Back to Journals » ImmunoTargets and Therapy » Volume 10
Pathophysiology of Non-IgE-Mediated Food Allergy
Authors Zhang S, Sicherer S, Berin MC, Agyemang A
Received 2 November 2021
Accepted for publication 24 December 2021
Published 29 December 2021 Volume 2021:10 Pages 431—446
DOI https://doi.org/10.2147/ITT.S284821
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Michael Shurin
Shouling Zhang, Scott Sicherer, M Cecilia Berin, Amanda Agyemang
Department of Pediatrics, Division of Allergy and Immunology, Icahn School of Medicine at Mount Sinai, Kravis Children’s Hospital, The Elliot and Roslyn Jaffe Food Allergy Institute, New York, NY, USA
Correspondence: Amanda Agyemang
Department of Pediatrics, Division of Allergy and Immunology, Icahn School of Medicine at Mount Sinai, Kravis Children’s Hospital, The Elliot and Roslyn Jaffe Food Allergy Institute, One Gustave L. Levy Place, Box 1198, New York, NY, 10029, USA
Tel +212 241-5548
Email [email protected]
Abstract: Non-IgE-mediated food allergies are a group of disorders characterized by subacute or chronic inflammatory processes in the gut. Unlike IgE mediated food allergies that may result in multi-organ system anaphylaxis, the non-IgE mediated food allergies primarily affect the gastrointestinal tract. This review outlines the clinical manifestations, epidemiology, pathophysiology, and management of non-IgE-mediated food allergies. An updated literature search of selected non-IgE-mediated food allergies was conducted for this review using PubMed database to the current year (2021). Reviewed disorders include food protein-induced enterocolitis syndrome (FPIES), food-protein enteropathy (FPE), food protein-induced allergic proctocolitis (FPIAP), and eosinophilic gastrointestinal disorders (EGIDs) such as eosinophilic esophagitis (EoE). While extensive gains have been made in understanding FPIES, FPIAP, FPE, and EoE, more information is needed on the pathophysiology of these food allergies. Similarities among them include involvement of innate immunity, T-lymphocyte processes, alteration of the intestinal lumen at the cellular level with the appearance of inflammatory cells and associated histologic changes, and specific cytokine profiles suggesting food-specific, T-cell, and immune-mediated responses. While FPIES and FPIAP typically resolve in early childhood, EGIDs typically do not. Emerging new therapies for EoE offer promise of additional treatment options. Further studies identifying the immunopathogenesis, associated biomarkers, and mechanisms of tolerance are needed to inform prevention, diagnosis and management.
Keywords: food protein-induced enterocolitis syndrome, FPIES, food protein-induced enteropathy, FPE, food protein-induced allergic proctocolitis, FPIAP, eosinophilic gastrointestinal disorders, EGIDs, eosinophilic esophagitis, EoE, pathophysiology
Introduction
Food allergies can be categorized by pathophysiology into IgE-mediated, non-IgE-mediated, or mixed IgE and non-IgE-mediated conditions. Non-IgE-mediated food allergies are characterized by subacute or chronic symptoms, whereas IgE-mediated food allergies are characterized by the rapid onset of symptoms following ingestion (eg, anaphylaxis). Symptoms of non-IgE-mediated food allergies are primarily localized to the gut but may also affect the skin or lungs.1,2 Mixed IgE and non-IgE-mediated food allergies include eosinophilic gastrointestinal disorders (EGIDs) such as eosinophilic esophagitis (EoE), and dermatologic conditions such as atopic dermatitis. In this review, we will discuss the clinical manifestations, epidemiology, pathophysiology, and management of non-IgE-mediated food allergies including food protein-induced enterocolitis syndrome (FPIES), food-protein enteropathy (FPE), and food protein-induced allergic proctocolitis (FPIAP). We will also briefly review non-IgE-mediated food allergies of eosinophilic origin, including eosinophilic esophagitis (EoE).
Food Protein-Induced Enterocolitis (FPIES)
Food protein-induced enterocolitis syndrome (FPIES) is a non-IgE-mediated gastrointestinal food hypersensitivity that affects the entire gastrointestinal tract.1 In 1967, one of the first case descriptions of FPIES by Gryboski described 21 hospitalized patients diagnosed with gastrointestinal milk allergy that presented with symptoms of chronic diarrhea and hematochezia.3 Eight of these children underwent sigmoidoscopy and rectal biopsy which revealed colitis that normalized after milk elimination. Respiratory and cutaneous symptoms were absent, unlike in IgE-mediated food allergy, and milk-induced colitis was identified as a distinct entity.3 Nearly a decade later, Powell described additional findings of increased peripheral leukocytosis, and neutrophilia, as well as increased presence of inflammatory cells in stool, among infants following a positive response to milk and/or soy challenge.4,5 The term, food protein-induced enterocolitis, was coined by McDonald et al in 1982.6 Further recognition of the constellation of symptoms and laboratory features seen in cases led to a refined understanding of the disease as a clinical syndrome by Sicherer et al in 1998.7
Clinical Manifestations
FPIES typically presents in infancy as either an acute or chronic phenotype.8 Acute FPIES is characterized by repetitive, profuse, and protracted vomiting starting approximately 2 hours following ingestion of the culprit food and may include later onset of watery diarrhea. The reaction may lead to dehydration, pallor, lethargy, and/or hypovolemic shock. Laboratory abnormalities include an elevated white blood cell count with neutrophilia and bandemia, and possibly acidemia and methemoglobinemia. Symptoms usually resolve within 24 hours as long as the culprit food is not re-introduced. Chronic FPIES is characterized by intermittent, yet progressive vomiting, and watery diarrhea, leading to weight loss, failure to thrive (FTT), lethargy, dehydration and/or metabolic derangements.8 Chronic FPIES symptoms are insidious and triggered by repeated ingestion of a culprit food, often cow milk or soy-based formula, over several days to weeks.8 Diagnosis is clinical and based on history and symptom resolution following elimination of the suspected triggering food(s).8 Table 1 compares features of acute and chronic FPIES based on international guidelines.8
 |
Table 1 Acute vs Chronic FPIES |
Epidemiology
FPIES awareness is increasing worldwide, and prevalence rates vary geographically.8,9 The cumulative worldwide incidence of FPIES is estimated at 0.015% to 0.7%, and the prevalence of FPIES is estimated to be 0.51% in US infants.10 In a retrospective Korean study of 142 infants admitted for vomiting and/or diarrhea, 11.3% of infants were diagnosed with cow’s milk protein-induced enterocolitis.11 In an Israeli prospective birth cohort, Katz et al noted an incidence of 3 per 1000 newborns (0.34%) with cases attributed to cow’s milk.12 In Australia, an estimated incidence of 15.4 per 100,000 has been reported.13 Chronic FPIES is less prevalent but appears more frequently in Japan and Korea.11,14 In an international survey of caregiver-reported FPIES in 441 children, most affected children were female (50.7%), white (86.2%), and atopic (54.8%) suggesting that various demographic and atopic risk factors may exist.15 In the United States, grains (oat, rice), cow’s milk (CM) and soy are most often implicated.15 Avocado is the most commonly avoided fruit and associated with increased likelihood of banana avoidance.15 In Mediterranean countries, such as Spain and Italy, fish is a common trigger, whereas in Australia, rice was the most common trigger observed.13,16,17 Geographic differences in FPIES prevalence and trigger foods may exist due to differences in feeding behaviors (eg, breastfeeding vs cow milk/soy formula use rates), timing of solid food introduction, intestinal microbiome, and genetics, but more studies are needed to ascertain the reasons for these regional variations.18
The typical ages for FPIES presentation may also vary by food trigger. For CM/soy FPIES, existing international studies report a range of 0.28–7 months for symptom onset.3,4,7,8,12,14,17,19–26 Conversely, in solid-food FPIES, an older age of presentation is more common with a reported range of 4.5–12.1 months.7,8,17,19,23–26 In adult-onset FPIES, seafood is the most common trigger.27
Pathophysiology
The pathogenesis of FPIES is poorly understood, but thought to be a combination of intestinal, innate, and cell-mediated pathways.8 FPIES appears to largely be a combination of innate and cellular immunity processes involving antigen-specific T cells and cytokines, leading to gut inflammation in the colon and ileum.3,28–34 This inflammation is believed to cause increased intestinal permeability and fluid shifts into the gastrointestinal lumen.35 In early studies, Chung et al demonstrated increased TNF-α expression coupled with diminished TGF-β1 receptors in the intestinal epithelium by gut immunohistochemistry in FPIES patients, supporting initial thoughts that this cascade of inflammatory factors weakens the integrity of the intestinal epithelial barrier and allows antigen in to propagate an inflammatory response.22 Studies in subsequent years have highlighted the complexity of potential pathways underlying the inflammatory cascade of FPIES. A Japanese study elucidating serum cytokine participants in FPIES pathophysiology demonstrated interleukin (IL)-2, IL-5, and IL-8 were increased in all four positive FPIES oral challenges obtained from 6 studied patients with FPIES.36 IL-8 was again noted to be elevated in patients with cow’s milk induced FPIES reactions, along with tryptase, supporting roles for neutrophils and mast cells, respectively, in FPIES reactions.37
Innate immune activation in FPIES reactions has been implicated in the pathogenesis of FPIES. Broad activation of the innate immune system was confirmed via CyTOF analysis of whole blood of 14 patients with FPIES by Goswami et al.38 This study demonstrated dominant activation of monocytes in addition to neutrophils, eosinophils, and natural killer cells after positive challenges with trigger foods.38 Mehr et al used transcriptional profiling of 36 children with FPIES to identify several genes associated with granulocytes and innate signaling (IL-10, TREM1).39 In this study, matrix metalloproteinase 9, IL-B, and STAT3 were identified as key factors in positive FPIES challenge responses.39 A number of additional innate cytokines (TNF-alpha, oncostatin M, leukemia inhibitory factor, IL-10, and IL-6) were identified as elevated in serum during and after FPIES reactions.40
Many studies have supported the involvement of T lymphocytes mediating FPIES reactions.41,42 Global activation of T lymphocytes and their extravasation from the peripheral blood after positive FPIES challenges are demonstrated through significant loss of circulating T lymphocytes and associated CD69 upregulation of remaining lymphocytes.38 However, evidence for pathways for T cell specific immune responses and the subtype of effector cells and cytokine profiles mediating this activation have conflicted. An early, double-blind, placebo-controlled food challenge to rice in Italy revealed Th2 activation in the form of immediate increase in IL-4 expression during a positive rice FPIES challenge.41 Broad investigation of antigen-specific lymphoproliferation and cytokine production profiles in patients with non-IgE-mediated gastrointestinal food allergies and patients with IgE-mediated allergy also found Th2 cytokines, such as IL-3, IL-5, and IL-13, were significantly produced in vitro by milk protein-stimulated, peripheral blood mononuclear cells from patients with non-IgE gastrointestinal food allergies.42 IgE or Th1 cytokines such as IFN-Y or IL-17 were not found to be significantly expressed in these same populations.42 Conversely, Adel-Patient et al found very weak expression of inflammatory Th2 and Th17 cytokines after stimulation of peripheral blood mononuclear cells with cow’s milk protein in subjects with cow’s milk FPIES.43 Other studies also demonstrated weak Th2 responses that were not significantly different from control populations.44
A recent prospective study of children with FPIES undergoing supervised oral food challenges offers new insights to an IL-17 signaling pathway in acute FPIES reactions. Berin et al used proteomic and flow cytometric analysis to examine peripheral blood samples from children (11 reactors and 12 outgrown) at baseline, symptom onset, and 4 hours after symptom onset in children undergoing FPIES food challenges.45 Specifically, acute FPIES reactions were associated with activated IL-17 pathway signaling, demonstrating significant elevations in chemokine CCL20 and TH-17-related cytokines (IL-17A, IL-22, IL-17C, and CCL20).45 Sources of IL-17 in peripheral cells were confirmed as primarily CD4+ TH17 cells.45 In this study, the IL-17 pathway was identified as a key feature of acute FPIES from symptom onset until resolution, which was not previously described.45
Achievement of immune tolerance may also be, in part, mediated by T lymphocytes. Mori et al observed increased serum IL-10 expression in a follow-up negative rice challenge, in an 8-month-old who reacted to rice 6 months prior, suggesting that IL-10 was associated with achieving tolerance.41 In addition, IL-10 serum levels have been demonstrated to increase in subjects after cow’s milk FPIES had resolved, further suggesting a role for IL-10 in food tolerance.37 Tregs have also been noted to be elevated during the course and or resolution of FPIES, suggesting roles for these cells in tolerance.41 T-lymphocyte processes overall appear central in our understanding of FPIES. Table 2 summarizes findings of cytokine profiles seen in FPIES.
 |
Table 2 Inflammatory Responses Implicated in FPIES |
Additional branches of the immune process have been speculated to be included in FPIES pathophysiology such as humoral immunity. The presence of specific IgE towards trigger foods is associated with persistent FPIES23 and up to 30% of individuals with FPIES also have low positive specific IgE levels.7,14,42 Atypical FPIES can also present with some degree of serum IgE positivity and IgE-mediated cutaneous reactions.10 Though associations between FPIES symptomatology and IgE presence are suggested, there have been no studies successfully describing a food-specific IgE-mediated response in FPIES. For instance, Adel-Patient et al did not detect IgE reactivity to 8 separate milk allergens or digested cow’s milk when comparing milk FPIES with IgE-mediated milk or peanut allergy.43
Studies suggesting involvement of other immunoglobulin isotypes are conflicting.10 Plasma cells producing IgA and IgG were noted in the intestinal mucosa in the earliest studies of subjects with cow’s milk malabsorption syndromes, along with increased IgA and IgM content of the stool and serum post-challenge with cow’s milk.46 Additionally, egg and soy induced FPIES reactions also demonstrated elevations in specific IgA and IgG post-challenge when compared to control subjects in a small study by McDonald et al.20 However, larger studies indicate no evidence for food-triggered antibody recognition.40 A comparison of individuals with challenge-proven milk FPIES and individuals with history of milk FPIES that had since resolved did not reveal significant differences in milk-specific IgG1, IgG, IgM, and IgA levels.44,47 There has even been suggestion of suppression of immunoglobulin responses, specifically IgG4 and IgA, when subjects with FPIES were challenged with their food triggers thus highlighting the lack of understanding of the role of humoral immunity in FPIES.47,48
Neuroimmune mechanisms are also suggested in the pathogenesis given the symptomatic improvement of vomiting, abdominal pain, and lethargy with ondansetron use in acute episodes of FPIES.49 However, this is also poorly understood. Rapid recovery of acute FPIES reactions in a case series of 5 consecutive patients undergoing oral food challenges at a pediatric allergy clinic was observed with ondansetron treatment, a selective serotonin 5-HT3 receptor antagonist.49
Existing imaging and biopsy studies have revealed additional insight to the gross pathology of FPIES. Initial rectal biopsies of children who underwent sigmoidoscopy with suspected FPIES identified colitis that normalized after a milk elimination diet.3 A review of 53 cases of children with allergic disorders of the GI tract, including 15 with allergic proctitis and 38 with allergic gastroenteritis, confirmed diffuse eosinophils in the lamina propria with focal infiltration of the epithelium by eosinophils on rectal mucosal biopsy.28 Proctosigmoidoscopy in four infants with suspected soy protein intolerance after soy formula challenge showed mucosal friability and loss of vascular pattern. Their rectal biopsies showed acute colitis with crypt abscesses, mucus depletion of rectal glands, and polymorphonuclear leukocytes within the lamina propria.29 Intestinal biopsies of 31 infants with cow’s milk protein intolerance confirmed mucosal damage with partial villous atrophy and blunting seen most often.30
Management
In acute management of FPIES reactions, the severity of presenting symptoms should be considered.8 For mild symptoms, oral rehydration, ondansetron (for ages 6 months and older), and close monitoring for 4–6 hours from onset of symptoms at home is suggested.8 For moderate or severe symptoms with greater than 3 episodes of emesis or lethargy, emergency care and/or hospitalization may be required.8 Aggressive fluid resuscitation in an emergency setting, a single dose of intravenous methylprednisolone, and ondansetron may be considered.8 In severe cases, close attention to electrolyte abnormalities such as acidemia, correction of methemoglobinemia, and intensive cardiorespiratory support may be indicated.8 Management of acute and chronic FPIES is also based on an elimination diet of the triggering food.8 Due to high milk and soy co-reactivity, transition to soy formula is not recommended in cases of FPIES to cow’s milk.8 Use of extensively hydrolyzed or amino-based formula may be considered in addition to breastfeeding.8 Guided introduction of low risk solids is recommended for long-term management, beginning with less allergenic foods (eg, berries, carrot, quinoa, lamb, and apple).8
Prognosis is favorable for FPIES. Most children outgrow FPIES by age 1–5 years, but timing varies by triggering food and patient. For example, CM-triggered FPIES tends to resolve by age 3–5 years, whereas rice FPIES typically resolves by age 5 in 50% of cases.1,8
Food Protein-Induced Allergic Proctocolitis (FPIAP)
Clinical Manifestations
Food protein-induced allergic proctocolitis (FPIAP) is a non-IgE-mediated, self-limited, food allergy of the rectum and colon. Lake et al first described FPIAP in 1982 in six exclusively breastfed infants who developed inflammatory changes in the rectum in the first month of life.50 FPIAP typically starts in the first 6 months of life with blood-streaked and/or mucus-containing stools.1 Breastfed infants are often older at initial presentation and with less severe histologic findings. Infants appear well but may have colicky behavior with increased bowel movements. FTT is absent, which is a key difference from other non-IgE-mediated food allergies. Anemia secondary to stool blood loss may rarely occur, requiring iron supplementation.1
Epidemiology
Estimates of prevalence in infants with rectal bleeding range widely from 18% to 64%.51,52 A recent prospective study of newborn infants identified a cumulative incidence of 17% (n = 153) for FPIAP when diagnosed clinically by community pediatricians without confirmatory oral challenge.53 This study likely over-represented the prevalence by including infants with occult or chronic blood in the stool without confirming food as a trigger. Varying practices in screening and diagnosis of FPIAP may account for different rates. Risk factors for FPIAP include atopy, such as having a first-degree relative with food allergy), eczema, or household pets at birth.53 Infants fed both breast milk and formula during the first 4 months of life in this recent prospective study were 56% less likely than exclusively formula-fed infants, and 38% less likely than exclusively breastfed infants to develop FPIAP.53 IgE-mediated allergy is also strongly associated with FPIAP, with Martin et al observing that infants with FPIAP were almost twice as likely to develop IgE mediated allergy.54
Pathophysiology
FPIAP pathology is mostly limited to the rectosigmoid colon for unknown reasons, and like other non-IgE-mediated food allergies, is poorly understood. Similar to FPIES, inflammatory cytokine TNF-α is overexpressed, whereas TGF-β1 receptor activity and TGF-β ligand expression are decreased in tissue and allergen-restimulated peripheral blood mononuclear cells in subjects with FPIAP.42,55 The hypothesized role for abnormal expression of TNF-α and TGF-β is to weaken the epithelial barrier of intestinal mucosa and promote fluid shifts resulting in diarrhea and hematochezia.
Endoscopy and biopsy are rarely undertaken because FPIAP is a clinical diagnosis. When biopsied, gross findings of focal erythema with lymphoid nodular hyperplasia are present.56 Signature findings on biopsy include marked eosinophilic infiltration and degranulation of the rectosigmoid colon in close proximity to the lymphoid nodules.28,51,57 A prospective study of 35 infants with rectal bleeding and allergic colitis revealed 31 infants with marked eosinophilic infiltrate (with >20 eosinophils per high-power field) on histopathology.57 No correlation between tissue and peripheral eosinophilia has been noted in FPIAP.56
There are currently no reported biomarkers identified to support FPIAP diagnosis.53 Children with an FPIAP diagnosis had a two-fold risk of developing IgE-mediated food allergy, even when accounting for atopic dermatitis as a risk.54 A shared pathophysiology may explain this, or the possibility that dietary elimination was a risk factor, which underscores the need for more studies in this area and consideration to rechallenge to the presumed trigger after symptom resolution to confirm the diagnosis.
Management
A maternal elimination diet of the triggering food in exclusively breastfed infants, or a trial of extensively hydrolyzed or amino acid-based cow’s milk formula in either breastfed or formula-fed infants, may be considered for the management of cow’s milk induced FPIAP.1 To establish a dietary trigger, a re-trial of the suspected food a few weeks after resolution of rectal bleeding is advised.58 Regarding maternal dietary restriction, some suggest waiting a month before starting dietary elimination, since bleeding may self-resolve, but individual approaches may vary.58,59
Prognosis is favorable, and cases usually resolve by 12 months of age.1 In fact, a recent US prospective study of FPIAP showed the median age of symptom resolution was potentially earlier, at 123 days.53 Infants are otherwise well-appearing without significant morbidity resulting from this self-limited condition.
Food Protein-Induced Enteropathy (FPE)
Food protein-induced enteropathy (FPE) is a non-IgE-mediated food allergy affecting the small bowel. The diagnosis is rarely made nowadays. FPE was first described by Kuitunen et al in 1975 among 54 infants with malabsorption syndrome and cow’s milk intolerance from 1962 to 1971 in Finland. Infants presented with diarrhea, FTT, vomiting and 20% had eczema and recurrent respiratory infections that resolved around age 1. Labs showed malabsorption, raised serum IgA and CM precipitants, while biopsies revealed damaged jejunal mucosa.60 Unlike FPIES or FPIAP, histological confirmation of FPE requires biopsy.1
Clinical Manifestations
FPE presents with protracted, non-bloody, diarrhea in the first 9 months of life. Symptoms typically present within the first 2 months, and usually within weeks of CM introduction.1,2,8,31,32,60 Other food triggers include soy, wheat, and egg.2 Up to half of affected infants have FTT, abdominal distention, and/or malabsorption.1 Additional features include protein-losing enteropathy, hypoalbuminemia, and FTT.1 FPE is characterized by abnormal small intestinal mucosa, improved after dietary avoidance.2
Epidemiology
FPE is a rare condition. An overall decline in FPE has been noted over the last few decades.61 Peak incidence was noted in the 1960s in Finland, followed by a gradual disappearance of cases of severe jejunal damage caused by CM in the following three decades.1,61 Personal history of atopy is estimated to be 22% in FPE, whereas family history of atopy is unknown.1
Pathophysiology
Distinguishing features of biopsies in patients with FPE include specific damage to villous architecture of the small intestine mucosa, unlike the other non-IgE mediated gastrointestinal allergies mentioned in this review.62,63 Specific histological findings include villous atrophy, lymphonodular hyperplasia of the duodenum and colon, increased intraepithelial lymphocytes (>25/100 epithelial cells) and marked eosinophil infiltration and degranulation in the mucosa.1,34,61,64–66 Suggested cellular etiologies for the inflammation induced structural damage include the presence of food (milk) specific T lymphocytes in duodenal tissue which express Th2 cytokines upon stimulation with milk.67 Increased intestinal intraepithelial CD8+ T cells that are food-allergen specific are noted in FPE, similar to celiac’s disease, and thus likely also contribute to the malabsorption.1,61,68,69 Expression of IFN-Y and IL-4 cytokines have also been identified in jejunal biopsy specimens.1,70 Overall, pathophysiology of FPE involves eosinophils, cow-milk specific T lymphocytes, and specific cytokine profiles.2,34,37,67
Management
Avoidance of the triggering food is recommended. Introduction of non triggering foods can occur without restriction, unlike FPIES which requires a more gradual approach of slow introduction of foods related to the trigger.44 Further management, including food introduction, has been discussed at length.8 Prognosis is favorable with resolution of symptoms usually reported by age 24–36 months.1
Eosinophilic Gastrointestinal Disorders (EGIDs)
The eosinophilic gastrointestinal disorders (EGIDs) include eosinophilic esophagitis (EoE), eosinophilic gastritis (EG), eosinophilic gastroenteritis (EGE), eosinophilic enteritis, and eosinophilic colitis (EC). These disorders are characterized by chronic eosinophilic inflammation and although IgE mediated allergy is not necessarily identified, they are typically categorized as “mixed” IgE and non-IgE mediated because allergic sensitization is often observed. EoE, the most well-studied, will be primarily discussed here. Eosinophilic esophagitis (EoE) is a chronic, immune-mediated esophageal disease characterized by symptoms related to esophageal dysfunction and eosinophil-predominant inflammation on histology.71
Clinical Manifestations
Symptoms include abdominal pain, dysphagia, nausea, emesis, esophageal food impaction, gastroesophageal reflux symptoms, diarrhea, chest pain, and bloody stools.72 Symptoms vary by age, with nonspecific gastrointestinal symptoms and FTT more common in childhood, and esophageal symptoms like heartburn, chest pain, and dysphagia more prevalent in older children and adults.72 Other immunological disorders, like celiac disease, and psychosocial comorbidities, such as depression and anxiety, are also associated with EoE.72 Eosinophilic gastritis/gastroenteritis (EG/EGE) are inflammatory disorders characterized by with eosinophilic infiltration within in the GI tract beyond the esophagus. In EGE, symptoms of abdominal pain are more common.73 Diagnosis of EoE is based on marked eosinophilic infiltrates on esophageal biopsy with ≥15 eosinophils per high-power field (hpf).74 Eosinophilia is localized to specific tissues in the GI tract in EGIDs, and is typically independent of peripheral blood eosinophilia.73
Epidemiology
The prevalence of EoE is increasing in the United States and is estimated at 0.5–1 case/1000 persons.75 There is limited data on the prevalence of other EGIDs such as EG/EGE due to their rarity, however EGE in the United States is estimated to be 22–28 per 100,000.76 Conflicting reports suggest lower prevalences of 6.3/100,000 for EG, 8.4/100,000 for EGE, and 3.3/100,000 for eosinophilic colitis.77 Most existing studies of EoE take place in North America, and more information is needed on worldwide prevalence rates and diagnostic screening recommendations. A large multicenter US EoE study revealed a predominantly male (68.2%) and white (87.9%) EoE phenotype.72 The median age of EoE symptom onset was 5 years (range 1–12 years) and the median age of EoE diagnosis was 8 years (range 3–15 years).72 Food allergy (67.0%), allergic rhinitis (60.3%), atopic dermatitis (46.4%), and asthma (45.4%) were common comorbidities.72
Pathophysiology
The pathogenesis of EGIDs, as with other non-IgE-mediated food allergies discussed, is not fully understood. The pathogenesis of EoE, the EGID most often evaluated by allergists, is multifactorial, involving a combination of genetic, host, and environmental factors.78,79 EoE is characterized by the endoscopic findings of eosinophilic infiltration of the esophagus.71 Additional endoscopic findings may include fixed and transient esophageal rings, whitish exudates, longitudinal furrows, edema, esophageal narrowing, and esophageal lacerations.71
The impaired epithelial barrier of the esophagus is central to EoE pathogenesis.78 There are several genes that are specific to the esophageal epithelium in which perturbations in expression are associated with EoE disease.78,80 Increased EoE susceptibility has been associated with pathogenic variants at gene loci of the esophageal-derived genes thymic stromal lymphopoietin (TSLP) encoded at gene locus 5q22, and calpain 14 protease (CAPN14) encoded at gene locus 2p23.74,78,81,82 Genes encoding for IL-1 family genes, serine peptidase inhibitors (SERPINs), serine protease inhibitors, Kazal-type-related inhibitors (SPINKs), and the calpain protease, CAPN14 part of the EoE transcriptome, serve as a collection of genes that are highly expressed in the esophagus, and when dysregulated, increase EoE risk.78,81,82 In particular, loss of SPINK7 function induces an epithelial barrier defect, proteolytic activity, and a proinflammatory Th2 cytokine response by epithelial cells of the esophagus, in individuals with EoE.78,83
Localized Th2 inflammation in the esophagus is also key in EoE, which includes the proinflammatory cytokine responses of IL-5 and IL-13. IL-5 traffics eosinophils to the esophagus, as identified in early mouse models, and in part promotes remodeling of the esophagus as noted by increased basal layer thickness and collagen accumulation in mice overexpressing IL-5.84,85 IL-13 is overexpressed in subjects with EoE and may serve as a downstream mediator of esophageal inflammation.74 T cell-mediated responses involving cytokines IL-5 and IL-13 are hypothesized to induce eotaxin 3, which then recruits eosinophils from the periphery into the tissue.86,87 Major degranulation of eosinophilic granules in the esophageal epithelia occurs upon arrival as evidenced by findings of increased major basic protein, eosinophil peroxidase, and cationic protein deposited in the tissue.78 Previous studies have also shown increased levels of IL-5, IL-13, IL-15, and plasma basic fibroblast growth factor in EoE.71,88–90
IL-13, in particular, also exacerbates the barrier disruption of the esophagus. IL-13 induces calpain 14, which as mentioned previously, is found specifically in the upper GI tract/esophageal epithelium.91,92 Calpain 14 acts as a proteolytic enzyme whose overexpression has been associated with decreased in vitro expression of desmoglein 1 and filaggrin proteins, and an associated impaired epithelial barrier in the esophagus.91–94 Filaggrin and desmoglein 1 were confirmed to be downregulated in expression in vivo in EoE, with the ability of IL-13 overexpression to independently decrease filaggrin.72
Tissue remodeling is seen in chronic EoE.95 Epithelial desquamation, basal zone hyperplasia, subepithelial fibrosis, angiogenesis, and/or smooth muscle hypertrophy have been reported in EoE.95 In a immunohistochemical analysis of esophageal biopsy specimens of children with and without EoE, biopsies from pediatric cases of EoE demonstrated increased subepithelial fibrosis and TGFβ expression compared to controls.96 There was also increased vascularity seen with increased vascular cell adhesion molecule 1 expression.96 Chehade et al found that esophageal subepithelial fibrosis was also common (57%) among a sample of 21 children with EoE undergoing distal esophageal biopsy, of which 42% had dysphagia.97 Fibrosis was correlated with esophageal eosinophil degranulation and activation.97 Aceves et al found that a rigid substrate matrix induces changes in esophageal smooth muscle cells leading to increased contraction and cellular hypertrophy.98 Clinically, tissue remodeling results in esophageal narrowing, strictures, dysmotility, and food impactions over time.95
The pathogenesis of other EGIDs is even less understood, although an allergic component has been suggested in some instances. An adult study of allergic EGE noted increased allergen-specific, IL-5-producing Th2 cells present in EGE as compared to increased non-IL-5-producing Th2 cells in IgE-mediated peanut allergy, which has yet to be further explored.99 Gastric biopsy samples in patients with EGE also showed increased Th2 cytokines (IL-4, IL-5, IL-13) and eosinophil-related chemokine eotaxin-3 upregulation.100 A Japanese case series of EGE revealed small intestinal villi flattening in 4 of 6 patients on endoscopy.101 A comparison of EGE patients with controls showed increased IL-3, GM-CSF, and IL-5 in 9 of 10 EGE patients, which was not observed in controls.102 While eosinophils are normally found to varying degrees in the lower gastrointestinal tract, uncertainty exists when assessing the origin of lower GI tract eosinophilia, since other non-EGID disorders may contribute such as parasitic infection, food or drug allergy, inflammatory bowel disease, or hypereosinophilic syndrome.103 Endoscopic and histopathologic features of nonesophageal EGIDs are being investigated, and there is an ongoing need for consensus of diagnostic criteria for these EGIDs.104 Table 3 summarizes the clinical features and pathophysiology of EGIDs.
 |
Table 3 Summary of EGIDs |
Management
Management options for EoE are diverse. The 2020 Joint Task Force (JTF) of the American Gastroenterological Association (AGA), American Academy of Allergy, Asthma, and Immunology (AAAAI), and American College of Allergy, Asthma, and Immunology (ACAAI) recently provided an updated practice parameter guideline for management of EoE.105 Swallowed topical steroids are considered first-line therapy for EoE, and the only therapy to receive a strong recommendation by the 2020 Joint Task Force.105,106
Elimination diets have been used with varying success rates for EGIDs, with most success reported in EoE.105–107 A six food empiric elimination diet (milk, egg, wheat, soy, peanut, tree nuts) has been effective in children and adults, with studies citing rates as high as 74% remission of disease.108 Less restrictive empiric eliminations diets, such as four food (milk, egg, wheat, soy) and two food (milk, wheat) eliminations diets, provide less conservative alternatives that can be stepped up or down and monitored for symptom response with endoscopy.107 Allergy test directed elimination diets are not recommended as skin prick testing, patch testing, and serum IgE have poor positive and negative predictive values for identifying food triggers.107 The use of elemental formula and food restriction is another less popular approach, given the significant restrictions it places on the individual. Elemental formula can be expensive, have limited insurance approval, and may be difficult to introduce due to unpleasant tastes associated with this formula type.109 Overall, elimination diets are only conditionally recommended with low to very low quality evidence to support its use.105 A comprehensive nutritional assessment is suggested with the use of any elimination diet to ensure adequate nutrition.110 Similarly, proton pump inhibitors and periodic esophageal dilation are additional therapies with conditional recommendations.105
Biologic treatment therapies are the new frontier of EoE management and are actively being explored. Lirentelimab, an anti–siglec-8 antibody that depletes eosinophils and acts as a mast cell inhibitor, has been shown to reduce gastrointestinal eosinophils in patients with eosinophilic gastritis or duodenitis in Phase 2 trials.111 The average percentage change in GI eosinophilia was −86% in the treatment group compared to 9% in the placebo group (P<0.001), which was the study’s primary endpoint.111 Targeted biologic therapies also being evaluated include dupilumab which targets anti-IL-4 and anti-IL-13.105,112,113 A phase 2 clinical trial of adults with active EoE undergoing dupilumab treatment assessed for a change from baseline to week 10 in Straumann Dysphagia Instrument patient-reported outcome score as its primary outcome.113 Phase 3 trials for dupilumab are ongoing, and has been suggested as a potential add-on or monotherapy for EoE patients refractory to standard interventions for the future.114 A reduction of mean value of 3.0 at week 10 compared with a mean reduction of 1.3 in the placebo group in patient-reported scores of dysphagia was seen with dupilumab use (P = 0.0304).113 Biologics with anti-IL-5 activity are also under investigation including benralizumab, mepolizumab, and reslizumab.115 Anti-IL-13 targeted biologics studied in adult EoE patients has demonstrated significant decrease in cellular markers of epithelial mesenchymal transition which mediates the complication of fibrostenosis in patients.116–118 Hirano et al’s study of a monoclonal antibody against IL-13 showed a dose-dependent reduction in mean esophageal eosinophil count per hpf at 16 weeks of 94.8 ± 67.3 compared to placebo.117 Additional therapeutic targets include TNF-α and chemoattractant receptors on Th2 cells (CRTH2).119–121 Investigations into omalizumab as treatment for EoE have not been fruitful, further supporting EoE being an non-IgE mediated process, despite its utilization of Th2 pathway.114,122,123 Table 4 provides a summary of biologics under consideration for EoE.89,105,111–122
 |
Table 4 Biologics Under Consideration for EoE |
Discussion and Conclusions
In this review, we summarize the clinical manifestations, epidemiology, and current understanding of the pathophysiology of FPIES, FPIAP, FPE, and EGIDs, including mainly EoE. With regard to epidemiology, overlapping features of common pediatric conditions in infancy (ie, reflux, colic, rash) and variations in infant dietary practices (ie, breastfeeding versus formula use) may affect existing prevalence rates. While gains have been made in understanding these disorders, more information is needed on the exact pathophysiology of these less well understood food allergies. Among the non-IgE-mediated food allergies, there are a variety of immunologic pathways that may underlie their pathophysiology. While FPIES is likely driven by innate immunity, EGIDs appear to be predominantly driven by Th2 processes (Table 5). At the tissue level, the morphology of the intestinal lumen integrity is altered in each disorder leading to findings which can be confirmed histologically. A significant eosinophilic presence is also seen among these non-IgE mediated disorders and in EGIDs to sustain inflammation and possible tissue remodeling.
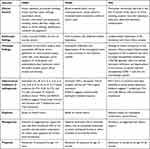 |
Table 5 Comparison of FPIES, FPIAP, and FPE |
Several gaps, however, remain in our understanding of non-IgE-mediated food allergy. For example, reasons for why these disorders localize to certain areas of the gut are unknown. Studies identifying why the rectosigmoid colon may be more affected in FPIAP versus the more extensive small and large intestinal involvement seen in FPIES and/or FPE small intestinal involvement are needed. Additional limitations include gaps in understanding the exact pathways involved in how these disorders develop. While activation of certain immune cell subtypes and cytokines have been identified these disorders, the mechanistic steps in which these cells may perceive food antigen, recruit cytokines to specific locations, and propagate inflammatory effects to their respective tissues are largely unknown.
For instance, in FPIES, the phenotype and mechanism of a pathogenic food-specific T cell response in the intestinal mucosa of affected individuals has not yet been demonstrated, which could likely offer many insights.124 Barriers, as previously noted, include the fact that the inflammatory cascade may be in part localized to the gastrointestinal tract, thus activated T cells cannot be easily accessed in the peripheral circulation for study.38 There is simultaneously a lack of access to gastrointestinal tissue of affected patients, since endoscopy and biopsy are not part of the routine clinical care of FPIES—thus limiting the ability for further studies.40 We thus rely on noninvasive means of identifying food-specific cells in the gastrointestinal tract during acute reactions.40
While FPIES, FPIAP, EGIDS and FPE have different timelines for symptom resolution (Table 5), it remains unclear exactly how tolerance develops, and how this may vary by disorder. Future studies aimed at understanding the exact immunological mechanisms of disease, in the hopes of identifying additional diagnostic markers and even therapeutic targets for intervention, are recommended. Future studies that distinguish between infants with varying feeding practices, such as those that are mostly breastfed versus formula fed, are also recommended in order to understand how small amounts of protein found in breast milk may still cause symptomatology among infants. For now, given the self-limited nature of many of these conditions, the mainstay of management is diagnosis based on clinical symptoms and food trigger avoidance.
Abbreviations
FPIES, food protein-induced enterocolitis; FPE, food-protein enteropathy; FPIAP, food protein-induced allergic proctocolitis; EGIDs, eosinophilic gastrointestinal disorders; EoE, eosinophilic esophagitis; EG, eosinophilic gastritis; EGE, eosinophilic gastroenteritis; EC, eosinophilic colitis; CM, cow’s milk; FTT, failure to thrive; OFC, oral food challenge.
Disclosures
Scott Sicherer – Reports royalty payments from UpToDate and from Johns Hopkins University Press; grants to his institution from the National Institute of Allergy and Infectious Diseases, from Food Allergy Research and Education; and personal fees from the American Academy of Allergy, Asthma and Immunology, outside of the submitted work.
M Cecilia Berin – Reports grants to her institution from the National Institute of Allergy and Infectious Diseases, outside of the submitted work.
The authors report no other conflicts of interest in this work.
Funding
There is no funding to report.
References
1. Nowak-Węgrzyn A, Katz Y, Mehr SS, Koletzko S. Non-IgE-mediated gastrointestinal food allergy. J Allergy Clin Immunol. 2015;135(5):1114–1124. doi:10.1016/j.jaci.2015.03.025
2. Connors L, O’Keefe A, Rosenfield L, Kim H. Non-IgE-mediated food hypersensitivity. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):56. doi:10.1186/s13223-018-0285-2
3. Gryboski JD. Gastrointestinal milk allergy in infants. Pediatrics. 1967;40(3):354–362. doi:10.1542/peds.40.3.354
4. Powell GK. Enterocolitis in low-birth-weight infants associated with milk and soy protein intolerance. J Pediatr. 1976;88(5):840–844. doi:10.1016/s0022-3476(76)81128-6
5. Powell GK. Milk- and soy-induced enterocolitis of infancy. Clinical features and standardization of challenge. J Pediatr. 1978;93(4):553–560. doi:10.1016/s0022-3476(78)80887-7
6. McDonald PJ, Powell GK, Goldblum RM. Serum D-xylose absorption tests: reproducibility and diagnostic usefulness in food-induced enterocolitis. J Pediatr Gastroenterol Nutr. 1982;1(4):533–536. doi:10.1097/00005176-198212000-00013
7. Sicherer SH, Eigenmann PA, Sampson HA. Clinical features of food protein-induced enterocolitis syndrome. J Pediatr. 1998;133(2):214–219. doi:10.1016/s0022-3476(98)70222-7
8. Nowak-Węgrzyn A, Chehade M, Groetch ME, et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2017;139(4):1111–1126.e4. doi:10.1016/j.jaci.2016.12.966
9. Baker MG, Nowak-Wegrzyn A. Food protein-induced enterocolitis syndrome: epidemiology and comorbidities. Curr Opin Allergy Clin Immunol. 2020;20(2):168–174. doi:10.1097/aci.0000000000000615
10. Nowak-Wegrzyn A, Berin MC, Mehr S. Food protein-induced enterocolitis syndrome. J Allergy Clin Immunol Pract. 2020;8(1):24–35. doi:10.1016/j.jaip.2019.08.020
11. Hwang JB, Lee SH, Kang YN, Kim SP, Suh SI, Kam S. Indexes of suspicion of typical cow’s milk protein-induced enterocolitis. J Korean Med Sci. 2007;22(6):993–997. doi:10.3346/jkms.2007.22.6.993
12. Katz Y, Goldberg MR, Rajuan N, Cohen A, Leshno M. The prevalence and natural course of food protein-induced enterocolitis syndrome to cow’s milk: a large-scale, prospective population-based study. J Allergy Clin Immunol. 2011;127(3):
13. Mehr S, Frith K, Barnes EH, Campbell DE. Food protein-induced enterocolitis syndrome in Australia: a population-based study, 2012–2014. J Allergy Clin Immunol. 2017;140(5):1323–1330. doi:10.1016/j.jaci.2017.03.027
14. Nomura I, Morita H, Hosokawa S, et al. Four distinct subtypes of non-IgE-mediated gastrointestinal food allergies in neonates and infants, distinguished by their initial symptoms. J Allergy Clin Immunol. 2011;127(3):
15. Maciag MC, Bartnikas LM, Sicherer SH, et al. A slice of Food Protein-Induced Enterocolitis Syndrome (FPIES): insights from 441 children with FPIES as provided by Caregivers in the International FPIES Association. J Allergy Clin Immunol Pract. 2020;8(5):1702–1709. doi:10.1016/j.jaip.2020.01.030
16. Vila L, García V, Rial MJ, Novoa E, Cacharron T. Fish is a major trigger of solid food protein-induced enterocolitis syndrome in Spanish children. J Allergy Clin Immunol Pract. 2015;3(4):621–623. doi:10.1016/j.jaip.2015.03.006
17. Sopo SM, Giorgio V, Dello Iacono I, Novembre E, Mori F, Onesimo R. A multicentre retrospective study of 66 Italian children with food protein-induced enterocolitis syndrome: different management for different phenotypes. Clin Exp Allergy. 2012;42(8):1257–1265. doi:10.1111/j.1365-2222.2012.04027.x
18. Mehr S, Frith K, Campbell DE. Epidemiology of food protein-induced enterocolitis syndrome. Curr Opin Allergy Clin Immunol. 2014;14(3):208–216. doi:10.1097/aci.0000000000000056
19. Nowak-Wegrzyn A, Sampson HA, Wood RA, Sicherer SH. Food protein-induced enterocolitis syndrome caused by solid food proteins. Pediatrics. 2003;111(4 Pt 1):829–835. doi:10.1542/peds.111.4.829
20. McDonald PJ, Goldblum RM, Van Sickle GJ, Powell GK. Food protein-induced enterocolitis: altered antibody response to ingested antigen. Pediatr Res. 1984;18(8):751–755. doi:10.1203/00006450-198408000-00016
21. Hwang JB, Sohn SM, Kim AS. Prospective follow-up oral food challenge in food protein-induced enterocolitis syndrome. Arch Dis Child. 2009;94(6):425–428. doi:10.1136/adc.2008.143289
22. Chung HL, Hwang JB, Park JJ, Kim SG. Expression of transforming growth factor beta1, transforming growth factor type I and II receptors, and TNF-alpha in the mucosa of the small intestine in infants with food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2002;109(1):150–154. doi:10.1067/mai.2002.120562
23. Caubet JC, Ford LS, Sickles L, et al. Clinical features and resolution of food protein-induced enterocolitis syndrome: 10-year experience. J Allergy Clin Immunol. 2014;134(2):382–389. doi:10.1016/j.jaci.2014.04.008
24. Fogg MI, Brown-Whitehorn TA, Pawlowski NA, Spergel JM. Atopy patch test for the diagnosis of food protein-induced enterocolitis syndrome. Pediatr Allergy Immunol. 2006;17(5):351–355. doi:10.1111/j.1399-3038.2006.00418.x
25. Mehr S, Kakakios A, Frith K, Kemp AS. Food protein-induced enterocolitis syndrome: 16-year experience. Pediatrics. 2009;123(3):e459–64. doi:10.1542/peds.2008-2029
26. Ruffner MA, Ruymann K, Barni S, Cianferoni A, Brown-Whitehorn T, Spergel JM. Food protein-induced enterocolitis syndrome: insights from review of a large referral population. J Allergy Clin Immunol Pract. 2013;1(4):343–349. doi:10.1016/j.jaip.2013.05.011
27. Tan JA, Smith WB. Non-IgE-mediated gastrointestinal food hypersensitivity syndrome in adults. J Allergy Clin Immunol Pract. 2014;2(3):355–7.e1. doi:10.1016/j.jaip.2014.02.002
28. Goldman H, Proujansky R. Allergic proctitis and gastroenteritis in children. Clinical and mucosal biopsy features in 53 cases. Am J Surg Pathol. 1986;10(2):75–86. doi:10.1097/00000478-198602000-00001
29. Halpin TC, Byrne WJ, Ament ME. Colitis, persistent diarrhea, and soy protein intolerance. J Pediatr. 1977;91(3):404–407. doi:10.1016/s0022-3476(77)81308-5
30. Fontaine JL, Navarro J. Small intestinal biopsy in cows milk protein allergy in infancy. Arch Dis Child. 1975;50(5):357–362. doi:10.1136/adc.50.5.357
31. Jenkins HR, Pincott JR, Soothill JF, Milla PJ, Harries JT. Food allergy: the major cause of infantile colitis. Arch Dis Child. 1984;59(4):326–329. doi:10.1136/adc.59.4.326
32. Coello-Ramirez P, Larrosa-Haro A. Gastrointestinal occult hemorrhage and gastroduodenitis in cow’s milk protein intolerance. J Pediatr Gastroenterol Nutr. 1984;3(2):215–218. doi:10.1097/00005176-198403000-00008
33. Richards DG, Somers S, Issenman RM, Stevenson GW. Cow’s milk protein/soy protein allergy: gastrointestinal imaging. Radiology. 1988;167(3):721–723. doi:10.1148/radiology.167.3.3363128
34. Chung HL, Hwang JB, Kwon YD, Park MH, Shin WJ, Park JB. Deposition of eosinophil-granule major basic protein and expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in the mucosa of the small intestine in infants with cow’s milk-sensitive enteropathy. J Allergy Clin Immunol. 1999;103(6):1195–1201. doi:10.1016/s0091-6749(99)70199-5
35. Powell GK, McDonald PJ, Van Sickle GJ, Goldblum RM. Absorption of food protein antigen in infants with food protein-induced enterocolitis. Dig Dis Sci. 1989;34(5):781–788. doi:10.1007/bf01540354
36. Kimura M, Ito Y, Shimomura M, et al. Cytokine profile after oral food challenge in infants with food protein-induced enterocolitis syndrome. Allergol Int. 2017;66(3):452–457. doi:10.1016/j.alit.2016.12.001
37. Caubet JC, Szajewska H, Shamir R, Nowak-Węgrzyn A. Non-IgE-mediated gastrointestinal food allergies in children. Pediatr Allergy Immunol. 2017;28(1):6–17. doi:10.1111/pai.12659
38. Goswami R, Blazquez AB, Kosoy R, Rahman A, Nowak-Węgrzyn A, Berin MC. Systemic innate immune activation in food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2017;139(6):1885–1896.e9. doi:10.1016/j.jaci.2016.12.971
39. Mehr S, Lee E, Hsu P, et al. Innate immune activation occurs in acute food protein-induced enterocolitis syndrome reactions. J Allergy Clin Immunol. 2019;144(2):600–602.e2. doi:10.1016/j.jaci.2019.04.021
40. Berin MC. Advances in understanding immune mechanisms of food protein-induced enterocolitis syndrome. Ann Allergy Asthma Immunol. 2021;126(5):478–481. doi:10.1016/j.anai.2021.01.033
41. Mori F, Barni S, Cianferoni A, Pucci N, de Martino M, Novembre E. Cytokine expression in CD3+ cells in an infant with food protein-induced enterocolitis syndrome (FPIES): case report. Clin Dev Immunol. 2009;2009:679381. doi:10.1155/2009/679381
42. Morita H, Nomura I, Orihara K, et al. Antigen-specific T-cell responses in patients with non-IgE-mediated gastrointestinal food allergy are predominantly skewed to T(H)2. J Allergy Clin Immunol. 2013;131(2):
43. Adel-Patient K, Lezmi G, Castelli FA, et al. Deep analysis of immune response and metabolic signature in children with food protein induced enterocolitis to cow’s milk. Clin Transl Allergy. 2018;8:38. doi:10.1186/s13601-018-0224-9
44. Caubet JC, Bencharitiwong R, Ross A, Sampson HA, Berin MC, Nowak-Węgrzyn A. Humoral and cellular responses to casein in patients with food protein-induced enterocolitis to cow’s milk. J Allergy Clin Immunol. 2017;139(2):572–583. doi:10.1016/j.jaci.2016.02.047
45. Berin MC, Lozano-Ojalvo D, Agashe C, Baker MG, Bird JA, Nowak-Wegrzyn A. Acute FPIES reactions are associated with an IL-17 inflammatory signature. J Allergy Clin Immunol. 2021;148(3):895–901.e6. doi:10.1016/j.jaci.2021.04.012
46. Savilahti E. Immunochemical study of the malabsorption syndrome with cow’s milk intolerance. Gut. 1973;14(6):491–501. doi:10.1136/gut.14.6.491
47. Konstantinou GN, Bencharitiwong R, Grishin A, et al. The role of casein-specific IgA and TGF-β in children with food protein-induced enterocolitis syndrome to milk. Pediatr Allergy Immunol. 2014;25(7):651–656. doi:10.1111/pai.12288
48. Shek LP, Bardina L, Castro R, Sampson HA, Beyer K. Humoral and cellular responses to cow milk proteins in patients with milk-induced IgE-mediated and non-IgE-mediated disorders. Allergy. 2005;60(7):912–919. doi:10.1111/j.1398-9995.2005.00705.x
49. Holbrook T, Keet CA, Frischmeyer-Guerrerio PA, Wood RA. Use of ondansetron for food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2013;132(5):1219–1220. doi:10.1016/j.jaci.2013.06.021
50. Lake AM, Whitington PF, Hamilton SR. Dietary protein-induced colitis in breast-fed infants. J Pediatr. 1982;101(6):906–910. doi:10.1016/s0022-3476(82)80008-5
51. Xanthakos SA, Schwimmer JB, Melin-Aldana H, Rothenberg ME, Witte DP, Cohen MB. Prevalence and outcome of allergic colitis in healthy infants with rectal bleeding: a prospective cohort study. J Pediatr Gastroenterol Nutr. 2005;41(1):16–22. doi:10.1097/01.mpg.0000161039.96200.f1
52. Arvola T, Ruuska T, Keränen J, Hyöty H, Salminen S, Isolauri E. Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics. 2006;117(4):e760–8. doi:10.1542/peds.2005-1069
53. Martin VM, Virkud YV, Seay H, et al. Prospective assessment of pediatrician-diagnosed food protein-induced allergic proctocolitis by gross or occult blood. J Allergy Clin Immunol Pract. 2020;8(5):1692–1699.e1. doi:10.1016/j.jaip.2019.12.029
54. Martin VM, Virkud YV, Phadke NA, et al. Increased IgE-mediated food allergy with food protein-induced allergic proctocolitis. Pediatrics. 2020;146(3). doi:10.1542/peds.2020-0202
55. Ozen A, Gulcan EM, Ercan Saricoban H, Ozkan F, Cengizlier R. Food protein-induced non-immunoglobulin E-mediated allergic colitis in infants and older children: what cytokines are involved? Int Arch Allergy Immunol. 2015;168(1):61–68. doi:10.1159/000441471
56. Nowak-Węgrzyn A. Food protein-induced enterocolitis syndrome and allergic proctocolitis. Allergy Asthma Proc. 2015;36(3):172–184. doi:10.2500/aap.2015.36.3811
57. Machida HM, Catto smith AG, Gall DG, Trevenen C, Scott RB. Allergic colitis in infancy: clinical and pathologic aspects. J Pediatr Gastroenterol Nutr. 1994;19(1):22–26. doi:10.1097/00005176-199407000-00004
58. Meyer R, Chebar Lozinsky A, Fleischer DM, et al. Diagnosis and management of Non-IgE gastrointestinal allergies in breastfed infants-An EAACI position paper. Allergy. 2020;75(1):14–32. doi:10.1111/all.13947
59. Miceli sopo S, Monaco S, Bersani G, Romano A, Fantacci C. Proposal for management of the infant with suspected food protein-induced allergic proctocolitis. Pediatr Allergy Immunol. 2018;29(2):215–218. doi:10.1111/pai.12844
60. Kuitunen P, Visakorpi JK, Savilahti E, Pelkonen P. Malabsorption syndrome with cow’s milk intolerance. Clinical findings and course in 54 cases. Arch Dis Child. 1975;50(5):351–356. doi:10.1136/adc.50.5.351
61. Savilahti E. Food-induced malabsorption syndromes. J Pediatr Gastroenterol Nutr. 2000;30(Suppl):S61–6. doi:10.1097/00005176-200001001-00010
62. Iyngkaran N, Abdin Z, Davis K, et al. Acquired carbohydrate intolerance and cow milk protein-sensitive enteropathy in young infants. J Pediatr. 1979;95(3):373–378. doi:10.1016/s0022-3476(79)80509-0
63. Iyngkaran N, Yadav M, Boey CG, Lam KL. Severity and extent of upper small bowel mucosal damage in cow’s milk protein-sensitive enteropathy. J Pediatr Gastroenterol Nutr. 1988;7(5):667–674. doi:10.1097/00005176-198809000-00008
64. Carroccio A, Mansueto P, D’Alcamo A, Iacono G. Non-celiac wheat sensitivity as an allergic condition: personal experience and narrative review. Am J Gastroenterol. 2013;108(12):
65. Fritscher-Ravens A, Schuppan D, Ellrichmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2014;147(5):1012–20.e4. doi:10.1053/j.gastro.2014.07.046
66. Molnár K, Pintér P, Győrffy H, et al. Characteristics of allergic colitis in breast-fed infants in the absence of cow’s milk allergy. World j Gastroenterol. 2013;19(24):3824–3830. doi:10.3748/wjg.v19.i24.3824
67. Beyer K, Castro R, Birnbaum A, Benkov K, Pittman N, Sampson HA. Human milk-specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. J Allergy Clin Immunol. 2002;109(4):707–713. doi:10.1067/mai.2002.122503
68. Kokkonen J, Haapalahti M, Laurila K, Karttunen TJ, Mäki M. Cow’s milk protein-sensitive enteropathy at school age. J Pediatr. 2001;139(6):797–803. doi:10.1067/mpd.2001.118882
69. Augustin MT, Kokkonen J, Karttunen TJ. Duodenal cytotoxic lymphocytes in cow’s milk protein sensitive enteropathy and coeliac disease. Scand J Gastroenterol. 2005;40(12):1398–1406. doi:10.1080/00365520510023765
70. Paajanen L, Vaarala O, Karttunen R, Tuure T, Korpela R, Kokkonen J. Increased IFN-gamma secretion from duodenal biopsy samples in delayed-type cow’s milk allergy. Pediatr Allergy Immunol. 2005;16(5):439–444. doi:10.1111/j.1399-3038.2005.00312.x
71. Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):
72. Chehade M, Jones SM, Pesek RD, et al. Phenotypic characterization of eosinophilic esophagitis in a large multicenter patient population from the consortium for food allergy research. J Allergy Clin Immunol Pract. 2018;6(5):1534–1544.e5. doi:10.1016/j.jaip.2018.05.038
73. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004;113(1):
74. O’Shea KM, Aceves SS, Dellon ES, et al. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology. 2018;154(2):333–345. doi:10.1053/j.gastro.2017.06.065
75. Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):201–218. doi:10.1016/j.gtc.2014.02.002
76. Spergel JM, Book WM, Mays E, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. 2011;52(3):300–306. doi:10.1097/MPG.0b013e3181eb5a9f
77. Jensen ET, Martin CF, Kappelman MD, Dellon ES. Prevalence of eosinophilic gastritis, gastroenteritis, and colitis: estimates from a national administrative database. J Pediatr Gastroenterol Nutr. 2016;62(1):36–42. doi:10.1097/mpg.0000000000000865
78. Rochman M, Azouz NP, Rothenberg ME. Epithelial origin of eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142(1):10–23. doi:10.1016/j.jaci.2018.05.008
79. Cianferoni A, Jensen E, Davis CM. The role of the environment in eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2021;9(9):3268–3274. doi:10.1016/j.jaip.2021.07.032
80. Kottyan LC, Parameswaran S, Weirauch MT, Rothenberg ME, Martin LJ. The genetic etiology of eosinophilic esophagitis. J Allergy Clin Immunol. 2020;145(1):9–15. doi:10.1016/j.jaci.2019.11.013
81. Kottyan LC, Davis BP, Sherrill JD, et al. Genome-wide association analysis of eosinophilic esophagitis provides insight into the tissue specificity of this allergic disease. Nat Genet. 2014;46(8):895–900. doi:10.1038/ng.3033
82. Sleiman PM, Wang ML, Cianferoni A, et al. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun. 2014;5:5593. doi:10.1038/ncomms6593
83. Azouz NP, Ynga-Durand MA, Caldwell JM, et al. The antiprotease SPINK7 serves as an inhibitory checkpoint for esophageal epithelial inflammatory responses. Sci Transl Med. 2018;10(444). doi:10.1126/scitranslmed.aap9736
84. Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168(5):2464–2469. doi:10.4049/jimmunol.168.5.2464
85. Mishra A, Wang M, Pemmaraju VR, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134(1):204–214. doi:10.1053/j.gastro.2007.10.002
86. Hogan SP, Mishra A, Brandt EB, Foster PS, Rothenberg ME. A critical role for eotaxin in experimental oral antigen-induced eosinophilic gastrointestinal allergy. Proc Natl Acad Sci U S A. 2000;97(12):6681–6686. doi:10.1073/pnas.97.12.6681
87. Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103(12):1719–1727. doi:10.1172/jci6560
88. Frischmeyer-Guerrerio PA, Guerrerio AL, Chichester KL, et al. Dendritic cell and T cell responses in children with food allergy. Clin Exp Allergy. 2011;41(1):61–71. doi:10.1111/j.1365-2222.2010.03606.x
89. Zuo L, Fulkerson PC, Finkelman FD, et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13R alpha 2-inhibited pathway. J Immunol. 2010;185(1):660–669. doi:10.4049/jimmunol.1000471
90. Huang JJ, Joh JW, Fuentebella J, et al. Eotaxin and FGF enhance signaling through an extracellular signal-related kinase (ERK)-dependent pathway in the pathogenesis of Eosinophilic esophagitis. Allergy Asthma Clin Immunol. 2010;6(1):25. doi:10.1186/1710-1492-6-25
91. Davis BP, Stucke EM, Khorki ME, et al. Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight. 2016;1(4):e86355. doi:10.1172/jci.insight.86355
92. Litosh VA, Rochman M, Rymer JK, Porollo A, Kottyan LC, Rothenberg ME. Calpain-14 and its association with eosinophilic esophagitis. J Allergy Clin Immunol. 2017;139(6):1762–1771.e7. doi:10.1016/j.jaci.2016.09.027
93. Sherrill JD, Kc K, Wu D, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2014;7(3):718–729. doi:10.1038/mi.2013.90
94. Wen T, Aronow BJ, Rochman Y, et al. Single-cell RNA sequencing identifies inflammatory tissue T cells in eosinophilic esophagitis. J Clin Invest. 2019;129(5):2014–2028. doi:10.1172/jci125917
95. Nhu QM, Aceves SS. Tissue remodeling in chronic eosinophilic esophageal inflammation: parallels in asthma and therapeutic perspectives. Front Med. 2017;4:128. doi:10.3389/fmed.2017.00128
96. Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119(1):206–212. doi:10.1016/j.jaci.2006.10.016
97. Chehade M, Sampson HA, Morotti RA, Magid MS. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45(3):319–328. doi:10.1097/MPG.0b013e31806ab384
98. Tkachenko E, Rawson R, La E, et al. Rigid substrate induces esophageal smooth muscle hypertrophy and eosinophilic esophagitis fibrotic gene expression. J Allergy Clin Immunol. 2016;137(4):1270–1272.e1. doi:10.1016/j.jaci.2015.09.020
99. Prussin C, Lee J, Foster B. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5(-) T(H)2 responses. J Allergy Clin Immunol. 2009;124(6):1326–32.e6. doi:10.1016/j.jaci.2009.09.048
100. Caldwell JM, Collins MH, Stucke EM, et al. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome. J Allergy Clin Immunol. 2014;134(5):1114–1124. doi:10.1016/j.jaci.2014.07.026
101. Ashitani K, Tsuzuki Y, Yamaoka M, et al. Endoscopic features and diagnostic procedures of eosinophilic gastroenteritis. Internal Med. 2019;58(15):2167–2171. doi:10.2169/internalmedicine.2298-18
102. Desreumaux P, Bloget F, Seguy D, et al. Interleukin 3, granulocyte-macrophage colony-stimulating factor, and interleukin 5 in eosinophilic gastroenteritis. Gastroenterology. 1996;110(3):768–774. doi:10.1053/gast.1996.v110.pm8608886
103. Pesek RD, Rothenberg ME. Eosinophilic gastrointestinal disease below the belt. J Allergy Clin Immunol. 2020;145(1):87–89.e1. doi:10.1016/j.jaci.2019.10.013
104. Pesek RD, Greuter T, Lopez-Nunez O, Bernieh A, Straumann A, Collins MH. Clinicopathologic correlations in eosinophilic gastrointestinal disorders. J Allergy Clin Immunol Pract. 2021;9(9):3258–3266. doi:10.1016/j.jaip.2021.06.002
105. Hirano I, Chan ES, Rank MA, et al. AGA institute and the joint task force on allergy-immunology practice parameters clinical guidelines for the management of eosinophilic esophagitis. Gastroenterology. 2020;158(6):1776–1786. doi:10.1053/j.gastro.2020.02.038
106. Chehade M, Aceves SS. Treatment of eosinophilic esophagitis: diet or medication? J Allergy Clin Immunol Pract. 2021;9(9):3249–3256. doi:10.1016/j.jaip.2021.07.029
107. Gonsalves NP, Aceves SS. Diagnosis and treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2020;145(1):1–7. doi:10.1016/j.jaci.2019.11.011
108. Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4(9):1097–1102. doi:10.1016/j.cgh.2006.05.026
109. McGowan EC, Platts-Mills TA. Eosinophilic esophagitis from an allergy perspective: how to optimally pursue allergy testing & dietary modification in the adult population. Curr Gastroenterol Rep. 2016;18(11):58. doi:10.1007/s11894-016-0531-z
110. Groetch M, Venter C, Skypala I, et al. Dietary therapy and nutrition management of eosinophilic esophagitis: a Work Group Report of the American Academy of Allergy, Asthma, and Immunology. J Allergy Clin Immunol Pract. 2017;5(2):312–324.e29. doi:10.1016/j.jaip.2016.12.026
111. Dellon ES, Peterson KA, Murray JA, et al. Anti-siglec-8 antibody for eosinophilic gastritis and duodenitis. N Engl J Med. 2020;383(17):1624–1634. doi:10.1056/NEJMoa2012047
112. Greuter T, Hirano I, Dellon ES. Emerging therapies for eosinophilic esophagitis. J Allergy Clin Immunol. 2020;145(1):38–45. doi:10.1016/j.jaci.2019.10.027
113. Hirano I, Dellon ES, Hamilton JD, et al. Efficacy of dupilumab in a Phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology. 2020;158(1):111–122.e10. doi:10.1053/j.gastro.2019.09.042
114. Clayton F, Fang JC, Gleich GJ, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147(3):602–609. doi:10.1053/j.gastro.2014.05.036
115. Zhang S, Assa’ad AH. Biologics in eosinophilic esophagitis. Curr Opin Allergy Clin Immunol. 2021;21(3):292–296. doi:10.1097/aci.0000000000000741
116. Rothenberg ME, Wen T, Greenberg A, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;135(2):500–507. doi:10.1016/j.jaci.2014.07.049
117. Hirano I, Collins MH, Assouline-Dayan Y, et al. RPC4046, a monoclonal antibody against IL13, reduces histologic and endoscopic activity in patients with eosinophilic esophagitis. Gastroenterology. 2019;156(3):592–603.e10. doi:10.1053/j.gastro.2018.10.051
118. Gann PH, Deaton RJ, McMahon N, et al. An anti-IL-13 antibody reverses epithelial-mesenchymal transition biomarkers in eosinophilic esophagitis: phase 2 trial results. J Allergy Clin Immunol. 2020;146(2):367–376.e3. doi:10.1016/j.jaci.2020.03.045
119. Choudhury S, Baker S. Eosinophilic esophagitis: the potential role of biologics in its treatment. Clin Rev Allergy Immunol. 2020;59(2):150–159. doi:10.1007/s12016-019-08749-6
120. Straumann A, Bussmann C, Conus S, Beglinger C, Simon HU. Anti-TNF-alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J Allergy Clin Immunol. 2008;122(2):425–427. doi:10.1016/j.jaci.2008.06.012
121. Straumann A, Hoesli S, Bussmann C, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68(3):375–385. doi:10.1111/all.12096
122. Loizou D, Enav B, Komlodi-Pasztor E, et al. A pilot study of omalizumab in eosinophilic esophagitis. PLoS One. 2015;10(3):e0113483. doi:10.1371/journal.pone.0113483
123. Rocha R, Vitor AB, Trindade E, et al. Omalizumab in the treatment of eosinophilic esophagitis and food allergy. Eur J Pediatr. 2011;170(11):1471–1474. doi:10.1007/s00431-011-1540-4
124. Berin MC. Immunopathophysiology of food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2015;135(5):1108–1113. doi:10.1016/j.jaci.2014.12.1948
125. Kelly KJ. Eosinophilic gastroenteritis. J Pediatr Gastroenterol Nutr. 2000;30(Suppl):S28–35. doi:10.1097/00005176-200001001-00005
126. Kuang FL, Legrand F, Makiya M, et al. Benralizumab for PDGFRA-negative hypereosinophilic syndrome. N Engl J Med. 2019;380(14):1336–1346. doi:10.1056/NEJMoa1812185
127. Stein ML, Collins MH, Villanueva JM, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118(6):1312–1319. doi:10.1016/j.jaci.2006.09.007
128. Assa’ad AH, Gupta SK, Collins MH, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141(5):1593–1604. doi:10.1053/j.gastro.2011.07.044
129. Straumann A, Conus S, Grzonka P, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59(1):21–30. doi:10.1136/gut.2009.178558
130. Spergel JM, Rothenberg ME, Collins MH, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(2):
131. Markowitz JE, Jobe L, Miller M, Frost C, Laney Z, Eke R. Safety and efficacy of reslizumab for children and adolescents with eosinophilic esophagitis treated for 9 years. J Pediatr Gastroenterol Nutr. 2018;66(6):893–897. doi:10.1097/mpg.0000000000001840
132. Fuchs GJ
133. Dellon ES, Collins MH, Rothenberg ME, et al. Long-term efficacy and tolerability of RPC4046 in an open-label extension trial of patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2021;19(3):473–483.e17. doi:10.1016/j.cgh.2020.03.036
134. Nhu QM, Chiao H, Moawad FJ, Bao F, Konijeti GG. The anti-α4β7 integrin therapeutic antibody for inflammatory bowel disease, vedolizumab, ameliorates eosinophilic esophagitis: a novel clinical observation. Am J Gastroenterol. 2018;113(8):1261–1263. doi:10.1038/s41395-018-0145-1
135. Taft TH, Mutlu EA. The potential role of vedolizumab in concomitant eosinophilic esophagitis and crohn’s disease. Clin Gastroenterol Hepatol. 2018;16(11):1840–1841. doi:10.1016/j.cgh.2018.06.022
136. Kim HP, Reed CC, Herfarth HH, Dellon ES. Vedolizumab treatment may reduce steroid burden and improve histology in patients with eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2018;16(12):1992–1994. doi:10.1016/j.cgh.2018.03.024
137. Mendoza Alvarez LB, Liu X, Glover S. Treatment-resistant eosinophilic oesophagitis successfully managed with tofacitinib. BMJ Case Rep. 2019;12(12):e232558. doi:10.1136/bcr-2019-232558
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
