Back to Journals » OncoTargets and Therapy » Volume 12
PAQR4 promotes cell proliferation and metastasis through the CDK4-pRB-E2F1 pathway in non-small-cell lung cancer
Received 25 July 2018
Accepted for publication 17 January 2019
Published 13 May 2019 Volume 2019:12 Pages 3625—3633
DOI https://doi.org/10.2147/OTT.S181432
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Leo Jen-Liang Su
Bin Wu,1 Rongyu Liu2
1Department of Pulmonary and Critical Care Medicine, Baoan Central Hospital of Shenzhen, Shenzhen 518102, China; 2Department of Pulmonary and Critical Care Medicine, First Affiliated Hospital of Anhui Medical University, Hefei 230022, China
Background: It is reported that progestin and adipoQ receptor 4 (PAQR4) has a tumorigenic effect on human breast cancer, but the role of PAQR4 in non-small-cell lung cancer (NSCLC) is unknown. The aim of this study was to investigate the role of PAQR4 in NSCLC.
Methods: Quantitative real-time PCR (qRT-PCR) and immunohistchemical (IHC) staining were used to analyze the expression of PAQR4 in HCC tissues and adjacent normal tissues. MTT, colony formation assay, flow cytometry (FCM), wound healing assays and transwell invasion assays were used to investigate the effects of PAQR4 on cell proliferation, colony formation, cell cycle, migration and invasion. Murine xenograft model assay was carried out to characterize the effects of PAQR4 knockdown on tumor growth in vivo.
Results: In this study, we found that the expression of PAQR4 was significantly upregulated in the NSCLC tissues of patients compared with that in the matched non-cancerous tissues. In addition, we found that PAQR4 was also significantly up-regulated in the NSCLC cell lines compared with normal human lung epithelial cells. Besides, we found that the over-expression of PAQR4 promoted promoted proliferation, colony formation, migration and invasion of the NSCLC cells, whereas the knockdown of PAQR4 inhibited proliferation, colony formation, migration and invasion of the NSCLC cells. Furthermore, mechanistic studies showed that the CDK4-pRB-E2F1 pathway was involved in NSCLC.
Conclusion: Hence, these results suggest that PAQR4 may be used as a new target in NSCLC therapy.
Keywords: PAQR4, cell proliferation, metastasis, CDK4-pRB-E2F1 pathway
Introduction
Non-small-cell lung cancer (NSCLC) is one of the most common respiratory neoplasms and a major cause of cancer-related morbidity and mortality worldwide.1–3 It is reported that the crude incidence of lung cancer in the European Union is 52.5/100,000 every year, with a yearly mortality of 48.7/100,000.4,5 Many factors are involved in triggering NSCLC.6,7 Despite great advances in understanding the disease, the long-term survival of patients with NSCLC is still very poor. As a result, effective therapeutic methods are in great need.
It is well known that abnormal expression of cancer-related genes is related to occurrence and development of a wide range of human malignancies.8–10 Progestin and adipoQ receptor 4 (PAQR4) is a member of the PAQR4 family.11,12 It is documented that the PAQR4 family plays an important role in a number of biological processes, including tumor development and metabolism.13–15 The CDK4-pRB-E2F1 pathway has been reported to be involved in several types of human tumors, including glioblastoma multiforme,16 melanoma,17 endometrial cancer,18 pituitary tumor,19 prostate cancer,20 liver cancer,21 and gastric cancer.22
It was reported that PAQR4 had a tumorigenic effect in human breast cancer, but the role of PAQR4 in NSCLC is unknown. This study aimed to investigate the role of PAQR4 in NSCLC and uncover the potential molecular mechanisms. In this study, we found that PAQR4 expression was significantly upregulated in NSCLC tissues. Moreover, the overexpression of PAQR4 promoted cell proliferation and metastasis, whereas the knockdown of PAQR4 inhibited these functions. Additionally, we found that the CDK4-pRB-E2F1 pathway was involved in the occurrence and development of NSCLC.
Materials and methods
Patients and tissue samples
NSCLC tissues and matched normal tissues were obtained from 60 patients who underwent surgical resection at First Affiliated Hospital of Anhui Medical University (Hefei, China). Tissues samples were stored in −80°C until further studies. All the patients gave their written informed consent. None of the patients received radiotherapy or immunotherapy. All the patients provided written informed consent. This study was approved by the Ethics Committee of Anhui Medical University. This work was conducted in accordance with the Declaration of Helsinki.
Immunohistochemistry (IHC)
The immunohistochemical staining was performed on 5 μm slides from formalin-fixed, paraffin-embedded lung tissue. In brief, after deparaffinization and dehydration, the slide preparations were incubated in 0.3% H2O2 to quench endogenous peroxidase activity. This step was followed by three washes with PBS. For antigen retrieval, sections were incubated with 10 mmol/L citrate buffer (pH 6.0) at 120°C for 20 minutes. Subsequently, the slides were exposed to blocking solution for 1 hour. The slides were incubated with the primary antibody using a 1:100 working dilution. The slides were incubated for 1 hour in a humidified chamber at room temperature, followed by three washes with PBS. The slides were then incubated under the same conditions with the secondary biotinylated antibody for 1 hour (avidin-biotin complex, Vectastain Elite kits; Vector Labs Inc., Burlingame, CA, USA).
Cell culture
Four NSCLC cell lines and one normal lung epithelial cell line BEAS-2B were obtained from Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai, China). All the cells were cultured in RPMI medium supplemented with 10% FBS, and incubated at 37°C in a humidified atmosphere with 5% CO2. Cells in logarithmic growth phase were used for further experiments.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA and microRNAs were extracted using the RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Following quantification by Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA), the extracted total RNA was reverse-transcribed using a TaqMan High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA, USA). For mRNA analysis, reverse transcription products were mixed with TaqMan Universal PCR Master Mix (Applied Biosystems), and qRT-PCR was performed on an Applied Biosystems 7,500 Fast Real-Time PCR systems (Applied Biosystems). The sequences of primers were as followed: PAQR4, forward 5′-CTCAGGTTGGGTCTAGGTG-3′ and reverse 5′-GCAGGGCTAGCTTTTGGATC-3′; β-actin, forward 5′-GGACTTCGAGCAAGAGATGG-3′ and reverse 5′-AGCACTGTGTTGGCGTACAG-3′. β-actin was used as an endogenous control to normalize ROCK1 expression levels. The relative expression level was calculated using the 2−ΔΔCt method.
Western blotting
Protein lysates were prepared from cells using 500 μL of RIPA buffer with 1 mM phenylmethane sulfonyl fluoride. Samples were subsequently sonicated for 2 minutes and centrifuged. The supernatants were collected and used for protein analysis. Lysates were separated on 8% polyacrylamide gels and transferred onto polyvinylidene fluoride membrane. The membranes were blocked with PBS containing 0.1% Tween-20 (PBST) and 5% nonfat milk (w/v) for 1 hour at room temperature. After they were washed with PBST, the membranes were probed with antibodies overnight at 4°C. Antibodies were all obtained from Abcam (Cambridge, UK). The membranes were washed again with PBST, then horseradish peroxidase-labeled IgG at 1:5,000 dilution was added at room temperature for 1 hour, and the blots were developed using enhanced chemiluminescence Western blotting reagents.
Cell proliferation
Cell proliferation was assessed using MTT Cell Proliferation and Cytotoxicity Assay Kit (Sigma-Aldrich Co., St Louis, MO, USA) according to the manufacturer’s protocols. Briefly, cells were seeded in each well of a 96-well plate at a density of 1×104 cells/well. Following incubation at 37°C for different periods of time, the culture medium was removed and MTT (20 μL, 5 mg/mL) was added to each well. After incubation at 37°C for another 4 hours, MTT solution was removed and replaced with dimethyl sulfoxide (150 μL, 4%; Sigma-Aldrich Co.). Absorbance was measured using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
Cell transfection
Cell transfection was performed using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) in accordance with the manufacturer’s instructions. PAQR4 expression vectors and short hairpin RNA (shRNA) specially targeting PAQR4 were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China). The pcDNA3.1 plasmids were used for the overexpression studies. The pLKO.3.1 plasmids were used for the knockdown studies. Cells were incubated for 24 hours and then collected for further studies.
Cell cycle analysis
For cell cycle analysis, cells were harvested at 48 hours post-transfection. The cells were washed with PBS and fixed in ethanol at −20°C. Then, the cells were washed with PBS, rehydrated, and stained in propidium iodide (BD Biosciences, San Jose, CA, USA). The stained cells (~1×105) were then examined using a flow cytometer and analyzed using ModFit software (BD Biosciences).
Colony formation
Clonogenic abilities of cells were examined by colony formation assays. In brief, 400 cells from each treatment were cultured in the six-well plates for 14 days. After 14-day culture, the cells were stained using crystal violet, and the stained cells were counted under an inverted microscope (Olympus, Tokyo, Japan).
Wound healing assays
Wound healing assays were conducted to examine the migration ability of cells. A scratch was made on the mono-layer of cells using a sterile plastic tip. After 24 hours of incubation, the closure of the scratch was observed. The scratch width was measured under an inverted microscope (Olympus), and cell migration was calculated.
Transwell invasion assays
Transwell assays were conducted to detect the invasion capabilities of cells. Cells (4×105) were plated within the top chamber coated with Matrigel membrane. FBS 10% was used as a chemoattractant in the lower chamber. Cells were incubated for 48 hours, and the cells that did not invade through the membrane were removed by a cotton swab. Cells on the lower chamber were then stained with crystal violet and counted under an inverted microscope (Olympus).
Statistical analysis
Data are expressed as mean ± SD. SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) was applied for statistical analysis. Student’s t-test was used to compare differences between two groups, and one-way ANOVA with post hoc Dunnett’s multiple comparison was performed to determine the differences among three independent groups. P<0.05 was considered statistically significant.
Results
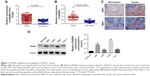
PAQR4 is significantly upregulated in NSCLC cells tissues
Although PAQR4 is reported to be involved in human breast cancer, the role of PAQR4 in human NSCLC remains unclear. The Cancer Genome Atlas information showed that the expression of PAQR4 was significantly upregulated in NSCLC tissues compared with the normal tissues (Figure 1A). As shown in Figure 1B, PAQR4 was significantly upregulated in NSCLC tissues compared with the normal tissues. The results of IHC displayed that PAQR4 expression was significantly upregulated in NSCLC tissues compared with the corresponding normal tissues (Figure 1C). As shown in Figure 1D, PAQR4 expression was dramatically upregulated in NSCLC cell lines compared with a human normal lung epithelial cell line. We found that PAQR4 expression was associated with pathological parameters, including tumor size, TNM stage, and lymph node metastasis (Table 1). These results suggest that PAQR4 is significantly upregulated in NSCLC tissues.
  | Table 1 The relationship between PAQR4 expression and pathological parameters of NSCLC patients |
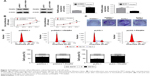
PAQR4 promotes cell proliferation, colony formation, and cell cycle
Next, we examined cell proliferation, colony formation, and cell cycle after overexpression or knockdown of PAQR4. Transfection efficacy was determined by Western blotting (Figure 2A). As shown in Figure 2B, the overexpression of PAQR4 promoted A549 cell proliferation compared with the control group, whereas the knockdown of PAQR4 inhibited H460 cell proliferation. As shown in Figure 2C, the overexpression of PAQR4 promoted A549 cell colony formation compared with the control group, but the knockdown of PAQR4 inhibited H460 cell colony formation. The results of flow cytometry demonstrated that the overexpression of PAQR4 boosted A549 cell cycle progression, whereas the knockdown of PAQR4 repressed H460 cell cycle progression (Figure 2D). These results suggest that PAQR4 promotes cell proliferation, colony formation, and cell cycle.
PAQR4 promotes cell migration and invasion
In this study, we evaluated cell migration and invasion after overexpression or knockdown of PAQR4. As shown in Figure 3A, cell migration was promoted in the pcDNA3.1-PAQR4 group compared with the control group, while cell migration was inhibited in the pLKO.1-PAQR4 group. Transwell invasion assays demonstrated that cell invasion was promoted in the pcDNA3.1-PAQR4 group compared with control treatment, whereas cell invasion was slowed down in the pLKO.1-PAQR4 group (Figure 3B). These results indicate that PAQR4 promotes cell migration and invasion.
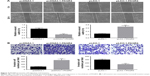
Knockdown of PAQR4 inhibits tumor growth
To investigate the effect of PAQR4 on tumor growth, we built murine xenograft models. We found that tumors of the pLKO.1-PAQR4 group grew much slower than those of the control group. Moreover, tumors of the pLKO.1-PAQR4 group were much heavier than those of the control group (Figure 4A). We found that Ki67 protein expression was decreased in the tumors of the pLKO.1-PAQR4 group compared with the control group (Figure 4B). These findings suggest that knockdown of PAQR4 inhibits tumor growth in vivo.
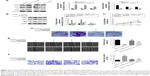
The CDK4-pRB-E2F1 pathway is involved in NSCLC
Finally, we examined the effect of PAQR4 on CDK4-pRB-E2F1 pathway. As shown in Figure 5A, the expression of CDK4, pRB, and E2F1 proteins was increased in the pcDNA3.1-PAQR4 group compared with the control group, whereas the expression of CDK4, pRB, and E2F1 proteins was decreased in the pLKO.1-PAQR4 group (Figure 5A). As shown in Figure 5B–F, CDK4 knockdown by shRNAs or inhibition of CDK4 by palbociclib promoted proliferation, colony formation, migration, and invasion of cells transfected with pLKO.1-PAQR4. There results manifest that the CDK4-pRB-E2F1 pathway is involved in NSCLC.
Discussion
NSCLC is the most common type of lung cancer, which poses a great threat to the health of the people around the world.23,24 Even though great advances have been made in the treatment of NSCLC, the long-term prognosis is still very poor.25 It is reported that recurrence and metastasis mainly contribute to the poor prognosis and therapeutic failure of NSCLC cases.26,27 As a conseqeunce, effective treatments are in great need. Up to now, modulation of key genes implicated in tumor development and progression is referred to as a promising therapeutic strategy.
PAQR4, known as a member of the PAQR4 family, plays crucial roles in many biological processes, including cell proliferation, cell cycle, cell differentiation, and cell death.11–15 A previous study reported that PAQR4 was significantly upregulated in human breast cancer tissues and served as an oncogene.11 However, the biological role of PAQR4 in NSCLC is still unclear. Therefore, better understanding the role of PAQR4 may be useful for improving the curative effects and lengthening overall survival of the NSCLC patients. In this study, we carried out qRT-PCR analysis and IHC assays, and found that PAQR4 is significantly upregulated in NSCLC tissues compared with the matched normal tissues. Interestingly, we found PAQR4 was also significantly upregulated in the NSCLC cell lines. These results indicate that PAQR4 may be involved in the occurrence and development of NSCLC.
Subsequently, we performed further studies. In this study, we found that the overexpression of PAQR4 promoted cell proliferation, colony formation, cell cycle, cell migration, and invasion, whereas the knockdown of PAQR4 inhibited cell proliferation, colony formation, cell cycle, cell migration, and invasion. Moreover, we found that the CDK4-pRB-E2F1 pathway was implicated in NSCLC. In this study, we downregulated the expression of CDK4 using pLKO.1-CDK4 and inhibited the pathway using CDK4 inhibitor, and discovered that inhibition of the CDK4-pRB-E2F1 pathway mitigated the promoting effect of PAQR4 on cell proliferation, migration, and invasion. Additionally, past studies have reported that the CDK4/pRB-E2F1 pathway plays key roles in diverse types of human neoplasms, such as glioblastoma multiforme,16 melanoma,17 endometrial cancer,18 pituitary tumor,19 prostate cancer,20 liver cancer,21 and gastric cancer.22 It is reported that the CDK4-pRB-E2F1 pathway is related to cell apoptosis and the progression of cell cycle.28,29
Conclusion
PAQR4 expression was significantly upregulated in human NSCLC tissues, and the expression of PAQR4 was also dramatically upregulated in NSCLC cell lines. Besides, this study uncovered that PAQR4 promotes proliferation and metastasis in NSCLC through the CDK4-pRB-E2F1 pathway. Hence, PAQR4 may be used as a potential diagnostic and curative target in NSCLC, and targeting the CDK4-pRB-E2F1 pathway may be a novel therapy for NSCLC.
Disclosure
The authors report no conflicts of interest in this work.
References
Tanvetyanon T, Lee JH, Fulp WJ, et al. Changes in the care of non-small-cell lung cancer after audit and feedback: the Florida initiative for quality cancer care. J Oncol Pract. 2014;10(4):e247–e254. | ||
Johnson DH. Evolution of cisplatin-based chemotherapy in non-small cell lung cancer: a historical perspective and the Eastern Cooperative Oncology Group experience. Chest. 2000;117(4 Suppl 1):133S–137S. | ||
Trippoli S, Vaiani M, Lucioni C, Messori A, et al. Quality of life and utility in patients with non-small cell lung cancer. Pharmacoeconomics. 2001;19(8):855–863. | ||
Behera D, Balamugesh T. Lung cancer in India. Indian J Chest Dis Allied Sci. 2008;46(4):269–281. | ||
Felip E, Garrido P, Trigo JM, et al. SEOM guidelines for the management of non-small-cell lung cancer (NSCLC). Clin Transl Oncol. 2009;11(5):284–289. | ||
Moreno M, Aristu J, Ramos LI, et al. Predictive factors for radiation-induced pulmonary toxicity after three-dimensional conformal chemoradiation in locally advanced non-small-cell lung cancer. Clin Transl Oncol. 2007;9(9):596–602. | ||
Yein CC. Role of CD151 in Epidermal Growth Factor-Induced Non-Small Cell Lung Cancer Cell Proliferation [master’s thesis]. Singapore: National University of Singapore; 2014. | ||
Sieg DJ, Hauck CR, Ilic D, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2(5):249–256. | ||
Ito T, Shiraki K, Sugimoto K, et al. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31(5):1080–1085. | ||
Kajita M, Itoh Y, Chiba T, et al. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153(5):893–904. | ||
Zhang H, Han R, Ling ZQ, et al. PAQR4 has a tumorigenic effect in human breast cancers in association with reduced CDK4 degradation. Carcinogenesis. 2018;39(3):439–446. | ||
Wang L, Zhang R, You X, et al. The steady-state level of CDK4 protein is regulated by antagonistic actions between PAQR4 and Skp2 and involved in tumorigenesis. J Mol Cell Biol. 2017;9(5):409–421. | ||
Tang YT, Hu T, Arterburn M, et al. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61(3):372–380. | ||
Xiang J, Qin HY, Han H. Advanced studies on PAQR membrane protein family. Progress in Modern Biomedicine. 2012;12(1):166–170. | ||
Yu X, Li Z, Chan MT, Wu WK. PAQR3: a novel tumor suppressor gene. Am J Cancer Res. 2015;5(9):2562–2568. | ||
Qiu S, Huang D, Yin D, et al. Suppression of tumorigenicity by microRNA-138 through inhibition of EZH2-CDK4/6-pRb-E2F1 signal loop in glioblastoma multiforme. Biochimica Et Biophysica Acta. 1832;2013(10):1697. | ||
Bartkova J, Lukas J, Guldberg P, et al. The p16-cyclin D/Cdk4-pRb pathway as a functional unit frequently altered in melanoma pathogenesis. Cancer Res. 1996;56(23):5475. | ||
Tsuda H, Yamamoto K, Inoue T, Uchiyama I, Umesaki N. The role of p16-cyclin d/CDK-pRb pathway in the tumorigenesis of endometrioid-type endometrial carcinoma. Br J Cancer. 2000;82(3):675–682. | ||
Fedele M, Visone R, De Martino I, et al. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell. 2006;9(6):459–471. | ||
Mao A, Liu Y, Wang Y, et al. miR-449a enhances radiosensitivity through modulating pRb/E2F1 in prostate cancer cells. Tumour Biol. 2016;37(4):4831–4840. | ||
Santoni-Rugiu E, Jensen MR, Thorgeirsson SS. Disruption of the pRb/E2F pathway and inhibition of apoptosis are major oncogenic events in liver constitutively expressing c-myc and transforming growth factor alpha. Cancer Res. 1998;58(1):123. | ||
Deng M, Zeng C, Lu X, et al. miR-218 suppresses gastric cancer cell cycle progression through the CDK6/Cyclin D1/E2F1 axis in a feedback loop. Cancer Lett. 2017;403:175–185. | ||
Arenberg DA, Kunkel SL, Polverini PJ, et al. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med. 1996;184(3):981–992. | ||
Ford CE, Lau SK, Zhu CQ, et al. Expression and mutation analysis of the discoidin domain receptors 1 and 2 in non-small cell lung carcinoma. Br J Cancer. 2007;96(5):808–814. | ||
Yuxia M, Zhennan T, Wei Z. Circulating miR-125b is a novel biomarker for screening non-small-cell lung cancer and predicts poor prognosis. J Cancer Res Clin Oncol. 2012;138(12):2045–2050. | ||
Chen LT, Xu SD, Xu H, et al. MicroRNA-378 is associated with non-small cell lung cancer brain metastasis by promoting cell migration, invasion and tumor angiogenesis. Med Oncol. 2012;29(3):1673–1680. | ||
Srivastava G, Rana V, Wallace S, et al. Risk of intracranial hemorrhage and cerebrovascular accidents in non-small cell lung cancer brain metastasis patients. J Thorac Oncol. 2009;4(3):333–337. | ||
Chen XC, Chen LM, Zhu YG, et al. Involvement of CDK4, pRB, and E2F1 in ginsenoside Rg1 protecting rat cortical neurons from beta-amyloid-induced apoptosis[J]. Acta Pharmacologica Sinica. 2003;24(12):1259. | ||
Hauck L, von Harsdorf R. E2F transcription factors and pRb pocket proteins in cell cycle progression. Methods Mol Biol. 2005;296:239–245. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.