Back to Journals » Patient Preference and Adherence » Volume 17
Patient and Physician Preferences for Acute Myeloid Leukemia Maintenance Treatments Following Hematopoietic Stem Cell Transplantation
Authors Saini L, Griffin JD, Pandya BJ, Shah MV, Zhou M, Yang H, Song Y , Marshall DA
Received 15 July 2023
Accepted for publication 14 October 2023
Published 6 November 2023 Volume 2023:17 Pages 2805—2819
DOI https://doi.org/10.2147/PPA.S421871
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Lalit Saini,1 James D Griffin,2 Bhavik J Pandya,3 Manasee V Shah,3 Mo Zhou,4 Hongbo Yang,4 Yan Song,4 Deborah A Marshall5
1London Health Sciences Centre, London, Ontario, Canada; 2Dana-Farber Cancer Institute, Boston, MA, USA; 3Astellas Pharma, Inc, Northbrook, IL, USA; 4Analysis Group, Boston, MA, USA; 5Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada
Correspondence: Bhavik J Pandya, Director, Oncology Health Economics and Clinical Outcomes Research, Astellas Pharma, Inc, 1 Astellas Way, Northbrook, IL, 60062, USA, Tel +1 847 226-0066, Email [email protected]
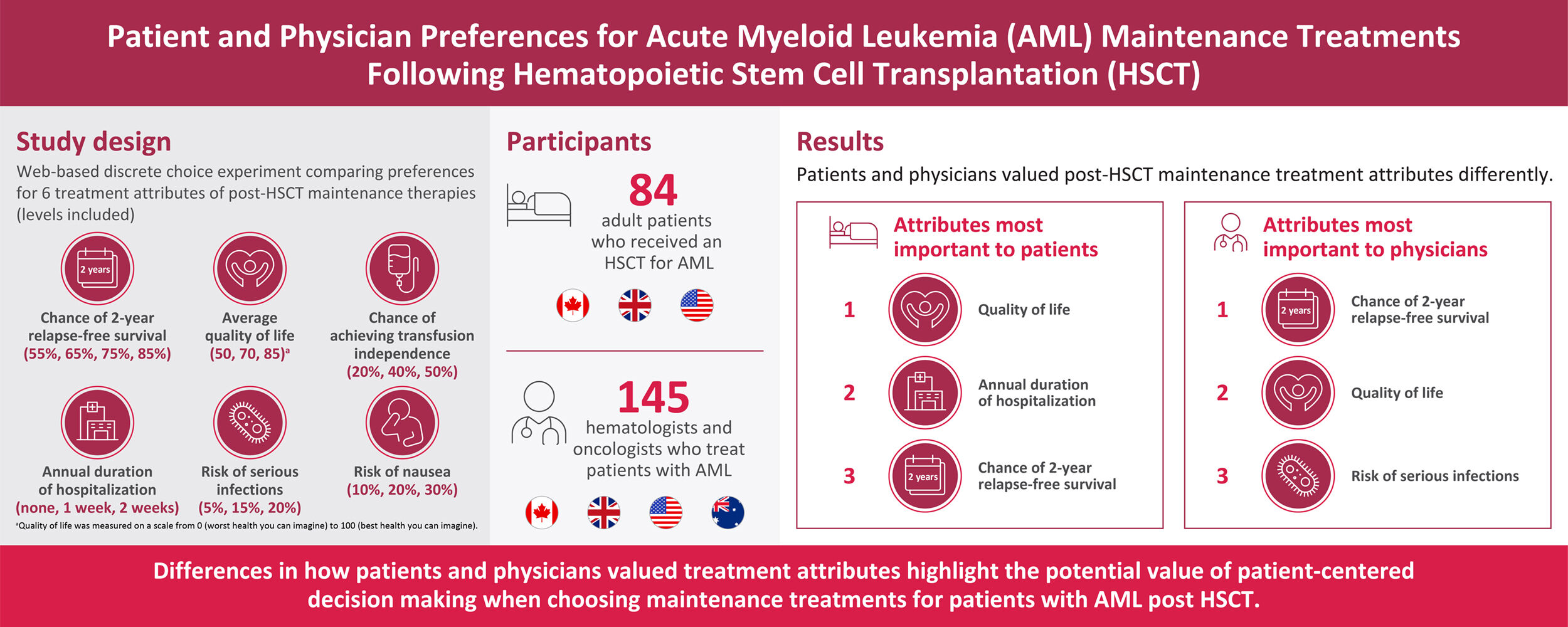
Purpose: This study assessed and compared preferences for treatment attributes of maintenance therapies post-hematopoietic stem cell transplantation (HSCT) in patients with acute myeloid leukemia (AML) and in physicians who treat these patients.
Patients and Methods: Patients with AML post HSCT and physicians from the United States, United Kingdom, Canada, and Australia (physicians only) completed a web-based discrete choice experiment (DCE). The DCE used inputs identified via a targeted literature review and qualitative interviews to ascertain relevant treatment attributes and associated levels. Six treatment attributes were selected (chance of 2-year relapse-free survival, quality of life [QoL], risk of serious infections, risk of nausea, chance of achieving transfusion independence, and duration of hospitalization annually), each with three or four levels. The experimental design included 36 choice tasks that presented a pair of hypothetical treatment profiles with varying attribute levels; participants chose a preferred treatment for each choice task. Choice tasks were divided into three blocks of 12 tasks each in the patient survey and 4 blocks of 9 tasks each in the physician survey; survey participants were randomly assigned to one of the blocks. Random parameter logit regression models were used to assess the impact of stated attributes on preferences for maintenance treatment post HSCT.
Results: Surveys from 84 patients and 149 physicians were assessed. For patients, QoL was the most important attribute, followed by duration of hospitalization and chance of 2-year relapse-free survival. For physicians, chance of 2-year relapse-free survival was the most important attribute, followed by QoL and risk of serious infections.
Conclusion: Differences in how patients and physicians valued post-HSCT maintenance treatment attributes were identified. These differences suggest that patient-centered decision-making may help physicians choose maintenance treatments for patients with AML post HSCT that better meet their treatment needs and improve their treatment satisfaction.
Plain Language Summary: Patients with acute myeloid leukemia (AML) are often treated with chemotherapy followed by stem cell transplantation (SCT). Following SCT, evidence suggests that some patients may benefit from maintenance therapy. In this study, researchers surveyed patients and physicians who treat patients with AML to understand what maintenance treatment characteristics they most valued following SCT. Characteristics evaluated during the survey were chance of survival without relapse, impact on quality of life, risk of serious infections, risk of nausea, chance of being free of needing blood transfusions, and impact on annual hospital length of stay. For patients, the most important maintenance treatment characteristics were better quality of life and fewer hospital days annually. For physicians, the most important maintenance treatment characteristic was a higher chance of survival without relapse. These results show differences in maintenance treatment preferences between patients and physicians, suggesting that patient-centered decision-making could be useful when selecting a maintenance treatment for AML after SCT that meets patients’ treatment needs and improves their treatment satisfaction.
Keywords: acute myeloid leukemia, hematopoietic stem cell transplantation, maintenance treatment, discrete choice experiment, quality of life, treatment preferences
Graphical Abstract:
Introduction
The treatment of patients with acute myeloid leukemia (AML) has changed considerably over the past decade from one focused on chemotherapy to one focused on individualized treatments.1,2 With this shift, treatment options now include standard chemotherapy regimens together with targeted therapies with greater emphasis on patient and disease characteristics.3,4
As of 2022, the current standard of care for fit patients with higher-risk AML includes induction chemotherapy followed by hematopoietic stem cell transplantation (HSCT).5 HSCT is a potentially curative option; however, patients with AML receiving HSCT after induction therapy are at risk of developing infections and acute or chronic graft-versus-host disease and may require blood transfusions and extended hospital stays.6,7 Although most patients with AML will obtain a complete remission with treatment, the risk of relapse varies according to age and genetic subtype and is seen in more than 60% of patients with higher-risk AML.1,4 For example, the presence of FMS-like tyrosine kinase 3 (FLT3) mutations, seen in around 30% of patients with AML, is associated with a poor prognosis.4
The discovery of oncogenes and targeted therapy has led to reevaluation of maintenance therapies for AML in the post-HSCT setting and a role for chemotherapies in some populations.8 Results from meta-analyses suggest that maintenance therapy with FLT3 inhibitors or hypomethylating agents improves outcomes compared with no maintenance therapy in AML post HSCT.9,10 For patients with FLT3-mutated AML, maintenance therapy with a FLT3 inhibitor post HSCT may be an option, with some study results suggesting these agents may have beneficial effects. Results from the SORMAIN trial11 showed a significant improvement in relapse-free survival with sorafenib compared with placebo when administered as maintenance therapy to patients with FLT3-internal tandem duplication–mutated AML post HSCT. Additionally, because FLT3 inhibitors are administered orally, these therapies have the potential to limit time spent in the hospital as patients can self-administer these therapies. Several ongoing trials are evaluating other FLT3 inhibitors such as gilteritinib (NCT02997202) and crenolanib (NCT02400255) as maintenance therapy post HSCT in patients with AML.12,13 As therapies are evaluated in the maintenance setting, it is important to understand the risks and benefits of available treatments and how these treatments meet patients’ needs.
Alongside treatment advancements, data suggest that patients who actively participate in their own care have improved outcomes and treatment satisfaction.14–16 A decrease in healthcare resource utilization was also reported when treatment aligned with patient preferences.17 Because patients and healthcare providers may value treatment attributes differently,18 a clear understanding of these values is needed to inform providers.
A discrete choice experiment (DCE) is a research method that can be used to understand preferences and trade-offs among a set of attributes that describe a treatment. A DCE allows estimation of the relative importance of different attributes and the trade-offs between these attributes of a product or service.19 In health, DCEs have been used to capture preferences for treatment attributes through hypothetical but realistic scenarios.20
With existing treatments and new drugs under investigation for maintenance therapy post HSCT, this study uses a DCE to gain a better understanding of what maintenance treatment attributes are most valued by patients and physicians.
Materials and Methods
Study Overview
This DCE was performed following good practices for preferences research, including qualitative research and experimental design.21–24 The identity of the human participants in this study cannot be directly ascertained, and the investigator did not contact or re-identify participants; therefore, the study received exemption from the Western Institutional Review Board. This study complies with the Declaration of Helsinki.
Study Design
Survey Development
The patient survey included questions to confirm eligibility, demographics, overall health status, disease characteristics, blood transfusion experience (including both blood cells and platelets), and DCE choice tasks to assess maintenance treatment preferences (Supplementary Table 1). The physician survey included questions to confirm eligibility, assess physician characteristics (eg, age, sex, geographic region, years of practice, and practice setting), and DCE choice tasks to assess maintenance treatment preferences. Although the online surveys did not allow participants to skip questions, the option to select “unknown/not sure” was available for some questions related to basic demographic information.
DCE Development
A targeted literature review was conducted to identify an initial list of AML maintenance treatment attributes along with their associated levels to include in the DCE. The targeted review was performed to identify studies evaluating sorafenib and midostaurin in patients with AML post HSCT and studies evaluating patient preferences and satisfaction with AML treatments. Searches were performed through PubMed and Google. To inform the DCE design, preliminary one-on-one phone interviews with six eligible patients and four eligible physicians were conducted to assess the initial list of attributes and their associated levels and to elicit additional treatment attributes before designing the final survey.23,25,26
Additionally, the literature supported including five to seven attributes in a DCE.25–28 Given the complexity of some of the attributes (eg, efficacy), six attributes were selected; three or four levels were included for each attribute based on the importance ranking obtained during the interviews. The final list of attributes included the top factors selected by participants that could impact treatment selection. The levels selected captured the range observed for existing and novel treatment options (Table 1).
 |
Table 1 Maintenance Treatment Attributes and Levels Included in the DCEs |
Following selection of importance of each attribute, participants were presented with a series of choice tasks that presented a pair of hypothetical treatment profiles (Treatment A vs Treatment B) with varying attribute levels (Figure 1). Participants were asked to choose a preferred treatment for each choice task. Choice tasks did not include an opt-out option in order to encourage participants to make trade-offs and maximize collection of data from a relatively limited sample size. However, a follow-up question was included after each choice task about whether participants would take (patients) or prescribe (physicians) the selected treatment if it were available in real-world practice.
 |
Figure 1 Example choice task from DCEs. Abbreviations: AML, acute myeloid leukemia; DCE, discrete choice experiment. |
The experimental design used to construct the choice tasks utilized the D-optimal main effects criteria using SAS Version 9.4 (SAS Institute, Inc., Cary, NC).24 Given the number of attributes and levels, the minimal number of choice tasks that met the condition for the existence of an orthogonal array was 36. The experimental design with 36 choice tasks that did not include dominant pairs (ie, one option better than the other on all attributes) and had the largest relative D-efficiency was then selected. All combinations in the final selected experimental design were plausible. All correlations between attributes were less than 0.07.
In consideration of respondent burden, choice tasks were divided into three blocks containing 12 choice tasks each in the patient survey and four blocks containing nine choice tasks each in the physician survey. A respondent was randomly assigned to one of the blocks. The order of choice tasks was randomized to avoid a potential ordering effect.
Two validation choice tasks were added to each block for the purpose of data quality checking,29 which included:
- Dominant profile: In one choice task, the attribute levels in one treatment profile (dominant profile) were better than the attribute levels in the other treatment profile (dominated profile). Participants were expected to choose the dominant profile.
- Test-retest: A participant was asked to respond to two choice tasks that had the same treatment profiles but in a reversed order. Participants were expected to make the same choice.
Survey Data Collection
Pretest
To ensure data quality, the logic and clarity of the survey was pretested among two eligible participants each in the United States (US) and the United Kingdom and one eligible participant in Canada. During each pretest, a moderator went through the questionnaire with a participant and documented their questions and concerns regarding the questionnaire. The cognitive burden of the questionnaire was assessed to ensure the difficulty level of the questions and the appropriateness of the number of questions. Both patient and physician surveys were revised based on the issues identified during the pretests.
Both the physician and patient surveys took around 20–30 min to complete. Both patients and physicians received an honorarium for their time. Responses to the online surveys were collected in a secure database and were deidentified. Participants provided consent to participate in the study prior to beginning the survey and could voluntarily withdraw from survey participation at any time.
Participants
Patients participating in the DCE were from Canada, the United Kingdom, and the US and had a self-confirmed AML diagnosis, were aged ≥18 years, were able to speak and read English, and received a stem cell transplant for AML. Patients were excluded if they were FLT3-mutation-negative.
Physicians participating in the DCE were from Australia, Canada, the United Kingdom, and the US and included hematologists or oncologists who treated a minimum of five patients with AML and more than three with a FLT3 mutation in the past 12 months. Physician participants were required to be proficient in spoken and written English.
Patients and physicians were recruited separately for interviews and online surveys through Global Perspectives™. Global Perspectives™, an IQVIA business, specializes in recruiting hard-to-reach patients for health economics and outcome research and market access projects and has access to online panels of physicians and patients in Australia, Canada, the United Kingdom, and the US. Physicians were recruited from an existing physician panel, and patients were recruited using a combination of existing patient panels, physician referral, patient association outreach, recruiter proprietary databases, and social media and online forums outreach. Patient and physician eligibilities were confirmed by checking their responses to the screener questions at the beginning of the online survey against the inclusion criteria described previously. Patients and physicians who were eligible and completed the online surveys received honorarium as compensation for their time based on fair market value.
Statistical Analyses
Patient and physician characteristics were summarized descriptively. Continuous variables were summarized using means and standard deviations (SD), whereas categorical variables were summarized using counts and proportions.
EQ-5D index scores (ie, utility values) were calculated using the value set from each participant’s country of residence according to the scoring manual.30 Absenteeism, presenteeism, work productivity loss, and activity impairment associated with blood transfusion measured by the Work Productivity and Activity Impairment (WPAI) questionnaire were calculated according to the scoring manual.31
Random parameter logit regression models were used to analyze the impact of treatment attributes on physicians’ and patients’ maintenance treatment choices. Preference weights were estimated for the attribute levels. All attribute levels were coded as categorical variables because the preference weights between the most preferred level and least preferred level may not be linear. Dummy coding was used with the expected least preferred level in each attribute omitted. The preference weights for all attribute levels were assumed to be normally distributed among respondents. Confidence intervals for the mean preference weights from the random parameter logit models were reported. The models were fitted using 50 Halton draws in Stata 14.2. The preference weights from the conditional logit model fitted using the same data were used as starting values. The models were fitted with different seeds until the preference weights were stable across models. Five different seeds (0, 10, 100, 1000, and 5000) were selected, all of which led to the same preference weights.
Data analysis other than the random parameter logit models was conducted using SAS Version 9.4 (SAS Institute, Inc., Cary, NC). A validity check was performed including a dominant pair and repeated task for test-retest assessment, excluding participants who failed the validity test (ie, provided different answers in two identical choice tasks) to evaluate the robustness of the results.
Results
Data from 84 patients (Canada, n=3; United Kingdom, n=30; US, n=51) with AML post HSCT and 149 physicians (Australia, n=16; Canada, n=31; United Kingdom, n=52; US, n=50) who treat patients with AML were included in the analysis.
Patients
The mean age of participating patients was 62.4 years (Table 2). Most were White/Caucasian (51.9%), male (60.2%), and had at least completed secondary school (98.8%); 51.2% of patients were retired. Most patients (97.6%) had at least one comorbidity, with hypertension (14.3%), diabetes (8.3%), and depression (6.0%) being reported by more than 5% of patients.
 |
Table 2 Patient Baseline Demographics and Clinical Characteristics |
The mean time from AML diagnosis to survey participation was 34.0 months (Table 2); 61.9% of patients had relapsed or were refractory to past treatments. Most patients (76.2%) had self-reported a FLT3 mutation; 23.8% self-reported either not being tested for a FLT3 mutation or being unknown/not sure of their FLT3-mutation status. At the time of survey completion, the average (SD) EQ-5D index score of patients (ie, utility) was 0.7 (0.2).
In the first-line setting, most patients reported receiving 7+3 (78.6%; cytarabine plus daunorubicin, doxorubicin, idarubicin, or mitoxantrone). At survey completion, two-thirds of patients were receiving treatment, of which 7+3 was the most common. Most patients (71.4%) participating in the DCE reported that intravenous infusions impacted their quality of life (QoL).
During the preceding 12 months, 79.8% of patients were hospitalized, and of these patients, 67.2% were hospitalized for 30 days or less (Table 2). Over the past 3 months, 59.5% of patients self-reported receiving at least one blood transfusion. Most patients receiving blood transfusions within the previous 3 months reported that blood transfusion affected their QoL and impacted their regular activities. The majority of those in the US receiving transfusions reported spending more than $500 per month out of pocket (OOP) for blood transfusions.
Physicians
Most physicians participating in the survey were hematologists (81.2%) (Supplementary Table 2); 78.1% were male with a mean (SD) age of 50.1 (8.3) years. On average, physicians practiced medicine for 19.8 (7.4) years, ranging from 4.0 to 40.0 years. Physicians reported treating a median of 30 patients with AML and eight patients with FLT3-mutated AML yearly.
Preferences
For patients, the most important maintenance treatment attributes as evaluated by this DCE were QoL, duration of hospitalization, and chance of 2-year relapse-free survival, while chance of achieving transfusion independence, risk of serious infections, and risk of nausea of all grades were considered lesser priorities (Figure 2). The increase in patient utility (ie, preference weight) from a 2-week reduction in hospitalizations per year (from 2 weeks per year to no hospitalizations per year) was larger and, therefore, more than compensated for the decrease in patient utility seen with a 15 percentage point increase in serious infection risk from treatment (from 5% to 20%) or a 30 percentage point reduction in 2-year relapse-free survival (from 85% to 55%) when other attributes were held constant. Similarly, the increase in patient utility from an increase in the chance of achieving transfusion independence from 20% to 50% (a 30 percentage point increase) was larger in this DCE and, therefore, more than compensated for an additional 15 percentage point increase in risk of serious infections (from 5% to 20%) or an additional 20 percentage point increased risk of nausea (from 10% to 30%) when other attributes were held constant.
 |
Figure 2 Preference weights of patients post-HSCT AML maintenance treatment. Abbreviations: AML, acute myeloid leukemia; HSCT, hematopoietic stem cell transplantation. |
Among physicians, the most important maintenance treatment attribute was chance of 2-year relapse-free survival, followed by average QoL and risk of serious infections (Figure 3).
 |
Figure 3 Preference weights of physicians for patients post-HSCT AML maintenance treatment. Abbreviations: AML, acute myeloid leukemia; HSCT, hematopoietic stem cell transplantation. |
If other maintenance treatment attributes were kept the same, the benefit of a 2-week reduction in hospitalizations (from 2 weeks per year to no hospitalizations) and an increase in chance of achieving transfusion independence from 20% to 50% (ie, 30 percentage point increase) can compensate for an additional 5 percentage point increase in the risk of serious infections (from 15% to 20%) or an additional 10 percentage point increase in the risk of nausea (from 10% to 20% or from 20% to 30%) from the treatment.
When assessing the importance of each maintenance treatment attribute individually for patients, most rated the chance of 2-year relapse-free survival (86.9%) and average QoL (73.8%) as being very important (Table 3). Most physicians also rated the chance of 2-year overall survival (85.2%) and average QoL (57.0%) as being very important when assessing the importance of each maintenance treatment attribute individually. More than half of patients considered the duration of hospitalization (67.9%) and risk of serious infections (56.0%) as being very important. When asked about chance of achieving transfusion independence, 94.0% of patients rated chance of achieving transfusion independence as being somewhat or very important. Less than 50% of physicians rated the risk of serious infections (47.0%) and chance of achieving transfusion independence (38.3%) as being very important and less than 25% rated hospitalization duration (23.5%) as very important.
 |
Table 3 Patient and Physician Ratings of Attribute Importance of AML Maintenance Treatments Post HSCT |
Results from the data validity analysis found that of the 84 patients and 149 physicians participating, 13 patients (15.5%) and five physicians (3.4%) failed the dominant profile test in the DCE (ie, chose the unambiguously worst choice task). Whereas two patients (2.4%) and 28 physicians (18.8%) failed the test-retest, providing different answers for two identical choice tasks. However, in the sensitivity analysis, preference weights did not change significantly when excluding these participants.
Discussion
This study assessed and compared preferences for maintenance treatment in patients with AML post HSCT and physicians who treat these patients. Results showed that among the six attributes included in the survey, the attribute most valued by patients with AML post HSCT was QoL, and by physicians, 2-year relapse-free survival. Physicians’ strong consideration for 2-year relapse-free survival was understandable, as it is a key clinical outcome. Physicians valued QoL secondarily, followed by hospitalization duration. Although maintenance treatments that improve or maintain QoL post HSCT were valued most by patients, it is equally important to consider those maintenance therapies that are most safe, well tolerated, can be administered in an outpatient setting, and delay the need for blood transfusions, while still extending survival.
Patients who participated in this study had an average age of 62 years and had previously been fit enough to undergo HSCT for AML; only 53.6% of patients were retired or disabled. Therefore, maintenance therapies that reduce the need for hospitalizations, intravenous therapies, and blood transfusion, and improve QoL could best meet their treatment needs and ultimately lead to improved outcomes.
In the post-HSCT setting for AML, a clear understanding of the maintenance treatment attributes most valued by patients and physicians can help guide therapy decisions as the number of available treatment options increases. This is the first study to evaluate maintenance treatment preferences in patients with AML post HSCT and in physicians treating patients with AML post HSCT. It is also unique in evaluating QoL and humanistic outcomes.
One previously published DCE that evaluated treatment preferences in patients with AML was identified.32 The DCE included 294 patients with AML; attributes included were event-free survival, complete response, hospital length of stay, and short- and long-term side effects. The most meaningful attribute identified by patients was a 10% improvement in chance of complete response. Several differences are worth noting between the population that participated in the study by Richardson et al32 and this study’s population. Most notably, this study focuses on post-HSCT treatments, while the study by Richardson et al focused on any AML treatment. Additionally, the mean time from diagnosis to survey participation was 7 years in the study by Richardson et al compared with slightly more than 2 years in this study, with no information provided on hospitalizations within the previous year. Finally, most patients in the study by Richardson et al reported being in remission, whereas two-thirds of patients participating in this survey reported actively receiving treatment, with the most common treatment being 7+3. Treatment with 7+3 suggests that the patients participating in this DCE were potentially receiving treatment for relapsed disease. Since the population in this study was more recently treated by HSCT, the burden of that therapy and the impact of hospitalizations for treatment and supportive care may have influenced their results more so than the population in the study by Richardson et al, which had a much longer disease history. Interestingly, in the study by Richardson et al, preference estimates for complete remission were lower and those for time in the hospital were higher for the group of patients diagnosed with AML within the previous 2 years.
A previously published DCE that evaluated physician treatment preferences for patients with relapsed/refractory multiple myeloma reported that overall survival was the highest priority when making treatment decisions.33 However, that DCE included clinical and safety outcomes such as overall survival, overall response rate, progression-free survival, thrombocytopenia, and neutropenia, which may not be equivocal to the preferences included for evaluation in the current study. Similarly, in a DCE evaluating patient frontline treatment preferences for acute lymphoblastic leukemia, overall survival was the highest priority.34 Again, preferences evaluated were clinical and safety outcomes such as overall survival, duration of remission, major cardiovascular events, and myelosuppression.
As part of this analysis, attributes reflective of humanistic burden, such as the impact of transfusions, QoL, and OOP costs, were evaluated from the patient perspective and from the physician perspective. Transfusion independence was rated as important or very important by more than 90% of participating patients and 87% of participating physicians. Although few patients received transfusions, transfusions negatively impacted patient QoL and the ability to participate in daily activities. Monthly OOP transfusion costs were >$500 for most US–based patients, with 43.2% of patients reporting costs >$2000. Patient OOP transfusion costs in the US would be expected to vary based on insurance coverage. These transfusion costs are similar to results from a retrospective database analysis (IBM MarketScan®, 2012–2017) from the US for patients with AML who relapsed post HSCT where monthly mean transfusion costs of around $1800 were reported.35 In the post-HSCT population, transfusion burden may increase in the event of relapse or refractory disease; therefore, maintenance therapies that prolong relapse-free survival may ultimately delay the need for transfusions and therefore decrease patient transfusion burden.
Strengths and Limitations
Several study limitations should be noted, including the selection bias inherent to surveys. Patients who were able to respond to the online survey may differ from patients who were not able to due to multiple factors, such as internet access, level of well-being or severity of illness, time constraints, or other technological challenges. Furthermore, given that the sample sizes of patients and physicians in some countries (eg, Canada and Australia) were relatively small, the preference results elicited in the survey may not be applicable to the entire patient and physician populations in those countries and in countries not included in the survey.
The assessment of patient and physician preferences for AML treatments was limited by the included treatment attributes. It is possible that additional treatment attributes not specified in the survey could impact preferences or alter the effects of existing attributes. To minimize participant burden, it was necessary to limit attributes identified from the target literature review and interviews. Treatment attributes were selected based on the importance rankings provided by patients and physicians in the initial interviews to minimize the impact of excluded treatment attributes on the preference results.
As is an inherent bias of surveys, this study relies on self-reported survey responses and could be subject to biases resulting from concerns common to all self-reported measures. Recall bias could exist, especially among patients who have longer durations of disease or who have discontinued treatment for some time.
Conclusion
These DCE results highlight treatment attributes valued by patients and physicians for maintenance therapy post HSCT. QoL, duration of hospitalization, and chance of 2-year relapse-free survival most significantly impacted patients’ choices of maintenance therapy post HSCT. Whereas chance of 2-year relapse-free survival was the single most influential attribute for physicians’ choices of maintenance therapy for patients post HSCT, QoL also significantly impacted their choices. In the context of patient-centered decision-making, considering patients’ preferences may add value as physicians aim to better meet patients’ needs, improve patients’ treatment satisfaction, and improve treatment outcomes.
Abbreviations
7+3, cytarabine plus daunorubicin, doxorubicin, idarubicin, or mitoxantrone; AML, acute myeloid leukemia; DCE, discrete choice experiment; FLAG, fludarabine, cytarabine, and filgrastim; FLT3, FMS-like tyrosine kinase 3; HSCT, hematopoietic stem cell transplantation; OOP, out-of-pocket; QoL, quality of life; R/R, relapsed or refractory; SD, standard deviation; US, United States; WPAI, Work Productivity and Activity Impairment; VAS, visual analog scale.
Data Sharing Statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com.
For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Acknowledgments
Medical writing/editorial support was provided by Sarah Thompson, PharmD, and Beth Lesher, PharmD, BCPS, of OPEN Health, Bethesda, MD, and Cheryl Casterline, MA, from Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and funded by the study sponsor.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or reviewed it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Astellas Pharma, Inc.
Disclosure
Lalit Saini received consulting fees and honoraria for participation on an advisory board from Astellas. James D. Griffin received consulting fees from Astellas and reports grants from Novartis Pharma, outside the submitted work. Bhavik J. Pandya is an employee of Astellas. Manasee V. Shah is a former employee of Astellas. Mo Zhou received consulting fees paid to Analysis Group from Astellas. Hongbo Yang received consulting fees paid to Analysis Group from Astellas. Yan Song received consulting fees paid to Analysis Group from Astellas. Deborah A. Marshall received consulting fees from Astellas. She also reports travel expenses and honorarium from ISPOR, travel expenses from Illumina, and consulting services paid to institution (UCalgary) from Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Molica M, Breccia M, Foa R, Jabbour E, Kadia TM. Maintenance therapy in AML: the past, the present and the future. Am J Hematol. 2019;94(11):1254–1265. doi:10.1002/ajh.25620
2. Tefferi A, Letendre L. Going beyond 7 + 3 regimens in the treatment of adult acute myeloid leukemia. J Clin Oncol. 2012;30(20):2425–2428. doi:10.1200/JCO.2011.38.9601
3. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi:10.1182/blood-2016-08-733196
4. Small D. FLT3 mutations: biology and treatment. Hematology. 2006;2006(1):178–184. doi:10.1182/asheducation-2006.1.178
5. National Comprehensive Cancer Network® (NCCN). NCCN clinical practice guidelines in oncology. Acute Myeloid Leukemia. v1; 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
6. Pandya BJ, Chen CC, Medeiros BC, et al. Economic and clinical burden of relapsed and/or refractory active treatment episodes in patients with acute myeloid leukemia (AML) in the USA: a retrospective analysis of a commercial payer database. Adv Ther. 2019;36(8):1922–1935. doi:10.1007/s12325-019-01003-7
7. Cieri N, Maurer K, Wu CJ. 60 years young: the evolving role of allogeneic hematopoietic stem cell transplantation in cancer immunotherapy. Cancer Res. 2021;81(17):4373–4384. doi:10.1158/0008-5472.CAN-21-0301
8. Lin TL, Levy MY. Acute myeloid leukemia: focus on novel therapeutic strategies. Clin Med Insights Oncol. 2012;6:205–217. doi:10.4137/CMO.S7244
9. Bewersdorf JP, Allen C, Mirza AS, et al. Hypomethylating agents and FLT3 inhibitors as maintenance treatment for acute myeloid leukemia and myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation-a systematic review and meta-analysis. Transplant Cell Ther. 2021;27(12):997.e991–997.e911. doi:10.1016/j.jtct.2021.09.005
10. Pasvolsky O, Shimony S, Yeshurun M, et al. Maintenance therapy after allogeneic hematopoietic transplant for acute myeloid leukemia: a systematic review and meta-analysis. Acta Oncol. 2021;260(10):1335–1341.
11. Burchert A, Bug G, Fritz LV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3–internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020;38(26):2993–3002. doi:10.1200/JCO.19.03345
12. Crenolanib maintenance following allogeneic stem cell transplantation in FLT3-positive acute myeloid leukemia patients. ClinicalTrials.gov identifier: NCT02400255. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02400255?term=NCT02400255&draw=2&rank=1.
13. A trial of the FMS-like tyrosine kinase 3 (FLT3) inhibitor gilteritinib administered as maintenance therapy following allogeneic transplant for patients with FLT3/internal tandem duplication (ITD) acute myeloid leukemia (AML). ClinicalTrials.gov identifier: NCT02997202. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02997202?term=gilteritinib+maintenance+AML&draw=4&rank=3.
14. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27(3 Suppl):S110–127. doi:10.1097/00005650-198903001-00010
15. Morelli E, Mulas O, Caocci G. Patient-physician communication in acute myeloid leukemia and myelodysplastic syndrome. Clin Pract Epidemiol Ment Health. 2021;17(1):264–270. doi:10.2174/1745017902117010264
16. Stewart MA. Effective physician-patient communication and health outcomes: a review. Can Med Assoc J 1995;152(9):1423–1433.
17. Elwyn G, Frosch DL, Kobrin S. Implementing shared decision-making: consider all the consequences. Implement Sci. 2016;11(1):114. doi:10.1186/s13012-016-0480-9
18. Harrison M, Milbers K, Hudson M, Bansback N. Do patients and health care providers have discordant preferences about which aspects of treatments matter most? Evidence from a systematic review of discrete choice experiments. BMJ Open. 2017;7(5):e014719–e014719. doi:10.1136/bmjopen-2016-014719
19. Ryan M. Discrete choice experiments in health care. BMJ. 2004;328(7436):360–361. doi:10.1136/bmj.328.7436.360
20. Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320(7248):1530–1533. doi:10.1136/bmj.320.7248.1530
21. Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health--a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013
22. Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–315. doi:10.1016/j.jval.2016.04.004
23. Hollin IL, Craig BM, Coast J, et al. Reporting formative qualitative research to support the development of quantitative preference study protocols and corresponding survey instruments: guidelines for authors and reviewers. Patient. 2020;13(1):121–136. doi:10.1007/s40271-019-00401-x
24. Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13. doi:10.1016/j.jval.2012.08.2223
25. Coast J, Al-Janabi H, Sutton EJ, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ. 2012;21(6):730–741. doi:10.1002/hec.1739
26. Coast J, Horrocks S. Developing attributes and levels for discrete choice experiments using qualitative methods. J Health Serv Res Policy. 2007;12(1):25–30. doi:10.1258/135581907779497602
27. Johnson R, Orme B Getting the most from CBC. Sequim: Sawtooth Software Research Paper Series, Sawtooth Software; 2003.
28. Marshall D, Bridges JF, Hauber B, et al. Conjoint analysis applications in health - How are studies being designed and reported?: an update on current practice in the published literature between 2005 and 2008. Patient. 2010;3(4):249–256. doi:10.2165/11539650-000000000-00000
29. Janssen EM, Marshall DA, Hauber AB, Bridges JFP. Improving the quality of discrete-choice experiments in health: how can we assess validity and reliability? Expert Rev Pharmacoecon Outcomes Res. 2017;17(6):531–542. doi:10.1080/14737167.2017.1389648
30. EuroQol Research Foundation. EQ-5D-5L User Guide; 2019. Available from: https://euroqol.org/publications/user-guides.
31. Reilly Associates. WPAI Scoring. Available from: http://www.reillyassociates.net/WPAI_Scoring.html.
32. Richardson DR, Crossnohere NL, Seo J, et al. Age at diagnosis and patient preferences for treatment outcomes in AML: a discrete choice experiment to explore meaningful benefits. Cancer Epidemiol Biomarkers Prev. 2020;29(5):942–948. doi:10.1158/1055-9965.EPI-19-1277
33. Batchelder L, Philpott S, Divino V, et al. Physician treatment preferences for relapsed/refractory multiple myeloma: a discrete choice experiment. Future Oncol. 2022;18(25):2843–2856. doi:10.2217/fon-2022-0378
34. Ashaye A, Thomas C, Dalal M, et al. Patient preferences for frontline therapies for Philadelphia chromosome-positive acute lymphoblastic leukemia: a discrete choice experiment. Future Oncol. 2022;18(17):2075–2085. doi:10.2217/fon-2022-0082
35. Griffin J, Shah M, Song Y, Yang H, Tang W Economic burden of relapse after hematopoietic stem cell transplantation (HSCT) in patients with acute myeloid leukemia (AML)- a retrospective claims-based analysis.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
