Back to Journals » Infection and Drug Resistance » Volume 11
Pathogens in the Meibomian gland and conjunctival sac: microbiome of normal subjects and patients with Meibomian gland dysfunction
Authors Jiang X, Deng A, Yang J, Bai H, Yang Z, Wu J, Lv H, Li X , Wen T
Received 10 January 2018
Accepted for publication 5 June 2018
Published 11 October 2018 Volume 2018:11 Pages 1729—1740
DOI https://doi.org/10.2147/IDR.S162135
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xiaodan Jiang,1,2,* Aihua Deng,3,* Jiarui Yang,1,2,* Hua Bai,3 Zhao Yang,3 Jie Wu,3 Huibin Lv,1,2 Xuemin Li,1,2 Tingyi Wen3,4
1Department of Ophthalmology, Peking University Third Hospital, Beijing 100191, China; 2Beijing Key Laboratory of Restoration of Damaged Ocular Nerve, Peking University Third Hospital, Beijing 100191, China; 3CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China; 4Savaid Medical School, University of Chinese Academy of Sciences, Beijing 100049, China
*These authors contributed equally to this work
Objective: To explore the composition of the ocular microbiome in normal subjects and patients with Meibomian gland dysfunction (MGD).
Subjects and methods: Seventy subjects (140 eyes) were enrolled in our study. Signs of dry eye were evaluated and bacterial species in the conjunctival sac (CS) and Meibomian gland (MG) secretions were then identified by 16S rRNA gene sequencing. Additionally, 17 subjects (34 eyes) were further evaluated to determine differences in the microbiomes in the surface and deep layers of MG using a segmental secretion analysis.
Results: The positive bacterial isolation rate was markedly higher in MG secretions than in the CS. The bacterial composition of the control and mild group was simple, whereas the composition of bacteria was more complex as the severity of MGD increased. The positive bacterial isolation rate and number of bacterial types were significantly higher in the severe MGD group than those in the control, mild and moderate MGD groups. Corynebacterium macginleyi was only detected in the severe MGD group, with an isolation rate of up to 26.3%. Furthermore, a new grading system for bacterial severity of MGD was proposed and the severity of MGD appeared to be positively correlated with a higher grade of bacterial severity. The segmental secretion analysis showed severe MGD had a significantly higher incidence of bacterial discordance rate.
Conclusion: The severity of MGD was positively correlated with a higher isolation rate, a greater number of bacterial species, and a higher grade of bacterial severity, which implied that MGD might be correlated with bacterial changes. This study provided some basis for the indications of antibiotic in clinical practice.
Keywords: Meibomian gland dysfunction, microbiome, pathogens, bacterial flora, 16S rRNA, scanning electron microscopy
Introduction
Dry eye is an ocular disease characterized by ocular discomfort due to an abnormal quantity or quality of tear film, which causes pathological changes in the ocular surface.1 The prevalence of dry eye has increased in recent years, and the global prevalence ranges from 6.8% to 35.0% depending on ages and areas.2,3 There are two major kinds of dry eye, aqueous deficiency dry eye and evaporative dry eye. Evaporative dry eye includes Meibomian gland dysfunction (MGD) and other genetic diseases caused less mature Meibomian glands (MGs) such as Eda-deficient disease.4 MGD is the major form of evaporative dry eye, and, therefore, a large amount of research has been undertaken to better understand the pathogenesis of MGD and appropriate treatment methods.1
A number of eye diseases have been reported to be related to ocular bacterial flora. Ocular microbes were reported to contribute to the occurrence of endophthalmitis after cataract surgery in early studies.5 Recently, an increasing number of studies have focused on the relationship between bacterial flora and dry eye due to the frequent occurrence of various infectious diseases in cases of dry eye, such as anterior blepharitis,6 MG disease,7 and keratitis.8 In healthy people, the ocular surface usually only contains commensal bacteria, and one of the most common genus is coagulase-negative Staphylococcus.9 However, a broad range of bacteria, such as Staphylococcus aureus and Klebsiella spp., have been identified on the ocular surface of patients with the infectious diseases mentioned above.10,11
Based on recent studies of ocular bacteria, antibiotics have been used as a treatment method for MGD; however, the precise pathologies of MGD remain largely unknown. Thus, the development of a more specific and feasible treatment method has been difficult. Currently, topical azithromycin alone or in combination with oral doxycycline has been reported to relieve the signs and symptoms of dry eye.12,13 However, the specific indications for the use of antibiotics to treat MGD in clinical practice remain poorly defined.
In previous studies, specimens of bacteria isolated from the ocular surface were primarily obtained from bacterial swabs of the conjunctival sac (CS) and eyelid margins,14–16 which may only represent a portion of the bacterial flora of the ocular surface. Few studies have explored the bacteria found in MG secretions.17,18 Therefore, the relationship between the bacterial flora found in MG secretions and the signs and symptoms of MGD remain unclear, and no study has yet established an appropriate method for defining the complete microbiome of the ocular surface. In this study, the microbiomes of the CS and MG from patients with MGD were investigated to establish a grading system for bacterial severity, which might provide a basis for clinical treatment of MGD.
Subjects and methods
This study was conducted according to the principles of the Declaration of Helsinki and was approved by the Human Research and Ethics Committee of Peking University Third Hospital. Written informed consent in Chinese was obtained from each participant (and a parent if the patient was under the age of 18) before enrollment.
Subjects
MGD patients were recruited from the outpatient department of the Department of Ophthalmology at Peking University Third Hospital consecutively between December 2015 and October 2017 and control volunteers were recruited by advertisement between December 2015 and December 2017. The inclusion criteria for the MGD were as follows: 1) adult patients with a chief complaint of one of the following symptoms: dryness, foreign body sensation, burning, or tearing for more than 6 months; 2) a diagnosis of MGD with two or more of the following signs in both eyes: redness or thickening of the lid margin, telangiectasia, reduced or no secretions, poor quality secretions, and gland capping;19 and 3) a willingness to cooperate with the doctors during the examination procedure. Control volunteers consisted of 29 (58 eyes) subjects recruited by advertisement from the Physical Examination Center of Peking University Third Hospital. The inclusion criteria for the controls were as follows: 1) no chief complaint of any dry eye symptoms or tear break up time (TBUT) was equal to or longer than 5 seconds without obvious dry eye symptoms; 2) MG associated assessments were not able to meet the criteria for the diagnosis of MGD; 3) corneal staining was negative; 4) a willingness to cooperate with the doctors during the examination procedure. Exclusion criteria of MGD patients and healthy controls included the following: 1) patients who used of any type of antibiotic drops or systemic antibiotics within the past month; 2) patients who currently use treatments for dry eye or MGD (other than artificial tears); 3) patients with active allergies, infections, or inflammatory diseases of the ocular surface unrelated to dry eye or MGD; 4) a history of ocular trauma or surgery within one year; 5) patients with alterations in the lacrimal drainage system such as punctal occlusion; 6) patients who used systemic medications that might alter the tear film; 7) patients who used contact lenses within the past month; 8) patients with systemic diseases affecting the ocular surface; and 9) pregnant or nursing mothers. Forty-one patients (17 males, 24 females) with MGD (82 eyes) were enrolled in this study. The mean ± SD age of MGD patients was 34.3±10.8 years (ranged from 17 to 60). In the control group, 13 were males and 16 were females, with a mean ± SD age of 31.8±8.7 years (ranged from 21 to 55).
Clinical evaluation
The clinical assessments of the enrolled subjects were conducted in the following order: collection of demographic information (including age and sex) and physical signs including conjunctival injection, upper and lower tear meniscus height (TMH),20,21 TBUT,1 corneal staining,22,23 lid margin,19 orifice, tear foam and MG assessments (detailed methodology is shown in Supplementary material).24 An interval of 5 minutes was required between the different examinations. All examinations were conducted and the data collected by two doctors (JXD and YJR), and the average values are presented as the final results.
Sample collection
Samples were collected in a sterile operation room by the same ophthalmologist (JXD) who was wearing sterile gloves and mask. Sterile transport swabs were used to collect bacterial samples. Sample collection was performed in the order described below. 1) The lower CS was swabbed at two different positions, and the swabs were swirled to obtain a specimen on each side of the swab. Care was taken to avoid contact with the eyelids and lashes when taking swabs from the CS. 2) After a drop of repivacaine hydrochloride (Benoxil; Santen Pharmaceutical Co., Ltd, Osaka, Japan) was applied to both eyes as topical anesthesia, each CS was successively irrigated with 0.9% medical saline, 0.025% iodophor and 0.9% medical saline for 1 minute. The eyelid margins (including the roots of the eyelashes) were sterilized using entoiodine. 3) A Meibomian massage was performed using a sterile eyelid plate in the upper eyelid to collect the MG secretions. A new sterile eyelid plate was used for the lower eyelid, after which the same procedure was repeated for the other eye. Segmental secretions were also collected from several patients using the same procedure described for the collection of normal MG secretions. The irrigation and sterilization procedures were performed again before the next MG secretion was collected. Additionally, sterile swabs were exposed in the air of the operation room for 10 seconds each time the sample was collected as blank control.
Strain isolation and 16S rRNA gene sequencing
After collecting the MG secretions, samples were stored at 4°C, and bacterial strains were isolated within 2 hours. The samples were plated onto agar plates containing 10 g of tryptone, 3 g of beef powder, 5 g of NaCl, 50 mL of defibrinated sheep blood, and 15 g of agar in 1 L of medium. After a 24–72-hour incubation at 37°C, colonies with different phenotypes were selected for further analysis. Bacterial morphologies were observed using an optical microscope (Olympus Corporation, Tokyo, Japan) and a high intensity focused ion beam/scanning electron microscopy (FIB/SEM, Zeiss Auriga Compact, Carl Zeiss Meditec AG, Jena, Germany). Genomic DNA was extracted from the isolated strains, and 16S rRNA genes were amplified using a previously described method.25 The PCR products were purified and sequenced using an ABI3730 genetic analyzer (Beijing Genomics Institute, Beijing, China). After the products were assessed for chimera formation using the Bellerophon server,26 and the 16S rRNA gene sequences were compared with the GenBank database to search for related sequences using the BLAST program. Based on the 16S rRNA gene sequences, the phylogenetic tree of the isolated strains and their closest species were constructed with the maximum-likelihood method using MEGA5.27 The bacterial culture of the blank controls showed no positive results.
Statistical analysis
ANOVA was used to compare the basic characteristics of patients with MGD and the number of isolated bacterial species of different severities. Fisher’s least significant difference post hoc test was used to compare patients with moderate or severe MGD with mild MGD. Eyelid margin characteristics and the correlations between the severity of MGD and specific bacteria were analyzed using the chi-squared test. Comparisons of specific bacterial isolation rates between the CS and MG were conducted using McNemar’s test. Segmental secretion analysis was performed using Student’s t-test. P<0.05 were considered statistically significant.
Results
Basic characteristics
Forty-one patients (17 males, 24 females) with MGD (82 eyes) were enrolled in this study; the mean ± SD age of MGD patients was 34.3±10.8 years. Twenty-nine healthy volunteers (13 males, 16 females) were enrolled in the control group with a mean ± SD age of 31.8±8.7 years (ranged from 21 to 55). The age and sex were matched between MGD and control (P=0.305, P=0.779, respectively). Of these 82 eyes with MGD, eight were subcategorized into the mild MGD group, 55 into the moderate MGD group and 19 into the severe MGD group. All patients completed the information collection process and examinations, and the results were shown in Table 1.
Analysis of the microbiomes of the CS and MG
The microbiomes isolated from CS and MG secretions are shown in Table 2. In the MG, S. epidermidis was the most frequently isolated bacterium and was observed in 46.4% of all eyes. By contrast, no bacteria were isolated from the CS of most patients (80.7%), and S. epidermidis was only detected in 10.7% of patients. In both the CS and MG, most of the isolated bacteria were Gram-positive, such as Staphylococcus, Corynebacterium and Microbacteriaceae. However, additional types of bacteria, including Gram-positive bacteria such as Bacillus, Paenibacillus and Lysinibacillus, were only isolated from the MG, indicating that the bacterial constituents of the MG were more complex than the bacterial constituents of the CS. The Gram-negative bacterium Moraxella osloensis was detected in the CS in three eyes, and Xanthomonadaceae was detected in the MG secretions from one eye (Figure 1). Based on these results, a greater number of bacteria with a more complex composition were isolated from MG secretions.
Compositions of the microbiomes of the mild, moderate, severe MGD groups and healthy controls
The relationships between MGD severity and bacteria isolated from MG secretions and between MGD severity and all isolated bacteria (from MG secretions and the CS) were separately analyzed. According to the results obtained from both analyses, a significantly greater number of types of bacteria was detected in the severe and moderate MGD groups than in the control and mild MGD group (P<0.05), and all the bacteria were used as an illustration (Table 3). Only the Gram-positive bacteria Staphylococcus and Lysinibacillus were isolated from the control and mild MGD group.
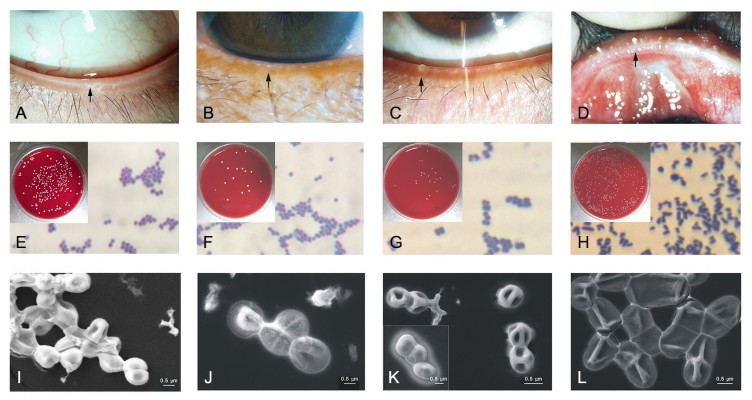
As shown in the Figure 2, S. epidermidis was isolated from eyes of the healthy control and mild MGD. As observed by optical microscopy and scanning electron microscopy, they showed similar morphology, such as round and agglomerated shape (Figure 2E, F, I and J), which is in accordance with the previous report.28
However, many types of bacteria, including the Gram-positive bacteria Staphylococcus (Figure 2C, J, K), Lysinibacillus, Microbacteriaceae and Bacillus, and the Gram-negative bacteria Moraxella osloensis and Xanthomonadaceae, were isolated from the control and moderate MGD group. Figure 2G and K show the morphology of S. aureus and S. hominis from eyes of moderate MGD. They were round, agglomerated and Gram-positive under optical microscopy, similar to S. epidermidis. Interestingly, the surfaces of S. hominis have holes and were clearly different to those of S. epidermidis and S. aureus.
With the exception of the bacteria isolated from the control, mild and moderate MGD groups, other Gram-positive bacteria, including Corynebacterium and Paenibacillus, were identified in the severe MGD group. To our knowledge C. macginleyi has been isolated from the ocular site in the previous study.29 C. macginleyi from eyes of severe MGD was rod-shaped and dispersed by optical microscopy and scanning electron microscopy (Figure 2H and L), which was significant different from microorganisms isolated from eyes of the healthy control, mild and moderate MGD. The incidence of C. macginleyi (Figure 2D, H and L) was significantly higher in the severe MGD group than in the control and moderate MGD groups (P<0.001 and P<0.001, respectively). The incidence of Lysinibacillus (G+) showed a significant difference among the mild, moderate and severe MGD groups (P=0.001). Thus, the composition of the microbiome in the control and the mild MGD group was simple, whereas it was more abundant and complex as the severity of MGD increased (Figure 3).
Correlation between the microbiome composition and MGD severity
The characteristics of the isolated microbiome in groups with different severities of MGD were investigated to further explore the relationship between the microbiome composition and MGD severity (Table 4; Figure 2). Because S. epidermidis has been reported to be the most frequently isolated bacteria in the normal flora,9 the types of isolated bacteria and their isolation rates were analyzed with or without the inclusion of S. epidermidis. With the inclusion of S. epidermidis, the positive isolation rate of bacteria in the severe MGD group was 89.5%, which was significantly higher than the isolation rate observed in the control (46.6%) and moderate MGD group (58.2%) (P=0.001 and P=0.006, respectively). In addition, more types of bacteria were isolated from the severe MGD group than in the control (P<0.001) and moderate MGD group (P=0.021). Although a higher positive bacterial isolation rate and more types of bacteria were identified in the severe MGD group than in the mild MGD group, the differences were not significant (P=0.099 and P=0.065, respectively). However, when the data were analyzed without S. epidermidis, the positive bacterial isolation rate was significantly higher for the severe group than that among the control (P<0.001), mild MGD groups (P=0.033), and moderate (P=0.013). Moreover, the types of isolated bacteria were also significantly more complex in the severe MGD group than those among the control (P<0.001) and the mild (P=0.007), and moderate MGD groups (P=0.006). Therefore, MGD severity was strongly correlated with an altered ocular microbiome that was specifically composed of non-S. epidermidis bacteria, such as C. macginleyi.
Relationship between commensalism and MGD severity
All of the eyes analyzed in this study were classified as grade 1 (no isolated bacteria), grade 2 (only S. epidermidis), grade 3 (S. epidermidis and other bacteria) and grade 4 (only other bacteria) to explore the relationship between bacterial commensalism and MGD severity. The overall P-value obtained from the chi-squared test was 0.004, and subgroup analyses were performed to explore the detailed differences. First, differences between MGD groups were explored (Table 5A). A higher incidence of grades 3 and 4 was observed in the moderate (18.2%, 7.3%) and severe MGD groups (31.6%, 31.6%) than in the control (12.1%, 3.4%) and mild MGD group (12.5%, 0%), and the difference between the control and severe group was significant (P<0.001). A significantly higher incidence of grades 3 and 4 was also observed in the severe MGD group than in the moderate MGD group (P=0.009). Second, differences between different commensalism grades were analyzed (Table 5B). We observed a significantly higher incidence of severe MGD and a lower incidence of control in grade four patients than those in grade 1. And a significant difference between grade 4 and grade 1, grade 3 and grade 1 (P<0.001, P=0.018, respectively), between grade 4 and grade 2 (P=0.018) was observed. Based on these results, higher commensalism grades are related to the severity of the MGD.
Analysis of segmental MG secretions
Seventeen patients (34 eyes), including 12 eyes as control, 12 eyes with moderate MGD and 10 eyes with severe MGD, were randomly selected from all of the enrolled subjects to analyze segmental MG secretions. As shown in Table 6, S. epidermidis was the most frequently isolated bacterium, and the Gram-negative bacterium Xanthomonadaceae was the only bacterium isolated from the surface layer of MG secretions in the moderate MGD group. Bacterial discordance was calculated: none (0%) indicated that bacterial results were consistent between the surface layer and the deep layer in both upper and lower Meibomian secretions; half (50%) indicated that bacterial results between the surface layer and the deep layer in one position (upper or lower Meibomian secretions) were consistent and in the other were different; and both (100%) meant bacteria between the surface layer and the deep layer in two positions (both upper and lower Meibomian secretions) were different. Severe MGD showed a significantly higher incidence of bacterial discordance (P=0.035).
  | Table 6 Differences of bacterial flora isolated from segmental Meibomian secretions Notes: n (%): number of eyes (percentage in the groups of control and bacterial severity). *P<0.05. |
Discussion
In this study, swabs of the CS and segmental MG secretions were confirmed. A correlation between the severity of MGD and the microbiome was observed, and we proposed a new grading system for bacterial severity which might be able to assess the severity of MGD.
Positive bacterial isolation rates for MG secretions (54.3%) were consistent with other published studies, in which the isolation rates for MG secretions ranged from 36.9% to 75.6%,18 which also implied that the sterilization procedure performed before sampling may not effectively sterilize the bacteria in the MG, particularly in patients with MGD. Thus, even when a standardized pre-operation sterilization procedure was implemented, the bacteria in the MG were not affected. During surgery, MG secretions containing bacteria might gradually contaminate the sterilized area and lead to severe consequences, such as endophthalmitis. Therefore, a stricter pre-operation evaluation of the MG may be required, as the bacterial isolation rate was highly correlated with MGD severity in our study, and severe MGD might increase the risk of infection. Stricter pre-operation preparation is also essential and should specifically include the use of a surgical adhesive membrane to cover the orifices of the MG. This preparation may reduce the risk post-operation infection, particularly in longer surgical procedures, such as vitreous and retina surgery.
According to our results, as the severity of MGD increased, the composition of the microbiome became more complex, and the bacterial abundance increased. However, mild MGD showed overall less abundance than the control as Microbacteriaceae and Bacillus were missing, and we thought the reason was the limitation of enrolled subjects in mild MGD. Meanwhile, the positive isolation rate of bacteria in mild MGD was still higher than the control, which indicated an altered microenvironment. Corynebacterium, particularly C. macginleyi, was only detected in the severe MGD group (31.6%), and this finding was significantly different between groups (P<0.001). Corynebacterium is a Gram-positive bacterium that widely colonizes the mucous membranes and skin; however, C. macginleyi is a pathogen that causes corneal ulcers and conjunctivitis in the ophthalmic area.30 Our study isolated C. macginleyi from MG secretions, and the positive rate was 26.3% in the severe MGD group. Thus, C. macginleyi isolation may be related to severe MGD and may be used as a biological marker for the evaluation of MGD severity; once C. macginleyi in MGD patients was isolated, it represented a more severe disease condition, which needs more attention from doctors.
Patients with MGD were previously shown to have a higher bacterial isolation rate in the MG than controls;18 however, our study is the first to analyze the number of types of isolated bacteria. Positive isolation rates and the number of different types of isolated bacteria were significantly higher in the severe MGD group than in the control and moderate MGD group. In the analysis that did not include S. epidermidis, positive bacterial isolation rates and the number of types of isolated bacteria were significantly higher in the severe MGD group than those among the control, mild and moderate MGD groups. The presence of more types of bacteria might represent an altered commensal microenvironment that may be caused by the alteration of Meibomian functions and further contribute to disease progress. Moreover, non-S. epidermidis bacteria might better reflect the severity of MGD and provide the basis for the indication for antibiotic treatments in patients with severe MGD. These results also explain why previous studies of the use of antibiotics showed therapeutic effects.12,13,31,32
A grading system for bacterial severity was first proposed and defined in our study, which might help to define the relative quantity of pathogens other than S. epidermidis in the MG commensal microenvironment. The grade of bacterial severity was a good indicator for the commensal microenvironment, according to our results. S. epidermidis was the bacterium that was most frequently isolated from the ocular surface in previous studies,14,15,18 and this bacterium is considered a component of the normal flora of the CS and MG. The presence of S. epidermidis with other bacteria might indicate that the commensal microenvironment has been partially altered, although the alterations were not extreme because S. epidermidis was still present. The presence of other bacteria in the absence of S. epidermidis indicates that the commensal microenvironment has been largely altered, and pathogens made up the majority of the bacterial community. When the concentrations of pathogens, such as Staphylococcus aureus, increase to a certain level, bacteria are able to initiate virulence and evade the host immune response,33 which might explain why the severity of MGD increased when pathogens constituted most of the microbiome and commensalism and pathogen concentrations were increased. Based on our results, the grade of bacterial severity was significantly correlated with MGD severity, which might offer some meaningful insights for clinical practice.
The true etiology of MGD remains uncertain, although it is thought to be due to a number of factors, including bacterial infection.34 Oral or topical antibiotics have been reported to be beneficial treatments for MGD.12,13 However, antibiotics are not effective for every patient, which is consistent with the results from our study, as not every patient in each group was present with a high grade of bacterial severity. Based on our results, a medical indication for antibiotics might be more suitable for patients with a high grade of bacterial severity (grade 3 or 4) or who have more types of isolated bacteria. Furthermore, a prospective study focusing on the effects of antibiotics on patients with MGD with high grades of bacterial severity or more types of isolated bacteria is required. We hope that precise medicines will become available for each patient in the future. Additionally, it is promising to explore the correlation between severity of other bacteria-correlated diseases and bacterial results using this grading system, which could give some implications for clinical practice.
Our study is the first to perform a segmental MG secretion analysis, and differences between the surface layer and deep layer of the MG were explored. Bacteria were isolated from both the surface layer and deep layer of the control and MGD groups. Some bacteria isolated from the deep layer were not detected in the surface layer, which strongly indicated that a bacterial microenvironment exists in the MG, rather than contaminated from the CS. Segmental secretions were less likely to be affected by the microbiome in the CS, which might be a reliable method for exploring the complexity of the microbiome of the MG in patients with MGD. Massage was performed to obtain secretions and proved to be effective in relieving MGD symptoms,32,35 which may help patients who are waiting for the bacterial results. Additionally, severe MGD showed a significantly higher incidence of bacterial discordance, which indicated that a more complex microbiome constitution and a deeper damage of MG occurred in severe MGD patients. More studies focusing on segmental bacterial analysis and the detailed bacterial species affecting the discordance in patients with different severities of MGD are required.
The severity of MGD is correlated with a higher bacterial isolation rate, a greater number of types of isolated bacteria, and a higher grade of bacterial severity. However, our study had some limitations. First, the numbers of enrolled eyes in mild MGD and patients who underwent the segmental secretion analysis were small. Second, due to the limitations associated with the detection device, we only explored aerobic bacteria isolated from the ocular surface, and further studies should also include anaerobic bacteria. Third, our study also lacked information regarding the patients’ living environments, financial statuses and careers, which might also influence the results. Fourthly, although standard sterilization procedure was performed and matched among groups, bacteria flora in the eyelid margin might not be removed completely,36 and the results could not fully represent the bacterial flora in MG. Last but not least, further studies are required to explore the use of antibiotic treatments for patients with high grades of bacterial severity and to investigate differences in the microbiomes in patients with different types of dry eye.
Conclusions
In this study, the microbiomes of the CS as well as the surface and deep layers of MG secretions were characterized by 16S rRNA gene sequencing. Bacterial isolation rates were significantly higher in MG secretions than in the CS and were higher in the severe MGD group than among the control, mild and moderate MGD groups. Based on the grading system for bacterial severity proposed in this study, the types of bacteria isolated in each patient (with or without S. epidermidis included) were calculated. The number of bacterial types and the grades of bacterial severity are positively correlated with the severity of MGD, which may provide some insights into the appropriate treatment of MGD. Furthermore, C. macginleyi may have potential value as a biological marker for the evaluation of MGD severity.
Acknowledgments
The authors would like to thank Jingnan Liang (Institute of Microbiology, CAS), Xu Tang, and Yixin Gu (Institute of Geology and Geophysics, CAS) for assistance with electron microscopy. This work was supported by grants from the Ministry of Science and Technology of China (2010Z × 09401–403) and grants from the National Science and Technology Major Project (2018Z × 10101004).
Disclosure
The authors report no conflicts of interest in this work.
References
Lemp MA, Baudouin C, Baum J. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5(2):75–92. | ||
Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. | ||
Lin PY, Cheng CY, Hsu WM. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai eye Study. Invest Ophthalmol Vis Sci. 2005;46(5):1593–1598. | ||
Sima J, Piao Y, Chen Y, Schlessinger D. Molecular dynamics of Dkk4 modulates Wnt action and regulates meibomian gland development. Development. 2016;143(24):4723–4735. | ||
Stern GA, Engel HM, Driebe WT. Recurrent postoperative endophthalmitis. Cornea. 1990;9(2):102–107. | ||
Groden LR, Murphy B, Rodnite J, Genvert GI. Lid flora in blepharitis. Cornea. 1991;10(1):50–53. | ||
Sharma S. Diagnosis of external ocular infections: microbiological processing and interpretation. Br J Ophthalmol. 2000;84(2):229. | ||
O’Callaghan RJ, Girgis DO, Dajcs JJ, Sloop GD. Host defense against bacterial keratitis. Ocul Immunol Inflamm. 2003;11(3):171–181. | ||
Hara J, Yasuda F, Higashitsutsumi M. Preoperative disinfection of the conjunctival sac in cataract surgery. Ophthalmologica. 1997;211(Suppl. 1):62–67. | ||
Kulaçoǧlu DN, Özbek A, Uslu H, et al. Comparative lid flora in anterior blepharitis. Turk J Med Sci. 2000;31:359–363. | ||
Pinna A, Sechi LA, Zanetti S, Carta F. Detection of virulence factors in a corneal isolate of Klebsiella pneumoniae. Ophthalmology. 2005;112(5):883–887. | ||
Foulks GN, Borchman D, Yappert M, Kim SH, Mckay JW. Topical azithromycin therapy for meibomian gland dysfunction: clinical response and lipid alterations. Cornea. 2010;29(7):781–788. | ||
Foulks GN, Borchman D, Yappert M, Kakar S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea. 2013;32(1):44–53. | ||
Suto C, Morinaga M, Yagi T, Tsuji C, Toshida H, Moeller CT, Branco BC, Mc Y. Conjunctival sac bacterial flora isolated prior to cataract surgery. Infect Drug Resist. 2012;5:37–41. | ||
Graham JE, Moore JE, Jiru X, et al. Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Invest Ophthalmol Vis Sci. 2007;48(12):5616–5623. | ||
Watters GA, Turnbull PR, Swift S, et al. Ocular surface microbiome in meibomian gland dysfunction in Auckland, New Zealand. Clin Exp Ophthalmol. 2017;45(2):105–111. | ||
Dougherty JM, Mcculley JP. Comparative bacteriology of chronic blepharitis. Br J Ophthalmol. 1984;68(8):524–528. | ||
Zhang SD, He JN, Niu TT, et al. Bacteriological profile of ocular surface flora in meibomian gland dysfunction. Ocul Surf. 2017;15(2):242–247. | ||
Asbell PA, Stapleton FJ, Wickström K, et al. The international workshop on meibomian gland dysfunction: report of the clinical trials subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2065–2085. | ||
Severinsky B, Wajnsztajn D, Frucht-Pery J. Silicone hydrogel mini-scleral contact lenses in early stage after corneal collagen cross-linking for keratoconus: a retrospective case series. Clin Exp Optom. 2013;96(6):542–546. | ||
Pult H, Purslow C, Murphy PJ. The relationship between clinical signs and dry eye symptoms. Eye. 2011;25(4):502–510. | ||
Caffery BE, Josephson JE. Corneal staining after sequential instillations of fluorescein over 30 days. Optom Vis Sci. 1991;68(6):467–469. | ||
Thomas ML, Szeto VR, Gan CM, Polse KA. Sequential staining: the effects of sodium fluorescein, osmolarity, and pH on human corneal epithelium. Optom Vis Sci. 1997;74(4):207–210. | ||
Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006–2049. | ||
Deng AH, Sun ZP, Zhang GQ, Wu J, Wen TY, et al. Rapid discrimination of newly isolated Bacillales with industrial applications using Raman spectroscopy. Laser Phys Lett. 2012;9(9):636–642. | ||
Huber T, Faulkner G, Hugenholtz P. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics. 2004;20(14):2317–2319. | ||
Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. | ||
Takahashi C, Kalita G, Ogawa N, et al. Electron microscopy of Staphylococcus epidermidis fibril and biofilm formation using image-enhancing ionic liquid. Anal Bioanal Chem. 2015;407(6):1607–1613. | ||
Suzuki T, Iihara H, Uno T, et al. Suture-related keratitis caused by Corynebacterium macginleyi. J Clin Microbiol. 2007;45(11):3833–3836. | ||
Joussen AM, Funke G, Joussen F, Herbertz G. Corynebacterium macginleyi: a conjunctiva specific pathogen. Br J Ophthalmol. 2000;84(12):1420–1422. | ||
Liu Y, Kam WR, Ding J, Sullivan DA, Yang L, Wendy RK, Juan D. One man’s poison is another man’s meat: using azithromycin-induced phospholipidosis to promote ocular surface health. Toxicology. 2014;320:1–5. | ||
Zhang SD, He JN, Niu TT, et al. Effectiveness of meibomian gland massage combined with topical levofloxacin against ocular surface flora in patients before penetrating ocular surgery. Ocul Surf. 2018;16(1):S154230195–30197. | ||
Reading NC, Sperandio V. Quorum sensing: the many languages of bacteria. FEMS Microbiol Lett. 2006;254(1):1–11. | ||
Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 20111922;52(4):e9. | ||
Thode AR, Latkany RA. Current and Emerging Therapeutic Strategies for the Treatment of Meibomian Gland Dysfunction (MGD. Drugs. 2015;75(11):1177–1185. | ||
Inagaki K, Yamaguchi T, Ohde S, et al. Bacterial culture after three sterilization methods for cataract surgery. Jpn J Ophthalmol. 2013;57(1):74–79. |
Supplementary material
Conjunctival injection and TMH
The degree of conjunctival injection was evaluated using a slit lamp microscope. The Institute for Eye Research Grading Scales1 were used to assess bulbar conjunctival redness with a score of 0 representing grade 1 redness, and a score of 3 representing grade 4 redness, which indicates severe redness of the bulbar conjunctiva. The central upper and lower TMH were measured using a slit lamp microscope (with a graticule in 0.05 mm units).2 Three consecutive readings were obtained, and the final results are presented as medians.
TBUT and corneal staining
A total of 5 µL of 2% sodium fluorescein was instilled onto the bulbar conjunctiva using a micropipette, without inducing reflex tearing. The patient was asked to blink naturally without squeezing 3–5 times and was then asked to stare straight ahead without blinking under the cobalt blue light until he or she received other instructions. A stopwatch was used to record the time between the last complete blink and the first appearance of a dry spot or disruption in the tear film.3 The procedure was repeated three times, and the final score is presented as an average value. For the corneal staining evaluation, the cornea was divided into five sectors.4 Each sector was graded from 0 to 3 using the following criteria: 0 representing no staining; 1 representing punctate or stippled staining; 2 representing ball and linear staining; and 3 representing coalesced staining.5
Eyelid margin, tear foam and MG assessments
According to the International Workshop on Meibomian Gland Dysfunction,6 six eyelid margin characteristics were assessed in this study: rounding of the posterior margin, irregularity or notching of the margin, telangiectasia or vascularity of the lid margin, trichiasis, hyperkeratinization and anterior blepharitis. Each sign was assigned a score of 0 or 1. A score of 0 indicated the absence of the particular eyelid margin characteristic (i.e. normal), whereas a score of 1 indicated the presence of the characteristic (i.e. abnormal). Tear foam was scored from 0 to 2. A score of 0 represented normal tear foam, whereas scores of 1 and 2 represented mildly abnormal and severely abnormal tear foam, respectively. MG assessments included: 1) the average number of lower lid orifices; 2) the quality of the expressed secretion; and 3) the degree of orifice obliteration. The quality of the expressed secretion was scored as follows: 0=clear; 1=cloudy; 2=granular; and 3=toothpaste-like. The degree of orifice obliteration was expressed as follows: 1=light pressure; 2=moderate pressure; and 3=heavy pressure. The grade of MGD severity was also expressed according to criteria from the Meibomian Gland Dysfunction Workshop reported by A. Tomlinson et al.7
References
Severinsky B, Wajnsztajn D, Frucht-Pery J. Silicone hydrogel mini-scleral contact lenses in early stage after corneal collagen cross-linking for keratoconus: a retrospective case series. Clin Exp Optom 2013;96(6):542–546. | ||
Pult H, Purslow C, Murphy PJ. The relationship between clinical signs and dry eye symptoms. Eye. 2011;25(4):502–510. | ||
Lemp MA, Baudouin C, Baum J, et al. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop. Ocul Surf. 2007;5:75–92. | ||
Caffery BE, Josephson JE. Corneal staining after sequential instillations of fluorescein over 30 days. Optom Vis Sci. 1991;68(6):467–469. | ||
Thomas ML, Szeto VR, Gan CM, Polse KA. Sequential staining the effects of sodium fluorescein, osmolarity, and pH on human corneal epithelium. Optom Vis Sci. 1997;74(4):207–210. | ||
Asbell PA, Stapleton FJ, Wickström K, et al. The international workshop on meibomian gland dysfunction: report of the clinical trials subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2065–2085. | ||
Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006–2049. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.