, and health literacy.7,9–13 More recent studies found an increase in positive patient perceptions about generic drugs.5,14 For example, in a national survey conducted in 2014, over 90% of US patients were comfortable asking their physicians to prescribe generic drugs. In addition, 87% of patients considered generic drugs to be as effective and safe as brand drugs and 84% of patients reported that their doctors should “always” or “usually” prescribe generic drugs when available.14
 |
Table 1 Approved Brand Drug Products and Their Generics |
Chronic obstructive pulmonary disease (COPD) and asthma and affect 15.7 million and 25 million Americans, respectively.15,16 Effective management of asthma and COPD often involves treatment with drug-device combination products. Drug-device combination products are products that consist of a drug constituent part and a device constituent part in which the drug constituent part operates as the primary mode of action. (For this article, “drug-device combination products” are referred to as “combination products.”). Although positive patient perceptions of generic drugs have increased over time, there have been limited studies conducted to assess patients’ perceptions of generic drug-device combination products, such as dry powder inhalers (DPIs) prescribed for asthma and COPD, and attitudes about DPI generic substitution. A few studies conducted in Europe found that asthma patients had negative perceptions of switching from brand to generic DPIs.17,18 In one study, four out of the five patients interviewed reported that they would be confused, worried, or unhappy if they received a generic DPI, and they would contact their doctor or pharmacist to receive training on how to use the generic device. Another survey indicated that about half (51%) of participants with asthma opposed substitution of their brand DPI with a generic DPI.17 These studies did not investigate participants’ attitudes about and perceptions of differences in design and usability features between the brand and generic devices, so the root cause for these negative perceptions is unclear. Prior research has found that patient satisfaction with inhaler devices and preferences for device characteristics tend to be consistent across indications, and attitudes toward switching to a generic DPI device should extend to both patient groups.19–21
This qualitative, enhanced focus group study was conducted in the United States among patients with COPD or asthma to advance the scientific understanding of patients’ attitudes about and perceptions of generic DPI substitution. The study design focused on learning how differences in design and usability features impact patients’ views of product quality, efficacy, and device usability.
Materials and Methods
Participants
To optimize our understanding of the impact of switching from a brand DPI to a generic DPI for all indicated user populations, we recruited groups of adult and adolescent patients experienced with different kinds of brand DPIs to ensure that all perspectives were heard. The focus was on representing DPI users and obtaining demographic diversity; as such, we did not have any set quotas for the number of COPD or asthmatic individuals and the final distribution reflects natural fallout of medical conditions. Our planned number of focus groups was informed by priori research and experience related to theme saturation (eg, research has found that two to three focus groups will capture at least 80% of themes on a topic and three to six will capture 90%).22 Because adult DPI users were more likely to have a range of medical conditions and experiences with a variety of devices than adolescent users, we held multiple groups for adults to capture that mixture of perspectives. The goal was to recruit two adolescent groups and five adult groups, however the final adolescent group scheduled for March 2020 had to be canceled due to COVID shutdowns. To understand how experiences and perceptions changed with different life stages/age groups, the study used a segmented recruitment strategy to include separate focus groups for adults ages 18 years and older (5 groups; n=36 participants) and adolescents ages 12 to 17 years (1 group; n=4 participants). A professional recruitment facility identified eligible participants who were English-speaking adults and adolescents who had experience using Advair® Diskus® (fluticasone propionate and salmeterol xinafoate inhalation powder [DPI]) or other brand DPIs (eg, fluticasone furoate and vilanterol, tiotropium bromide, salbutamol sulfate).
Participants were recruited, first by prioritizing the market research firm’s existing consumer research panels; which include self-identified information on health conditions and concerns, then through community outreach with partners, including healthcare provider offices, and paid recruitment advertisements that were placed on social media (eg, Facebook ads) or through social media groups with relevant interests. Adolescents obtained permission from a parent or guardian before being recruited to participate. Individuals who worked in the healthcare, marketing, advertising, or pharmaceutical industries, and individuals who worked for the Department of Health and Human Services were excluded from the study because of their potentially relevant knowledge and experience that might not reflect that of average consumers. To minimize the potential for trained responses or social desirability bias, individuals who participated in an interview or a focus group about prescription or over-the-counter (OTC) drugs or their medical condition during the previous three months were also excluded from the study.
Overall Study Design
To achieve geographic diversity, we collected data across six focus groups in two cities located in different parts of the United States (Minneapolis, MN, and Atlanta, GA). RTI International’s Institutional Review Board approved the study, and the study was submitted under “generic clearance” to the Office of Management and Budget (OMB) for review and approval before the start of any data collection (OMB Control number: 201712–0910-001). All participants signed the informed consent form (adults) or the assent form (adolescents; parent/guardian signed the informed consent form). Consent forms describe the study and participant protections, including a guarantee that individual participants will not be identified in any published or presented materials. The study complies with all relevant aspects of the Declaration of Helsinki. To keep groups a manageable size, each focus group included up to 10 participants. Groups consisted of either adolescent DPI users or adult DPI users who were actively using the brand Advair Diskus (fluticasone propionate and salmeterol DPI) or actively using a different brand DPI. Within each group, the recruitment strategy targeted a distribution of participants across age groups, sex, and education level. Focus groups took place in professional recruitment facilities during October 2019 and February 2020. One experienced moderator and one notetaker attended in person, and other team members observed remotely via livestreaming. The same experienced moderator led each of the focus group discussions, which lasted approximately 90 minutes and were audio recorded and transcribed. Participants received $125 reimbursement for their time.
Focus Group Sessions
Before the start of each focus group session, participants completed a brief pre-group questionnaire that asked broadly about their experience with their current brand DPI. Once the focus group session started, the moderator facilitated the flow of the discussion using a semi-structured interview guide that included probes to extract more in-depth information (eg, “Tell me more about that.” “Can you share an example?”). At the start of the focus group session, the moderator focused on generating discussion among participants about their overall perceptions of generic drugs to better understand participants’ overall feedback about (and reactions to) generic drugs before introducing the scenario of switching to a generic combination DPI.
Next, the moderator presented a journey mapping exercise to focus group participants23 with three “touchpoints” (steps) to project participants’ attitudes and opinions about generic devices. This exercise presented a potential real-life journey of salience for each participant—receiving a generic DPI for the first time instead of their current brand DPI. Traditionally, the journey mapping process has been used in market research to understand consumer perceptions and decision-making.24,25 More recently, this technique has been adapted for use in other contexts, including efforts to better understand how patients navigate complex systems, how patients make health-related decisions, and how to improve patient experiences.26,27 The moderator presented a simplified case study where the hypothetical “journey” described a multistep scenario in which a brand DPI was unexpectedly replaced with a generic DPI (Step 1: ordering a refill for their prescription brand DPI; Step 2: picking up the brand DPI and finding out it had been switched to a generic DPI; and Step 3: using the generic DPI for the first time). At each step, participants explored their perceptions and barriers of switching to a generic DPI by describing their thoughts, feelings, and other reactions. The moderator asked participants about the questions they would ask, the challenges they would face, how they would get information, and the actions they would take. Beginning in Step 2 of the journey, participants were able to physically hold and manipulate both a brand DPI (Advair Diskus) and a generic DPI (Wixela Inhub). This hands-on comparison facilitated the discussion about potential questions and barriers (see Figure 1 for a photo of devices used). The focus group sessions concluded with a discussion summarizing the journey process that allowed participants to provide final thoughts.
Data Analysis
Focus group audio recordings were used to generate verbatim transcripts of each session, and investigators compiled notes from each session. A coding scheme was developed based on the objectives of the study, the moderator’s guide questions, and session notes. NVivo qualitative data analysis software (QSR International, Version 12) was used as a data management tool to aid the analysis. Grounded theory in qualitative analysis guided data coding and analysis, which were conducted in phases.28 Coding via thematic analysis29 was conducted by two RTI team members, using the focus group session (rather than individuals) as the unit of analysis.30,31 Categories were iteratively refined to reflect distinct themes and then broken down into concrete, specific subcategories. After completing training on the codebook and applying the codes in NVivo software, half of the transcripts (n=3) were selected for double-coding by the two RTI team members to establish inter-rater reliability (assessed using Cohen’s Kappa). A third RTI team member, who was not present during the focus groups sessions, examined the double-coding to assess inter-rater reliability, identify discrepancies, and facilitate discussion with coders to resolve the discrepancies. After inter-rater reliability was established with the first three transcripts, the remaining transcripts were coded independently by one of the two RTI team members. The two RTI team members met regularly with the third RTI team member and the RTI project director to review the coded transcripts, address questions, and resolve discrepancies.
Results
Participant Demographics
Forty participants (four adolescents, 36 adults) took part in the six focus group sessions. The number of participants in each focus group session ranged from four to 10. Table 2 shows the distribution of participants, segmented by adolescent and adult DPI users, along with participant characteristics. When asked when they were first diagnosed and prescribed a DPI to treat their condition, adult DPI users reported a range between less than one and 70 years prior to the focus groups (1950 to 2019). Adolescent DPI users reported being diagnosed between less than one and 12 years prior to the focus groups, (2008 to 2019), with an average indicating approximately 6 years of use.
 |
Table 2 Demographics of Participants in the Focus Groups Sessions (n=40) |
Based on responses from the pre-group questionnaire, the majority of adolescent and adult DPI users were either somewhat or very satisfied with their current brand DPI and had received training before using it (Table 3). Of the 18 adults who received training when prescribed their first inhaler device, half (9, 50%) received training from a doctor, one-third from a nurse (6, 33%), and the remainder from a pharmacist (3, 17%). Almost all (16, 89%) reported that they were either “somewhat” or “very” satisfied with the training. Both of the adolescents who received training were trained by a nurse and indicated that they were somewhat or very satisfied with the training.
 |
Table 3 Experience with Current DPI (n=30) |
General Perceptions of Generic Drugs
The first part of each focus group assessed participants’ overarching understanding and perceptions of generic drugs. Participants in the adolescent group were not familiar with the term “generic” as it relates to generic products, so a brief introduction was provided to orient them to the concept before asking about specific drugs. All groups with adult participants were familiar with the term and were asked directly about their perspectives on and experiences with generic drugs. Participants in all groups mentioned their perceptions of (a) the financial impact or the overall lower cost of generic drugs when compared with brand drugs, (b) the role that health insurance has in determining whether brand or generic medications are given to patients, and (c) how insurance coverage affects cost and availability. Participants across groups also compared generic and brand medications; some participants noted similarities (eg, ingredients, quality, efficacy) or described aspects of generic drugs that they questioned or perceived as inferior to the brand medications (eg, quality, efficacy) (Table 4).
 |
Table 4 Most Frequent Responses to Thoughts About Generic Drugs |
Journey Mapping Step 1: Needing a Refill for a DPI Medication
In Step 1 of the journey mapping exercise, participants described their thoughts when they reached the point of needing a refill for their prescription DPI medication (assuming that they did not need to return to a provider’s office to obtain the prescription refill). This step was used to frame the topic for participants and to set them up for the remaining journey mapping steps.
Journey Mapping Step 2: Receiving a Generic DPI
In Step 2, participants were asked to imagine the following situation: they receive their prescription DPI refill directly from the pharmacist, and the DPI product is a generic version instead of the brand DPI that they typically receive. Below we report the participants’ anticipated feelings or other reactions, questions, and ways they said they would obtain additional information.
Reactions
Table 5 summarizes the emotional reactions and thoughts from participants along with sample quotes illustrating the theme. Reactions to unexpectedly receiving a generic DPI were mixed; participants across groups described both positive and negative thoughts and/or feelings. At least one participant in each of the groups described an anticipated negative reaction, most often related to general confusion about why they received a different DPI product than expected and what this meant for them as patients. In half of the groups, participants expressed disappointment, anger, or frustration with being switched to a generic. In a third of the groups, participants expressed frustration with not being consulted about the product change, and in one group, they expressed distrust or skepticism toward people or organizations (such as their insurance companies) that played a role in the change. In two-thirds of the groups, participants described feelings of doubt or anxiety, particularly related to their ability to use the delivery device effectively.
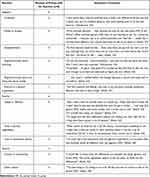 |
Table 5 Journey Mapping Step 2: Unexpected Receipt of a Generic DPI—Reactions and Emotions |
However, across all groups, participants also expressed anticipated positive feelings at this step. Most frequently, participants indicated that they would be happy or relieved particularly about the potential for a less expensive medicine option and the opportunity to have a choice for their DPI medication. In some groups, participants noted that they trusted their providers’ recommendations, assuming that their provider prescribed or recommended use of the generic version of the DPI product. Other positive emotions included being generally hopeful and excited about trying something new.
Less often, participants in some groups anticipated more neutral reactions to the potential switch to a generic DPI, including feelings associated with questions they would have about how the delivery device is different and curiosity about how to use it.
Questions, Anticipated Challenges, and Information Sources
In Step 2, participants discussed questions prompted by hands-on review of the devices, and they described anticipated challenges in making the switch from the brand DPI to the generic DPI. Questions and anticipated challenges focused on usability, efficacy, and cost.
Across all groups, participants talked about differences in the usability of the devices. Discussion topics ranged from how easy or difficult the generic device would be to operate to questions about the nuances of using it. For participants in most groups, questions related to device usability represented the biggest challenge, and participants generally noted that they would need additional information to ensure that they were operating the generic device correctly and that the medication was dispensed effectively:
At first, looking at the generic one thought … okay, so you just keep it level like this, like then inhale it … or do you have to … turn vertical, or is there a more effective way? Because, it’s unlike [the brand DPI] that has one single orifice where it comes out … Where [the generic DPI] has two, so it’s like, is it more effective or less effective? [Adult, G3]
It’s like getting used to a new gadget so to speak … [I’d wonder] whether the way to use it would be different. [Adult, G4]
In nearly all groups, when discussing potential questions they might have if they unexpectedly received the generic DPI, participants broadly questioned whether the efficacy of the brand and generic products would be different (without suggesting that one was better or worse). For example, one participant asked, “Does it work the same? Do I get the same results?” (Adult, G3). In addition, some participants wondered how long it had been on the market and how the side effects compared with the brand drug.
Consistent with the previous discussion about perceptions of generic drugs, participants frequently mentioned cost considerations. Most participants suggested that the cost of the generic DPI would be better or equal to the brand DPI:
What was the copay on this compared to my [the brand DPI]? [Adult, G3]
My copay is like $40, and then my generic is $5 … so that’s a significant difference for each [inhaler]. [Adult, G3]
Participants across all focus groups said that they would look for information or answers to their questions through a pharmacist or healthcare provider.
If I had a question, immediately I would just ask to have a consultation with the pharmacist … and, if I wasn’t satisfied or if I did not understand something, then I would consult with the physician. But first I would ask the pharmacist if I had any type of apprehension as far as how to use it. [Adult, G4]
Some of these participants also noted that the pharmacist would be likely to proactively notify them of the change or consult with their prescriber before filling or delivering the prescription.
I think initially, the pharmacist, then if they weren’t able to answer the questions, I probably honestly would go online, and then after that, I’d go to the doctor. [Adult, G3]
I’d probably look online. I know that the drug companies, they have a legal obligation to talk about [some things], but I still like getting it from a third-party source … [Adult, G4]
Other resources mentioned by a few participants included information distributed with the prescription drug product (eg, the patient package insert) and the insurance company.
Journey Mapping Step 3: Using the Generic DPI
In Step 3, participants were asked to envision the next step in the journey. In this hypothetical scenario, they picked up their prescription for the generic DPI device, and it was time to use it for the first time.
Reactions
Table 6 summarizes the emotional reactions and thoughts discussed and includes sample quotes from the discussions. As with the Step 2 discussion, reactions were mixed with participants describing both positive and negative feelings. Participants in most focus group sessions described an anticipated negative reaction. Most frequently, these negative reactions were related to anxiety or hesitation about using the generic DPI. Participants expressed frustration about potential usability differences and their ability to use the generic product correctly, as well as concerns about the efficacy of the generic product. Some participants also said they anticipated being skeptical about the generic DPI itself (eg, its reliability). Other anticipated negative feelings included disappointment with the generic DPI’s usability.
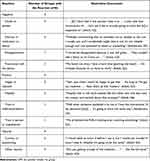 |
Table 6 Journey Mapping Step 3: Using a Generic DPI—Reactions and Emotions |
Participants in most groups also expressed anticipated positive feelings. These feelings ranged from feeling happy or relieved that they had their medication (whether it was the generic or brand DPI) to feeling hopeful that the generic DPI would work well (in some cases, this reaction was also related to potential financial benefits provided by generic products). Other positive emotions included trusting organizations with oversight (eg, the Food and Drug Administration [FDA]) that would ensure safety and effectiveness.
Some participants also expressed more neutral reactions associated with using the generic DPI. These included feelings associated with questions or curiosity about how it would feel to use it and what differences they may experience compared with the brand DPI. Other feelings and thoughts included wondering if they were using the generic DPI correctly.
Questions, Anticipated Challenges, and Information Sources
In Step 3, participants continued to discuss their observations related to the differences between the DPI products in the context of potentially using the generic DPI for the first time. As in Step 2, the focus of much of the discussion across all groups was on the usability of the devices, including questions about how to hold it (eg, vertically or horizontally), the number of steps, and the coordination or strength needed to operate it successfully:
Yeah, when you open a [the brand DPI] dispenser, you know to put it in your mouth and take your dose. It’s easy, just visual. You can see how they take it. [With the generic DPI], you got to figure out how do I open it, and what do I do … you got to put your finger here, you got to hold it and do that, so it’s maybe like a puzzle. [Adult, G6]
Yeah, the functionality of it … because when you’re pulling it, it’s like then you’re also you’re like closing it at the same time … I get frustrated like really easy when I’m taking my medicine … you know [this] would drive me nuts. [Adult, G2]
In Step 3, the discussion of efficacy also echoed previous conversations. Participants wondered how well the generic DPI would work and whether they would notice a difference from their experience with the brand DPI. For participants who felt that the efficacy of the generic DPI would be better or equal to the brand DPI, the financial implications of the switch were raised again in the Step 3 discussions. As one participant noted,
I’d be happy just paying less for the same thing [Adult, G6]
However, others indicated that they still had questions, and some participants reported that they would take steps to ensure that there were no unexpected differences in the medication (ie, the drug inside the DPI device):
More so than normal, I’ll just pay real close attention to just make sure how it’s working and … just make mental notes of what’s going on while I’m on it, just so I can report if anything’s different. [Adult, G6]
Just because it’s a different medication you don’t really know. Usually generics work, but still, you’re putting something new in your body. [Adult, G3]
When discussing how they would react at the point of taking the medication, some participants said that they would also consider potential safety and side effects (during this discussion, the moderator clarified that although the medication delivery device would change, the medication inside [drug constituent] would not):
Is it really the same? I would compare. I’d take the two of them and read the labels and all. [Adult, G1]
Are there any different side effects that I should look for? [Adult, G3]
To get answers about how to use the brand and generic DPIs during Step 3, most participants said that they would review the patient package insert or talk to pharmacists to get training or a description of differences. Some participants said they would turn to online sources, including videos, or rely on their own (or others’) experiences using the brand name device:
I guess I would just ask the pharmacist for a look on the box about the ingredients … [Adult, G3]
Yeah, I would read the directions … I appreciate the pictures; it shows you exactly what to do … [Adult, G2]
The instructions also, and if I had any other questions, I would ask my mom or my brother. They both use inhalers too. [Adolescent, G5]
I would go to the manufacturer’s website or video tutorial. [Adult, G4]
Reactions to Differences in User Interface
Participants were able to hold and manipulate the actual brand and generic DPI devices (emptied of any medication) beginning in Step 2. Most participants described differences between the look or feel of the user interfaces. Some participants noted aspects of the generic DPI devices that they preferred or that they thought would be better or equal to the brand DPI device:
I like the generic one because it covers the mouthpiece up, keeps it from getting dirty. [Adult, G6]
[The generic] feels better in my hand … looks like it’s made of heavier plastic. [Adult, G1]
Well, I like how [with] the generic, this little counter is five times the size of the one that’s on [the brand DPI]. [For] older people, it’s [the branded DPI] harder to see. [Adult, G3]
In contrast, in most focus group sessions, participants also suggested that there were aspects of the look and feel of the generic DPI’s user interface that they felt were inferior to the brand DPI’s user interface. These participants primarily focused on the size and weight of the generic DPI device, which could affect portability and convenience:
And then it’s bigger and that would be a lot less convenient to fit in my purse or in my pocket if I needed to. [Adolescent, G5]
[The generic DPI] is a lot heavier too. [Adult, G2]
The [brand DPI] is very lightweight, so if you’re driving and you’re trying to … administer a dosage, [the generic DPI] is weighty. So, it makes me kind of wonder, “’Am I going to be able to do this and drive at the same time?’ [Adult, G6]
Other participants commented on design elements, including lack of a sliding mouthpiece cover on the generic DPI device. Participants in some groups also reported that the quality of the generic DPI device seemed inferior:
The thing I don’t like about it … if you’re not holding it, [the powder will] just fall out. [Adolescent, G5]
[the generic DPI] feels more fragile to me. [Adult, G3]
I think [the brand DPI] has a more comfortable mouthpiece … it looks thicker, like more mouth shaped versus [the generic DPI]. [Adult, G1]
Facilitators and Barriers
After completing the journey mapping exercise, participants were asked to think about the process as a whole and identify potential facilitators and barriers to switching from the brand DPI to a generic DPI. This part of the focus group was frequently framed as a comparison of the two types of devices and discussion on the aspects of each they preferred.
Facilitators
The potential cost savings were highlighted again as facilitating the switch from a known brand DPI to a new generic DPI. Many participants reiterated that the potential reduced cost of the generic DPI was a benefit that they would consider as a primary factor in their decision-making.
If it was truly significantly more affordable, I think there’d be some relief for many people. [Adult, G3]
Barriers
Consistent with the journey mapping discussion, questions about usability were most frequently discussed as a potential barrier. Participants in most focus group sessions preferred the more familiar brand DPI device (a few participants acknowledged the differences but thought that the generic DPI device was not more difficult, or it was not a meaningful difference).
Actually administrating the ingredients, the use of it is totally different to me with that cap [on the generic device] the way it is, it’s very cumbersome. I think it would break fast. [Adult, G4]
With [the generic DPI], from the get-go, many of us were saying, “wait which way” [is it held]? But with [the brand DPI], you don’t have those questions, and then again … you know with [the brand DPI] there’s a click and it’s like, “yep, got it”. [Adult, G2]
The [brand DPI] seems a little bit more convenient … easier to use. [Adolescent, G5]
Some participants framed their concerns about unknown efficacy as a potential barrier to generic DPI adoption, and, in some cases, these concerns also related to concerns about usability.
One of the things we notice here is that some of us were going to use it the wrong way, without turning it correctly to use it. So the effectiveness of the delivery of the medication might be different if you took it the wrong way. [Adult, G6]
I was just nervous about using something different. [Adolescent, G5]
Discussion
We conducted a qualitative study of six focus groups to explore patients’ attitudes about and perceptions of generic DPI substitutions, including how differences between design and usability features of the generic DPI versus the brand DPI affect their views of product quality, efficacy, and device usability. Overall, we found that patients viewed generic drugs as more affordable and cost-effective than brand drugs, in part, because they were seen as more likely to be covered by insurance. They generally suggested that generic medications were similar to their brand counterparts, with the same active ingredients, although perceptions of efficacy, safety, and quality were mixed. Some participants also noted that generic drugs are more available than brand drugs, even if they are less well known, because they are not widely advertised to consumers.
Patient perceptions of generic DPIs seemed to be based on mental models,32,33 built on their experiences with other generic medications (both prescription and over the counter) and past experience with off-brand consumer products.2,14,34 Adolescent patients had limited experience ordering and refilling their medications, and they were unfamiliar with the term “generic”, although after an introduction and orientation using other consumer products (eg, cereal, soap) as examples and a reference point, their perceptions were similar to adult participants’ views.
We found that patients are interested in the potential cost savings associated with switching to generic DPIs but may have questions about the usability, efficacy, and their own ability to use the generic DPI correctly. Building on recent studies that found patients are comfortable asking their provider to prescribe generic medications,14 our study found that some patients would reach out to their healthcare provider or pharmacist to learn more about a generic DPI’s usability, efficacy, and quality. Although some participants suggested that they would question or research the efficacy of the medication’s quality, participants were not highly concerned that there would be issues with the generic device. Our study expanded on previous research by providing participants with the opportunity to see and physically manipulate sample generic (Wixela Inhub) and brand (Advair Diskus) DPI devices during the focus group session. This exercise prompted discussions about perceived differences between the brand and generic DPI devices. Most participants commented on the look or feel of the user interface and discussed aspects of the generic DPI’s interface that they felt were inferior (eg, size and weight). Often, these aspects tended to relate to convenience (ie, fitting the device in a purse) and ease of use (ie, fewer steps to perform) more so than proper use. However, participants showed some hesitation in their ability to properly use the generic device, particularly for the first time. Challenges related to usability were key throughout the discussion, including questions about how to hold the device (eg, vertically or horizontally), the number of steps, and the coordination or strength needed to operate it successfully. Nonetheless, in many cases, participants seemed willing to make the change to the generic DPI, particularly if they thought there would be a financial benefit.
The focus of this study was on patients’ initial reactions when presented with a generic DPI instead of their brand DPI. Most participants noted that they would look for additional information to learn more about the generic device or seek training and guidance (eg, from pharmacists, online sources). Although we asked some basic questions about previous training from healthcare professionals, additional details about the type, content, and depth of training were outside the scope of this study but may be an area for future research. During the generic drug application review process, FDA compares the device design and use process for the proposed generic combination product and its brand product to ensure that a patient or caregiver will understand how to use the generic product without additional training when generic substitution occurs. When a difference exists that affects steps involved with drug administration, FDA requests additional data to show that the difference will not increase the risk for mistakes when using the generic combination product administer the correct drug dose.35
In line with previous studies,17,18 participants expressed some negative perceptions of switching from brand DPIs to generic DPIs, but much of that seemed to stem from not being asked or informed about the switch first. One key finding was the need to include participants in the decision-making process, particularly when it came to whether they had an option to choose the brand versus a generic DPI product. Participants appeared to like the idea that they might have a choice between the brand DPI and generic DPI, although they generally understood that the choices may be limited based on what their prescriber recommended or what their health insurance would cover. The range of anticipated negative reactions (ie, confusion, anger, disappointment, frustration) to the scenario of unexpectedly receiving a generic DPI demonstrated that patients do not want to be surprised with a switch to the generic DPI, although some acknowledged they had experienced a switch to other generic medications without prior notification or approval.
Entry of generic versions of drug products into the market is associated with a decrease in cost, and this can result in significant reduction of financial burden to patients and enhance treatment adherence. Advair Diskus (fluticasone propionate and salmeterol xinafoate) inhalation powder has been one of the most prescribed drugs of any class and is used for treatment of both COPD and asthma. When Wixela Inhub (fluticasone propionate and salmeterol xinafoate) Inhalation Powder, the first approved generic for Advair Diskus,36 entered the market in 2019, the cost savings over the first year reached 941 million dollars with an average 66% reduction in unit cost ($115 for Wixela Inhub vs $334 for Advair Diskus).37 Generic product cost savings generally are not fully realized until three or four generic versions of a drug-device combination product enter the market.38 The cost savings associated with generic substitution are appealing to patients but do not eliminate concerns that may arise when a patient unexpectedly receives a generic inhaler that has user interface design differences compared to the brand inhaler.
Like all research, our study had limitations. First, the focus group sessions consisted mostly of adult patients. Therefore, the findings presented in this article may not adequately reflect the views of adolescent patients and are limited in being able to distinguish real comparisons between adolescent and adult patients. For clarity, we highlighted emerging themes mentioned in the adolescent patient group only. Further, this is a qualitative study conducted with a self-selected sample, which limits the generalizability of findings. As such, the results of this study may not represent all patients with asthma or COPD. The sample was predominately made up of asthmatic individuals, and no formal comparisons between asthmatic and COPD subjects were made. The focus of the study was on patients’ experience with the drug devices and not their perception of or experience with their conditions. Though there are important differences between these two conditions, their similarities, including previously reported preferences for and challenges with using DPIs in both populations39–41 indicate that studying them together may be beneficial in providing insights that are relevant for both groups. In addition, the in-depth nature of these qualitative data provides a rich understanding of real-world experiences among asthma and COPD patients who use inhalation medications, including DPIs to manage their respiratory health. Future research may consider differences between these populations. Second, although coders underwent training and double-coded three focus group transcripts to establish inter-rater reliability, differences between individuals could result in differences in coding. To minimize differences in coding, the RTI team met regularly to review the coded transcripts together, address questions, and resolve discrepancies.
Conclusion
This is one of few published studies where patients with COPD or asthma, who actively use brand DPIs, explored and shared their perceptions about being switched to a generic DPI. Patients are interested in cost savings offered with use of generic DPIs but want to be informed by their healthcare provider or pharmacist about changes to their medicines (including generic substitution) before they occur. They want to understand how switching to a generic version of their medicine will affect them and their health condition and want to play a role in the decision-making process.
Participants expressed uncertainties about how generic versions of a drug product differ from the name brand product, which caused anticipatory anxiety and frustration. These uncertainties contributed to participant concerns about generic DPI usability and efficacy and about their ability to use the generic DPI correctly. Outcomes from these focus groups revealed opportunities for FDA, healthcare professional organizations, and patient advocacy organizations to improve generic drug literacy among adults, adolescents, and healthcare providers. In addition, our results support the need for ongoing research by FDA and other research organizations to expand scientific understanding of how differences between name brand and generic DPI devices affect patients’ abilities to navigate a switch to a generic version of their inhaler medication and use it correctly without additional training or instruction.
Abbreviations
COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; FDA, Food and Drug Administration; US, United States.
Disclosure
The authors report no conflicts of interest in this work. Views expressed in this article are from the authors and do not necessarily reflect the official policies of the Department of Health and Human Services, the National Institutes of Health, and the FDA, nor does any mention of trade names, commercial practices, or organization imply endorsement by the US Government.
References
1. Brennan TA, Lee TH. Allergic to generics. Ann Intern Med. 2004;141(2):126–130. doi:10.7326/0003-4819-141-2-200407200-00011
2. Shrank WH, Cox ER, Fischer MA, Mehta J, Choudhry NK. Patients’ perceptions of generic medications. Health Aff. 2009;28(2):546–556. doi:10.1377/hlthaff.28.2.546
3. Maly J, Dosedel M, Kubena A, Vlcek J. Analysis of pharmacists’ opinions, attitudes and experiences with generic drugs and generic substitution in the Czech Republic. Acta Pol Pharm. 2013;70(5):923–931.
4. Roman B. Patients’ attitudes towards generic substitution of oral atypical antipsychotics: a questionnaire-based survey in a hypothetical pharmacy setting. CNS Drugs. 2009;23(8):693–701. doi:10.2165/00023210-200923080-00006
5. Dunne SS, Dunne CP. What do people really think of generic medicines? A systematic review and critical appraisal of literature on stakeholder perceptions of generic drugs. BMC Med. 2015;13(1):173. doi:10.1186/s12916-015-0415-3
6. Ganther JM, Kreling DH. Consumer perceptions of risk and required cost savings for generic prescription drugs. J Am Pharm Assoc. 2000;40(3):378–383. doi:10.1016/S1086-5802(16)31086-5
7. Losifescu A, Halm EA, McGinn T, Siu AL, Federman AD. Beliefs about generic drugs among elderly adults in hospital-based primary care practices. Patient Educ Couns. 2008;73(2):377–383. doi:10.1016/j.pec.2008.07.012
8. Keenum AJ, Devoe JE, Chisolm DJ, Wallace LS. Generic medications for you, but brand-name medications for me. Res Social Adm Pharm. 2012;8(6):574–578. doi:10.1016/j.sapharm.2011.12.004
9. Sewell K, Andreae S, Luke E, Safford MM. Perceptions of and barriers to use of generic medications in a rural African American population, Alabama, 2011. Prev Chronic Dis. 2012;9:E142. doi:10.5888/pcd9.120010
10. Piette J. Cost-related medication underuse. A window into patients’ medication related concerns. Diabetes Spectr. 2009;22(2):77–80. doi:10.2337/diaspect.22.2.77
11. Figueiras MJ, Alves NC, Marcelino D, Cortes MA, Weinman J, Horne R. Assessing lay beliefs about generic medicines: development of the generic medicines scale. Psychol Health Med. 2009;14(3):311–321. doi:10.1080/13548500802613043
12. Omojasola A, Hernandez M, Sansgiry S, Jones L. Perceptions of generic prescription drugs and utilization of generic drug discount programs. Ethn Dis. 2012;22(4):479–485.
13. Gaither CA, Kirking DM, Ascione FJ, Welage LS. Consumers’ views on generic medications. J Am Pharm Assoc. 2001;41:729–736. doi:10.1016/S1086-5802(16)31308-0
14. Kesselheim AS, Gagne JJ, Franklin JM, et al. Variations in patients’ perceptions and use of generic drugs: results of a national survey. J Gen Intern Med. 2016;31(6):609–614. doi:10.1007/s11606-016-3612-7
15. Wheaton AG, Cunningham TJ, Ford ES, Croft JB. Employment and activity limitations among adults with chronic obstructive pulmonary disease—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(11):289.
16. Centers for Disease Control and Prevention. Most recent national asthma-data 2018; 2021. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm.
17. Booker R. Do patients think that dry powder inhalers can be used interchangeably? Int J Clin Pract. 2005;59(s149):30–32. doi:10.1111/j.1368-504X.2005.00725.x
18. Doyle S, Lloyd A, Williams A, et al. What happens to patients who have their asthma device switched without their consent? Prim Care Respir J. 2010;19(2):131–139. doi:10.4104/pcrj.2010.00009
19. Ding B, Small M, Scheffel G, Holmgren U. Maintenance inhaler preference, attribute importance, and satisfaction in prescribing physicians and patients with asthma, COPD, or asthma–COPD overlap syndrome consulting for routine care. Int J Chron Obstruct Pulmon Dis. 2018;13:927. doi:10.2147/COPD.S154525
20. Kaplan A, van Boven JF. Switching inhalers: a practical approach to keep on UR RADAR. Pulm Ther. 2020;6(2):381–392. doi:10.1007/s41030-020-00133-6
21. Lavorini F, Braido F, Baiardini I, Blasi F, Canonica GW. Asthma and COPD: interchangeable use of inhalers. A document of Italian Society of Allergy, Asthma and Clinical Immmunology (SIAAIC) & Italian Society of Respiratory Medicine (SIMER). Pulm Pharmacol Ther. 2015;34:25–30. doi:10.1016/j.pupt.2015.07.005
22. Guest G, Namey E, McKenna K. How many focus groups are enough? Building an evidence base for nonprobability sample sizes. Field Methods. 2017;29(1):3–22. doi:10.1177/1525822X16639015
23. Parvanta C, Nelson DE, Harner RN. Public Health Communication: Critical Tools and Strategies. Burlington, MA: Jones & Bartlett Learning; 2017.
24. Følstad A, Kvale K. Customer journeys: a systematic literature review. J Serv Theory Pract. 2018;28(2):196–227. doi:10.1108/JSTP-11-2014-0261
25. Rosenbaum MS, Otalora ML, Ramírez GC. How to create a realistic customer journey map. Bus Horiz. 2017;60(1):143–150. doi:10.1016/j.bushor.2016.09.010
26. Bearnot B, Mitton JA. “You’re always jumping through hoops”: journey mapping the care experiences of individuals with opioid use disorder-associated endocarditis. J Addict Med. 2020;14(6):494–501. doi:10.1097/ADM.0000000000000648
27. Ridder EF, Dekkers T, Porsius JT, Kraan G, Melles M. The perioperative patient experience of hand and wrist surgical patients: an exploratory study using patient journey mapping. Patient Exp J. 2018;5(3):97–107. doi:10.35680/2372-0247.1273
28. Chun Tie Y, Birks M, Francis K. Grounded theory research: a design framework for novice researchers. SAGE Open Med. 2019;7:2050312118822927. doi:10.1177/2050312118822927
29. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi:10.1191/1478088706qp063oa
30. Morgan DL. Focus Groups as Qualitative Research.
31. Kidd PS, Parshall MB. Getting the focus and the group: enhancing analytical rigor in focus group research. Qual Health Res. 2000;10(3):293–308. doi:10.1177/104973200129118453
32. Jones NA, Ross T, Lynam PP, Leitch A. Mental models: an interdisciplinary synthesis of theory and methods. Ecol Soc. 2011;16:art46. doi:10.5751/ES-03802-160146
33. Johnson-Laird P. Mental Models. Cambridge, MA: Harvard University Press; 1983.
34. Colgan S, Faasse K, Martin LR, Stephens MH, Grey A, Petrie KJ. Perceptions of generic medication in the general population, doctors and pharmacists: a systematic review. BMJ Open. 2015;5(12):e008915. doi:10.1136/bmjopen-2015-008915
35. GUIDANCE D. Comparative analyses and related comparative use human factors studies for a drug-device combination product submitted in an ANDA. Center for Drug Evaluation and Research (CDER); 2017.
36. Donohue JF, Burgoyne DS, Ward JK, Allan R, Koltun A, Cooper A. Wixela inhub: a generic equivalent treatment option for patients with asthma or COPD. Pulm Ther. 2021;7(1):47–57. doi:10.1007/s41030-020-00142-5
37. Wang Z, Ahluwalia SK, Newman B, Dhapare S, Zhao L, Luke MC. Medication cost-savings and utilization of generic Inhaled Corticosteroid (ICS) and Long-Acting Beta-Agonist (LABA) drug products in the USA. Ther Innov Regul Sci. 2022;56(2):346–357. doi:10.1007/s43441-021-00372-y
38. Conrad R, Lutter R. Generic competition and drug prices: new evidence linking greater generic competition and lower generic drug prices; 2019.
39. Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102(4):593–604. doi:10.1016/j.rmed.2007.11.003
40. Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017;27(1):1–10. doi:10.1038/s41533-017-0016-z
41. Schulte M, Osseiran K, Betz R, et al. Handling of and preferences for available dry powder inhaler systems by patients with asthma and COPD. J Aerosol Med Pulm Drug Deliv. 2008;21(4):321–328. doi:10.1089/jamp.2007.0634
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

