Back to Journals » International Journal of General Medicine » Volume 10
Parecoxib relieves pain and has an opioid-sparing effect following major gastrointestinal surgery
Authors Essex MN , Xu H , Parsons B , Xie L, Li C
Received 13 June 2017
Accepted for publication 22 August 2017
Published 28 September 2017 Volume 2017:10 Pages 319—327
DOI https://doi.org/10.2147/IJGM.S143837
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Margaret Noyes Essex,1 Hao Xu,2 Bruce Parsons,1 Li Xie,3 Chunming Li4
1Global Medical Affairs, Pfizer, New York, NY, USA; 2Department of General Surgery, Jiangsu Province Hospital, Nanjing, China; 3Medical Affairs, Pfizer Investment Co. Ltd., Beijing, China; 4Statistics, Pfizer, Madison, NJ, USA
Purpose: Parecoxib provides analgesia following a variety of surgeries, including minor gastrointestinal procedures. To our knowledge, there is no data on parecoxib following major gastrointestinal surgery. This study assessed the efficacy and opioid-sparing effects of parecoxib following major gastrointestinal surgeries.
Patients and methods: Patients in this analysis were a subset from a large, randomized, double-blind, placebo-controlled trial of parecoxib following noncardiac surgeries and consisted of those undergoing a variety of major gastrointestinal surgeries via laparotomy. Pain, pain interference with function, supplemental opioid utilization, opioid-related symptoms, and Patient/Physician Global Evaluation of Study Medication were compared between placebo and parecoxib groups in the 2−3 days following surgery.
Results: Significantly (p<0.001) lower pain scores were observed in the parecoxib group (n=111), relative to placebo (n=126), on Day 2 (−33%) and Day 3 (−35%). Pain interference with function scores was also significantly (p<0.001) lower among patients receiving parecoxib compared with placebo on Day 2 (−29%) and Day 3 (−36%). At 24, 48, and 72 hours, the cumulative amount of supplemental morphine consumed was 45%, 41%, and 40% less in patients receiving parecoxib compared with placebo (all p<0.001). The risk of experiencing ≥1 opioid-related symptoms was also significantly lower with parecoxib than with placebo on Day 2 (relative risk=0.75; p<0.001). Specifically, the risks of fatigue and drowsiness were significantly (both p<0.05) lower in patients receiving parecoxib compared to those receiving placebo. Patient and Physician Global Evaluation of Study Medication scores were significantly better in the parecoxib group than in the placebo group (p<0.001).
Conclusion: This study is the first to demonstrate that multiple-dose parecoxib, initiated upon recovery from anesthesia, provides analgesia and opioid-sparing effects following a variety of major gastrointestinal surgeries employing laparotomy.
Keywords: parecoxib, gastrointestinal, laparotomy, postoperative pain, opioid sparing
Introduction
Pain is a frequent complication following surgery and is a key concern of patients.1 Inadequately controlled postoperative pain can increase length of stay, total health care costs, and the risk of developing chronic pain.2,3 Despite these concerns, postoperative pain is often undermanaged.1 Laparoscopic techniques are associated with less postoperative pain and analgesic requirements, compared with traditional open laparotomy.4,5 Laparotomy, however, is still required in some cases, including a variety of major gastrointestinal (GI) procedures.
Due to the risk of specific adverse events that can delay recovery, current analgesic guidelines seek to reduce the amount of opioids utilized in the postoperative setting.6 The risk of opioid-related adverse events is particularly worrisome following GI surgery, since they include nausea, vomiting, and constipation.7 As a result, a multimodal analgesic approach is recommended that involves regular administration of nonselective nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase (COX)-2-specific inhibitors (COXIBs), or acetaminophen, unless contraindicated, to reduce the amount of opioids necessary to provide adequate pain relief.6
Though nonselective NSAIDs are effective for the management of postoperative pain, they are associated with specific GI-related events including ulceration and hemorrhage.8,9 This is thought to be attributed to inhibition of COX-1, which is constitutively expressed throughout the body and plays an important role in protection of the GI mucosal lining.9,10 COX-2 expression, in contrast, is largely limited to sites of inflammation, and there is less risk of GI-related adverse effects associated with the use of COXIBs, as compared with nonselective NSAIDs.8,10–12 Nonselective NSAIDs may also increase postoperative bleeding via COX-1–mediated inhibition of platelet aggregation.13
Parecoxib, an injectable COXIB, is approved in over 80 countries for the treatment of postoperative pain. Previous studies have shown that parecoxib reduces postoperative pain and opioid requirements following a variety of surgery types, including gynecologic and orthopedic procedures.14–19 Studies have also shown the utility of parecoxib following specific minor GI procedures such as laparoscopic cholecystectomy and endoscopic retrograde cholangiopancreatography.20–24 To our knowledge, however, there are no published reports of placebo-controlled trials examining the use of parecoxib in patients following major GI surgery. Therefore, this analysis examined the analgesic efficacy and potential for opioid sparing of parecoxib in patients, following a variety of diverse major GI surgeries requiring laparotomy.
Patients and methods
Data source
This is a subset analysis of patients undergoing major GI surgery by laparotomy requiring general, spinal, or epidural anesthesia. Patient data were derived from a large, randomized, double-blind, placebo-controlled trial of parecoxib for the treatment of postoperative pain following noncardiac surgery. Full details of the methods can be found in the original publication.25 The study was approved by an Institutional Review Board at each study site (Table S1), and written informed consent was obtained from all subjects.
Treatment
Patients were randomized to parecoxib/valdecoxib or matching placebo after they recovered from anesthesia. The parecoxib/valdecoxib treatment regimen consisted of the following (Table 1): an initial 40 mg intravenous (IV) dose of parecoxib on Day 1 (the day of surgery after recovery from anesthesia); 20 mg IV or intramuscular (IM) doses of parecoxib every 12 hours thereafter (through at least Day 3); and 20 mg oral doses of valdecoxib every 12 hours (until Day 10). Patients were transitioned from IV/IM parecoxib to oral valdecoxib once they could tolerate oral medication, but no sooner than Day 4. Supplemental analgesia was allowed during the IV/IM and oral phases of the study. This consisted of morphine via patient-controlled analgesia (PCA) or bolus administration in the IV/IM phase and codeine with acetaminophen or hydrocodone with acetaminophen during the oral phase. It should be noted that all outcomes in this analysis were assessed at a time when patients were receiving IV/IM parecoxib (Days 2 and 3) and had not yet received valdecoxib. Thus, the parecoxib/valdecoxib group will simply be referred to as the parecoxib group from here on.
Assessments
Daily, patients rated their pain on a scale from 0=none to 3=severe pain at 2, 4, 8, 12, and 24 hours after the first daily dose of study medication. Summed pain intensity (SPI) over 24 hours (SPI-24) was calculated as described previously and was compared between placebo and parecoxib-treatment groups on Day 2 (the day following surgery) and Day 3.25 Briefly, the SPI scores were calculated from the five pain assessments recorded each day. Each rating was weighted by the number of hours between the time it was obtained and the time the previous rating was obtained, so that the SPI score=(2-hour rating×2)+(4-hour rating×2)+(8-hour rating×4)+(12-hour rating×4)+(24-hour rating×12). The potential range of these scores was 0–72.
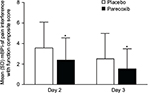
The question on pain interference with function in the modified Brief Pain Inventory-short form was used to generate a composite pain interference with function score for both treatment groups. The five items assess pain interference with general activity, mood, walking ability, relations with others, and sleep. The composite score was compared between treatment groups on Days 2 and 3.
The cumulative amount of supplemental morphine consumed was determined at 24, 48, and 72 hours post-initial dose of study treatment and was compared between the placebo and parecoxib groups.
The frequency of opioid-related symptoms was assessed on Day 2 using the Opioid-Related Symptom Distress Scale. These symptoms included drowsiness, retching/vomiting confusion, dizziness, itching, difficulty with urination, constipation, inability to concentrate, nausea, and fatigue.
Finally, both physicians and patients evaluated the study medication at the time of transition from IV/IM to oral dosing using a scale from 1=poor to 4=excellent.
Statistical analyses
Statistical analyses were performed on all randomized patients who received at least one dose of study medication. When necessary, missing data was imputed using a last observation carried forward approach. SPI-24 scores and the amount of morphine consumed were compared between treatment groups using a general linear model with treatment and country as factors. Composite modified Brief Pain Inventory-short form pain interference with function scores were compared between groups using a general linear model with treatment and country as factors. A relative risk (RR; parecoxib versus placebo) was calculated for each opioid-related symptom, based on the percentage of patients experiencing a specific symptom in each treatment group, using a 2×2 table, and groups were compared using a Fisher’s exact test. A RR (parecoxib versus placebo) of experiencing ≥1, ≥2, and ≥3 opioid-related symptoms was also determined for Day 2. Global evaluation of study medication scores was compared between treatment groups using a Cochran–Mantel–Haenzel test controlling for country. Additional summaries were conducted using descriptive statistics.
Results
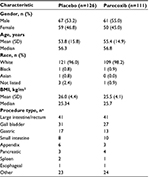
The placebo and parecoxib treatment groups comprised 126 and 111 patients, respectively. Patient demographics are shown in Table 2 and were similar between treatment groups. The types of surgical procedures performed were also similar between treatment groups.
Significantly lower mean pain intensity scores were evident for patients in the parecoxib group, compared with placebo, on both Days 2 and 3 following surgery (Figure 1). Mean SPI-24 scores in the parecoxib group were 33% and 35% lower than placebo on Days 2 and 3, respectively (both p<0.001). The absolute difference between treatment groups was 11.0 on Day 2 and 9.3 on Day 3. Pain interference with function scores were also significantly lower among patients receiving parecoxib compared with placebo (Figure 2). On Day 2, the mean composite pain interference with function score was 1.1 points lower in the parecoxib group than in the placebo group, which represents a 29% relative reduction (p<0.001). Likewise, the mean composite pain interference with function score was 0.9 points lower in the parecoxib group than in the placebo group on Day 3, which represents a 36% relative reduction (p<0.001).
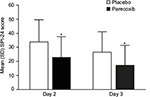  | Figure 1 Mean (SD) SPI-24 scores on Days 2 and 3 following surgery. Note: *p<0.001 versus placebo. Abbreviation: SPI-24, summed pain intensity over the previous 24 hours. |
At each time point examined, the cumulative amount of morphine consumed was significantly less in patients receiving parecoxib, relative to placebo (Figure 3). The relative reduction was 45% at 24 hours, 41% at 48 hours, and 40% at 72 hours (all p<0.001). The absolute reduction of morphine in milligrams was 8.3 mg at 24 hours, 14.3 mg at 48 hours, and 16.9 mg at 72 hours. The risk of experiencing ≥1 opioid-related symptoms was also lower in the parecoxib group than in the placebo group on Day 2 (RR=0.75; p<0.001). Regarding specific opioid-related symptoms, the risks of fatigue (p<0.05) and drowsiness (p<0.05) were significantly lower in patients receiving parecoxib compared to those receiving placebo (Figure 4).
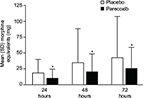  | Figure 3 Cumulative morphine consumption at 24, 48, and 72 hours post-initial dose of study medication. Note: *p<0.001 versus placebo. |
  | Figure 4 Risk of opioid-related symptoms on Day 2 following surgery. Notes: Relative risk (parecoxib versus placebo) is shown in the graph. *p<0.05 versus placebo. |
Patient (Figure 5A) and Physician (Figure 5B) Global Evaluation of Study Medication scores were significantly better in the parecoxib group than in the placebo group at the time of transition from IV/IM to oral dosing (both p<0.001). A greater percentage of patients in the parecoxib group (44%) rated their treatment as “Excellent”, compared with the placebo group (17%). Likewise, fewer patients rated the treatment as “poor” or “fair” in the parecoxib group (8%) than in the placebo group (36%). Physician ratings were similar to the patient ratings.
Discussion
Pain following major GI surgery can cause patient distress, delay mobilization, and lengthen recovery times. This study is the first, to our knowledge, to demonstrate that parecoxib is effective at relieving pain following a variety of diverse major GI surgeries involving laparotomy. In the days immediately following surgery, patients receiving parecoxib + morphine PCA reported significantly less pain than patients receiving placebo + morphine PCA. SPI scores were 33% and 35% lower in the parecoxib group, relative to placebo, on postoperative Days 2 and 3, respectively. A similar reduction in composite pain interference with function scores was also evident with parecoxib treatment, relative to placebo, on Day 2 (a 29% reduction) and Day 3 (a 36% reduction). This composite score takes into account the effects of pain on general activity, mood, walking ability, and relations with others. These data suggest that the analgesic benefit provided by parecoxib was associated with improvements in overall patient function.
Current multimodal analgesic recommendations aim to reduce the postoperative consumption of opioids and opioid-related adverse events that can stress the patient and delay recovery.26 In our study, patients receiving parecoxib consumed 40%–45% less morphine PCA, relative to placebo, over the first 24–72 hours post-initial dose of study medication. Thus, parecoxib provided a significant opioid-sparing effect. On postoperative Day 2, the risk of experiencing ≥1 opioid-related symptoms was significantly reduced in the parecoxib group compared with placebo. When specific opioid-related symptoms were reviewed, only the risks of fatigue and drowsiness were significantly reduced with parecoxib. However, these two symptoms were, by far, the most commonly reported events by patients in the placebo group (>50% of patients). The risk of most other opioid-related symptoms, with the exceptions of vomiting and itching, was lower with parecoxib compared to placebo, but did not reach the level of statistical significance. It is possible that the occurrence of certain symptoms (such as nausea, vomiting, and constipation) was a result of the GI procedure itself, as opposed to a side effect of opioid treatment. This may explain, at least in part, why there was not a decreased risk of these symptoms associated with parecoxib, even though it provided a significant opioid-sparing effect.
The overall benefits of IV/IM parecoxib treatment were recognized by both patients and physicians, as evidenced by their Global Evaluation of Study Medication scores just prior to switching to oral treatment. While these findings are encouraging, they are also limited in that our analysis was based on a subset of patients from a larger clinical trial that was not designed or powered to specifically assess the endpoints examined here. The patient sample size in each treatment group, however, was relatively large and equivalent to previous trials of parecoxib. In addition, our findings in patients undergoing major GI surgery are in agreement with previous studies of parecoxib in other surgical models, including major and minor gynecologic surgery,14–16 total knee replacement,17 total hip replacement,18,19 and minor GI surgeries.23,24,27 In these studies, both single-dose14,15,23,24,27 and multiple-dose15–19 regimens of IV parecoxib have been shown to be effective against postoperative pain and, often, were associated with an opioid-sparing effect.16–18 Indeed, guidelines from the Procedure-Specific Postoperative Pain Management group recommend the use of NSAIDs and/or COXIBs, such as parecoxib, as part of a multimodal analgesic approach following a variety of surgeries including major GI surgery (e.g., colonic resection).28
Conclusion
This study is the first to demonstrate that a multiple-dose regimen of IV parecoxib, initiated upon recovery from anesthesia, provides a significant analgesic benefit and opioid-sparing effect in the 2–3 days immediately following a variety of diverse major GI surgeries employing laparotomy.
Acknowledgments
This study was sponsored by Pfizer. Medical writing support was provided by Matt Soulsby PhD, CMPP, of Engage Scientific Solutions and was funded by Pfizer.
Disclosure
MNE, BP, and CL are full-time employees of, and own stock in, Pfizer. LX was a full-time employee of Pfizer at the time the work was done; current affiliation is Takeda Pharmaceutical (China) Company Limited. The authors report no other conflicts of interest in this work.
References
Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–540. | ||
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. | ||
Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North Am. 2005;23(1):21–36. | ||
Agha R, Muir G. Does laparoscopic surgery spell the end of the open surgeon? J Royal Soc Med. 2003;96(11):544–546. | ||
Hayden P, Cowman S. Anaesthesia for laparoscopic surgery. Continuing Educ in Anaesth Crit Care & Pain. 2011;11(5):177–180. | ||
The American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248–273. | ||
Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159–180. | ||
Rostom A, Muir K, Dube C, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: a Cochrane Collaboration systematic review. Clin Gastroenterol Hepatol. 2007;5(7):818–828. | ||
Bjarnason I. Gastrointestinal safety of NSAIDs and over-the-counter analgesics. Int J Clin Pract Suppl. 2013(178):37–42. | ||
Hawkey CJ. COX-1 and COX-2 inhibitors. Best Pract Res Clin Gastroenterol. 2001;15(5):801–820. | ||
Stoltz RR, Harris SI, Kuss ME, et al. Upper GI mucosal effects of parecoxib sodium in healthy elderly subjects. Am J Gastroenterol. 2002;97(1):65–71. | ||
Moore RA, Derry S, McQuay HJ. Cyclo-oxygenase-2 selective inhibitors and nonsteroidal anti-inflammatory drugs: balancing gastrointestinal and cardiovascular risk. BMC Musculoskelet Disord. 2007;8:73. | ||
Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209–219. | ||
Barton SF, Langeland FF, Snabes MC, et al. Efficacy and safety of intravenous parecoxib sodium in relieving acute postoperative pain following gynecologic laparotomy surgery. Anesthesiology. 2002;97(2):306–314. | ||
Bikhazi GB, Snabes MC, Bajwa ZH, et al. A clinical trial demonstrates the analgesic activity of intravenous parecoxib sodium compared with ketorolac or morphine after gynecologic surgery with laparotomy. Am J Obstet Gynecol. 2004;191(4):1183–1191. | ||
Snabes MC, Jakimiuk AJ, Kotarski J, Katz TK, Brown MT, Verburg KM. Parecoxib sodium administered over several days reduces pain after gynecologic surgery via laparotomy. J Clin Anesth. 2007;19(6):448–455. | ||
Hubbard RC, Naumann TM, Traylor L, Dhadda S. Parecoxib sodium has opioid-sparing effects in patients undergoing total knee arthroplasty under spinal anaesthesia. Br J Anaesth. 2003;90(2):166–172. | ||
Malan TP, Jr., Marsh G, Hakki SI, Grossman E, Traylor L, Hubbard RC. Parecoxib sodium, a parenteral cyclooxygenase 2 selective inhibitor, improves morphine analgesia and is opioid-sparing following total hip arthroplasty. Anesthesiology. 2003;98(4):950–956. | ||
Viscusi ER, Gimbel JS, Halder AM, Snabes M, Brown MT, Verburg KM. A multiple-day regimen of parecoxib sodium 20 mg twice daily provides pain relief after total hip arthroplasty. Anesth Analg. 2008;107(2):652–660. | ||
Joshi GP, Viscusi ER, Gan TJ, et al. Effective treatment of laparoscopic cholecystectomy pain with intravenous followed by oral COX-2 specific inhibitor. Anesth Analg. 2004;98(2):336–342. | ||
Gan TJ, Joshi GP, Zhao SZ, Hanna DB, Cheung RY, Chen C. Presurgical intravenous parecoxib sodium and follow-up oral valdecoxib for pain management after laparoscopic cholecystectomy surgery reduces opioid requirements and opioid-related adverse effects. Acta Anaesthesiol Scand. 2004;48(9):1194–1207. | ||
Papadima A, Lagoudianakis EE, Antonakis PT, et al. Parecoxib vs. lornoxicam in the treatment of postoperative pain after laparoscopic cholecystectomy: a prospective randomized placebo-controlled trial. Eur J Anaesthesiol. 2007;24(2):154–158. | ||
Lin S, Hua J, Xu B, et al. Comparison of bupivacaine and parecoxib for postoperative pain relief after laparoscopic cholecystectomy: a randomized controlled trial. Int J Clin Exp Med. 2015;8(8):13,824–13,829. | ||
Amornyotin S, Chalayonnawin W, Kongphlay S. A randomized controlled trial of preprocedure administration of parecoxib for therapeutic endoscopic retrograde cholangiopancreatography. J Pain Res. 2012;5:251–256. | ||
Nussmeier NA, Whelton AA, Brown MT, et al. Safety and efficacy of the cyclooxygenase-2 inhibitors parecoxib and valdecoxib after noncardiac surgery. Anesthesiology. 2006;104(3):518–526. | ||
Feldheiser A, Aziz O, Baldini G, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60(3):289–334. | ||
Shuying L, Xiao W, Peng L, Tao Z, Ziying L, Liang Z. Preoperative intravenous parecoxib reduces length of stay on ambulatory laparoscopic cholecystectomy. Int J Surg. 2014;12(5):464–468. | ||
PROSPECT Colonic Resection Summary Recommendations. Available from: http://www.postoppain.org/sections/?root_id=62,600& section=13. Accessed January 4, 2017. |
Supplementary material
  | Table S1 List of institutional review board or ethics committees approving the study |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.