Back to Journals » Drug Design, Development and Therapy » Volume 14
Panax Notoginseng Ameliorates Podocyte EMT by Targeting the Wnt/β-Catenin Signaling Pathway in STZ-Induced Diabetic Rats
Authors Xie L , Zhai R, Chen T , Gao C, Xue R, Wang N, Wang J, Xu Y, Gui D
Received 21 October 2019
Accepted for publication 19 January 2020
Published 5 February 2020 Volume 2020:14 Pages 527—538
DOI https://doi.org/10.2147/DDDT.S235491
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianbo Sun
Ling Xie, 1, 2 Ruonan Zhai, 3 Teng Chen, 3 Chongting Gao, 3 Rui Xue, 3 Niansong Wang, 3 Jianbo Wang, 4 Youhua Xu, 5 Dingkun Gui 3
1College of Fisheries and Life Science, Shanghai Ocean University, Shanghai, People’s Republic of China; 2Department of Nephrology, Shanghai Sixth People’s Hospital East Campus, Shanghai, People’s Republic of China; 3Department of Nephrology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China; 4Department of Interventional Radiology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China; 5Faculty of Chinese Medicine, State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, Taipa, Macau, People’s Republic of China
Correspondence: Dingkun Gui
Department of Nephrology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, 600 Yishan Road, Shanghai 200233, People’s Republic of China
Tel +86 021-64369181
Email [email protected]
Jianbo Wang
Department of Interventional Radiology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, 600 Yishan Road, Shanghai 200233, People’s Republic of China
Tel +86 21-6436 9181
Email [email protected]
Introduction: Epithelial–mesenchymal transition (EMT) may contribute to podocyte dysfunction in diabetic nephropathy (DN). Aiming to identify novel therapeutic options, we investigated the protective effects of Panax notoginseng (PN) on podocyte EMT in diabetic rats and explored its mechanisms.
Methods: Diabetes was induced in rats with streptozotocin (STZ) by intraperitoneal injection at 55 mg/kg. Diabetic rats were randomly divided into three groups, namely, diabetic rats, diabetic rats treated with beraprost sodium (BPS) at 0.6 mg/kg/d or PN at 0.4 g/kg/d p.o., for 12 weeks. Urinary albumin/creatinine ratio (ACR), biochemical parameters, renal histopathology, and podocyte morphological changes were evaluated. Protein expression of EMT markers (desmin, α-SMA, and nephrin) as well as components of the Wnt/β-catenin pathway (wnt1, β-catenin, and snail) was detected by immunohistochemistry and Western blot, respectively.
Results: In diabetic rats, severe hyperglycemia and albuminuria were detected. Moreover, mesangial expansion and podocyte foot process effacement were found markedly increased in diabetic kidneys. Increased protein expression of wnt1, β-catenin, snail, desmin, and α-SMA, as well as decreased protein expression of nephrin was detected in diabetic kidneys. All these abnormalities found in DN rats were partially restored by PN treatment.
Conclusion: PN ameliorated albuminuria and podocyte EMT in diabetic rats partly through inhibiting Wnt/β-catenin signaling pathway. These findings provide experimental arguments for a novel therapeutic option in DN.
Keywords: Panax notoginseng, diabetic nephropathy, podocyte, Wnt/β-catenin, epithelial–mesenchymal transition
Introduction
Diabetic nephropathy is a major cause of end-stage renal disease1 and becomes a global health problem. However, therapeutic strategies for preventing its progression are limited. The morphological abnormalities of DN include mesangial expansion, glomerular endothelial cell injury, glomerular basement membrane thickening, and podocytes depletion.2,3 Podocytes are important functional cells in the glomerulus and cannot regenerate when they suffer from injury. The clinical study demonstrated that podocyte loss contributed to the progression of DN.4 Many studies indicated that podocyte injury played an important role in the development and progression of DN.5–9 Recently, epithelial–mesenchymal transition has been demonstrated to play an important role in renal fibrogenesis.10 Podocyte EMT was also associated with the development of albuminuria in DN.11 Moreover, EMT was a crucial mechanism leading to podocyte depletion and proteinuria in DN.12 Thus, podocytes EMT may contribute to podocyte dysfunction, which represents a novel mechanism leading to DN. This novel discovery may offer promising insights to the development of new therapeutic options for the treatment of DN. It was reported that podocyte depletion was in association with the development of DN.13 Therefore, there is a pressing need for the development of novel and effective interventions for podocyte injury. Panax notoginseng, an edible and medicinal Chinese herb, is widely used in China for physical fitness. PN has been included in the list of health Chinese herbs with drug and food properties announced by the National Health Commission of the People’s Republic of China. Our previous study found that Notoginsenoside R1, one of the major components of PN, ameliorated glucose-induced podocyte adhesive dysfunction and subsequent podocyte detachment.14 Notoginsenoside R1 was also reported to attenuate renal ischemia-reperfusion injury in rats.15 Panax notoginseng saponins, the traditional Chinese medical compound consisting of total saponins from PN, protected against kidney injury in diabetic rats through upregulation of silent information regulator and activation of antioxidant proteins.16 However, the protective effects of PN on podocyte EMT in diabetic rats have not been investigated yet. Thus, this study aimed to investigate the effects of PN on albuminuria and podocyte EMT in diabetic rats. We hypothesized that PN attenuated albuminuria and podocyte EMT in diabetic rats partly through inhibiting Wnt/β-catenin signaling pathway, which will provide a potential new treatment for DN.
Materials and Methods
Materials
Panax notoginseng granule was purchased from Sanjiu Medical & Pharmaceutical Co Ltd (Shenzhen, China, the approval number of the Chinese Pharmaceutical Regulatory Department:1702002S). The total saponin content in PN granule was greater than 3.75%. Beraprost sodium was purchased from Beijing Tide Pharmaceutical Co Limited (Beijing, China). Streptozotocin (STZ) (1000254870) was acquired from Sigma (Sigma, USA). Bicinchoninic acid (BCA) (BL521A) was acquired from Biosharp (Biosharp, China). ECL (BL520A) was acquired from Biosharp (Biosharp, China). Bovine serum albumin (BSA)(4240GR100) was acquired from BioFroxx (BioFroxx, Germany). Nitrocellulose membrane acquired from Millipore (Millipore, USA). Anti-Wnt1 (sc-514531) antibody was purchased from Santa Cruz (Santa Cruz, USA). Anti-Nephrin (PRS2265) antibody was purchased from sigma (Sigma, USA), and the secondary antibodies were purchased from Cell Signaling (Cell Signaling, USA). Antibodies of β-catenin (ab32572), snail (ab53519), desmin (ab15200), α-SMA (ab5694), β-actin (ab8226), α-tubulin (ab7291) and the secondary antibodies (ab6721) were obtained from Abcam (Abcam, USA). Blood glucose meter (ACCU-CHEK, Germany); Automatic biochemistry analyzer (HITACHI 7600-120E, Japan); light microscopy (Leica, Germany); electron microscopy (Philip, Netherlands).
Animal Studies
All animal experiments were performed in accordance with the National Institutes of Health guide for the care and use of laboratory animals. All experimental protocols were approved by the Animal Ethics Committee of Shanghai sixth people’s hospital (laboratory animal permit: SYXK (Shanghai) 2011-0128). Healthy male Sprague-Dawley (SD) rats weighing 180 to 200 g were provided by Experimental Animal Center of our hospital. Animals were fed in room temperature at 23°C. Rats were fed with free water and standard food. Diabetes rats were induced with STZ (55 mg/kg) dissolved in 0.1 M citrate buffer (pH 4.5) by a single intraperitoneal. Three days after STZ injection, the blood glucose (BG) level was measured from the tail vein using a blood glucose meter. Rats with a blood glucose beyond 16.7 mmol/L were considered as diabetic rats. Diabetic rats were then randomly divided into three groups (n=7/each group): (1) diabetic rats (DN); (2) diabetic rats treated with BPS at 0.6 mg/kg/d (DN+BPS); (3) diabetic rats treated with PN at 0.4 g/kg/d (DN+PN). Normal Sprague-Dawley rats were considered as normal control rats (NC). BPS and PN were started after blood glucose beyond 16.7 mmol/L and were treated by oral garage once daily for 12 weeks. Rats were kept in individual metabolic cages for 24 hrs urine collection at the end of 3 and 12 weeks of treatment. Urine was centrifuged at 1500 rpm for 5 mins at 4°C. Whole urine was stored at −80°C. Urinary albumin/creatinine ratio (ACR) was measured by using an automatic biochemistry analyzer. Rats were then anesthetized with pentobarbital sodium and the blood samples were taken through the abdominal aorta for measuring creatinine by using an automatic biochemistry analyzer. All rats were then sacrificed and the kidneys were harvested immediately.
Histological Studies
Tissues were fixed with 10% buffered formalin and embedded in paraffin, cut into 4 μm sections and stained with hematoxylin and eosin (HE) and periodic acid-schiff (PAS). The stained sections were then detected by light microscopy. Glomerular injury was evaluated by mesangial expansion in sections stained with HE and PAS. The sections were then observed independently by two blinded investigators. Glomerular mesangial expansion was scored semi-quantitatively as previously described in the literature.17
Electron Microscopy Studies
The renal cortex was cut into pieces on ice, fixed with 2% glutaraldehyde dissolved in PBS 2 hrs at 4°C and washed twice in the same buffer. The tissue fragments were post-fixed in 1% PBS-buffered OsO4 for 2 hrs at 4°C and washed twice in the same buffer. Dehydrate with different concentration of ethanols. Epoxy propane replacement twice. Saturate with epoxy propane and epoxy resin 618, embedded in epoxy 48 hrs at 60°C. Ultrathin sections were stained with uranyl acetate and lead citrate and examined by electron microscopy. The number of podocyte foot processes present in each micrograph was divided by the total length of GBM regions in each image to determine the average density of podocyte foot processes. The electron microscope photos were evaluated in a blinded fashion.
Western Blot Studies
Kidney cortex was homogenized in lysis buffer on ice with a homogenizer. The supernatants were collected after centrifuging at 10,000 rpm for 10 mins at 4°C. Protein concentration of the supernatants was measured by the BCA kit assay. The whole tissue lysates were mixed with SDS loading buffer (30% Acrylamide, 1 M Tris–HCl6.8, 1.5 M Tris–HCl8.8, 10% SDS, 10% Ammonium persulfate, TEMED, ddH2O). Samples were separated by sodium dodecyl sulfate (SDS)/polyacrylamide gel electrophoresis and electro-transferred to a nitrocellulose membrane. The membranes were blocked with 5% bovine serum albumin in TBST for 1.5 hrs. After washing 5 mins for three times with TBST, the blots were incubated overnight at 4°C with anti-nephrin (1:2000), anti-desmin (1:5000), anti-α-SMA (1:5000), anti-wnt1 (1:1000), anti-β-catenin (1:5000), anti-snail (1:3000), and anti-β-actin (1:5000) and α-tubulin (1:5000) antibodies at the dilutions by 5% bovine serum albumin in TBST. After washing 5 mins for five times with TBST, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000) at the dilutions by 5% bovine serum albumin in TBST for 1 hr at room temperature. After washing 5 mins for five times with TBST, protein bands were visualized by ECL. Optical density of the bands was measured by a Bio-Rad gel imaging system. Equality of loading was ensured by using the antibody to β-actin or α-tubulin. Protein expression was quantified as the ratio of specific band to β-actin or α-tubulin.
Immunohistochemistry Studies
Paraffin-embedded of kidney tissues were deparaffinized with rehydrated, xylene, and ethanol. Anti-nephrin (1:200), anti-desmin (1:200), anti-α-SMA (1:200), anti-wnt1 (1:200), anti-β-catenin (1:500) and anti-snail (1:100) antibodies were diluted in 1% BSA, all antibodies were incubated during 45 mins at room temperature. Antigen repaired for 10 mins by boiling water bath. Primary antibodies were diluted in 2% BSA and incubated overnight at 4°C. The secondary antibodies (1:1000) were incubated 60 mins at 37°C. Use diaminobenzidine and hematoxylin staining, and then rinsing, redyeing, dehydration, transparency, and tablet sealing. The immunohistochemical staining in each glomerulus was scored semi-quantitatively as previously described in the literature.18 All slides were observed independently by two blinded investigators.
Statistical Analysis
All data were expressed as mean ± standard error of the mean (SEM). Statistics were conducted by SPSS version 18.0. The significance of differences in continuous variables among groups was tested by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple range test. A P value < 0.05 was considered statistically significant.
Results
Effects of PN on Serum and Urine Levels of Biochemical Markers in Diabetic Rats
The STZ-induced diabetic rats developed severe hyperglycemia and albuminuria. Level of blood glucose in DN, DN+BPS, and DN+PN groups were prominently increased in STZ-induced diabetic rats when compared with control rats (Figure 1A). No significant difference in the level of creatinine were observed between PN treated and untreated diabetic rats, which indicated that PN did not cause apparent toxicity to the kidney (Figure 1B). The diabetic rats showed severe albuminuria when compared with the control rats. However, PN or BPS significantly reduced ACR in diabetic rats at the end of 3 weeks and 12 weeks (Figure 2) of treatment. Thus, PN treatment attenuated albuminuria in diabetic rats.
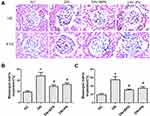
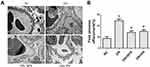
Effects of PN on Renal Histopathology and Podocyte Foot Process Effacement in STZ-Induced Diabetic Rats
Diabetic rats exhibited focal mesangial matrix expansion. However, PN or BPS markedly ameliorated mesangial expansion when compared with untreated STZ-induced diabetic rats (Figure 3). Moreover, PN or BPS also ameliorated podocyte foot process effacement in diabetic rats (Figure 4). These results indicated that PN significantly attenuated renal histopathology and podocyte foot process effacement in diabetic rats.
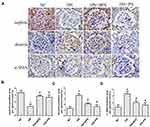
Effects of PN on Podocyte EMT in Diabetic Rats
Compared with the control rats, we observed that the protein of nephrin was markedly reduced in diabetic rats. Nevertheless, treatment with PN increased the protein expression of nephrin (Figure 5A) in diabetic rats. Compared with the normal control rats, the protein expression of α-SMA and desmin was increased in diabetic rats. However, DN+PN group showed decreased protein expression of desmin (Figure 5B) and α-SMA (Figure 5C) in diabetic rats. The findings of immunohistochemical staining further confirmed the above findings (Figure 6). Decreased protein expression of nephrin, and increased protein expression of desmin and α-SMA were tested in kidneys from diabetic rats. Furthermore, these abnormalities were restored by treatment with PN. These results demonstrated that PN might ameliorate podocyte EMT in diabetic rats.
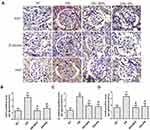
Effects of PN on Wnt/β-Catenin Signaling Pathway in STZ-Induced Diabetic Kidneys
By Western blot analysis, we found that the protein expression of wnt1, β-catenin, and snail was increased in the renal tissue from STZ-induced diabetic rats when compared with the control rats. Most importantly, PN treatment suppressed protein expression of wnt1 (Figure 7A), β-catenin (Figure 7B) and snail (Figure 7C) in the kidneys from diabetic rats. These findings were further confirmed by the results of immunohistochemistry analysis (Figure 8). We observed that the expression of wnt1, β-catenin, and snail was markedly increased in the renal tissue from diabetic rats when compared with the control rats. However, PN treatment decreased expression of wnt1, β-catenin, and snail in diabetic rats. The above results indicated that the protective effect of PN on podocyte EMT was associated with the inhibition of Wnt/β-catenin signaling pathway in diabetic rats.
Discussion
Diabetes mellitus (DM) has become an important global health problem.19 Clinical hallmarks of DN include proteinuria and decline in glomerular filtration rate leading to ESRD.20,21 These functional changes developed a consequence of structural abnormalities, including mesangial expansion and podocyte injury.22,23 Recently, much work has underlined the important role of podocyte in the development and progression of DN.5–9 Thus, podocytes become a promising target for the development of novel treatment for DN.
The novel finding of this study was the discovery of a natural medicine for treatment of DN. We reported that PN ameliorated podocyte dysfunction and EMT partly by inhibiting Wnt/β-catenin signaling pathway in STZ-induced diabetic rats. This conclusion was based upon the following findings: (i) Treatment with PN attenuated the functional and structural abnormalities of DN, such as albuminuria, mesangial expansion, and podocyte foot process effacement; (ii) Treatment with PN ameliorated the protein expression of desmin, α-SMA, and nephrin in diabetic rats. (iii) Treatment with PN significantly inhibited protein expression of wnt1, β-catenin, and snail. Therefore, PN might be a novel drug to attenuate podocyte EMT partly through inhibiting Wnt/β-catenin signaling pathway in diabetic rats. PN is a promising drug candidate for DN according to the results obtained in an animal model of DN.
Beraprost sodium is an orally active prostacyclin (prostaglandin I2; PGI2) analogue. Previous study reported that BPS ameliorated proteinuria and renal histologic lesions in diabetic rats.24 Experimental study documented that BPS had a beneficial effect on DN.25 Recent study demonstrated that BPS attenuated characteristics of metabolic syndrome in obese rats.26 Thus, BPS was used as a positive control in this study to investigate the protective effect of PN on the functional and structural abnormalities of DN. We found that treatment with PN or BPS attenuated the functional and structural abnormalities of DN. The previous study found that the BPS alleviated constriction effect of angiotensin II on efferent glomerular arteriole and attenuated glomerular hyperfiltration, as well as inhibited growth of mesangial cells by platelet-derived growth factor to decrease albuminuria in the patients of incipient DN.27 However, there were different mechanisms of PN and BPS in the treatment of DN. Our study demonstrated that PN protected DN through inhibiting Wnt/β-catenin signaling pathway.
Loss of podocytes by EMT characterizes the early stages of DN. To identify new therapeutic strategies, we firstly investigated the effects of PN on podocyte EMT in diabetic rats. Podocyte injury resulted in abnormal permeability of the GBM, terminally leading to proteinuria.28,29 Importantly, podocyte injury was closely associated with the progression of glomerular diseases.30 Many studies demonstrated that EMT was associated with podocyte loss and proteinuria in DN.31–33 During EMT, podocytes lose the expression of nephrin, while augmenting the expression of desmin and α-SMA.34–36 Taken together, podocyte EMT is recognized to play a crucial role in the development of DN and targeted inhibition of podocyte EMT may provide a new therapeutic approach for DN. In the present study, we observed that α-SMA and desmin expression were increased while nephrin expression was decreased in diabetic rats. Treatment with PN for 12 weeks decreased renal expression of α-SMA and desmin, preserved nephrin expression, as well as ameliorated albuminuria in diabetic rats. These results demonstrated that PN ameliorated podocyte EMT in diabetic rats.
We next investigated the mechanisms underlying the action of PN on podocyte EMT in diabetic rats. Dysfunction of Wnt signaling may contribute to a wide range of pathologies of human diseases.37 Emerging evidence demonstrated that Wnt/β-catenin signaling played a critical role in mediating podocyte dysfunction and albuminuria.38 Wnt signaling pathway was also associated with podocyte injury and dysfunction in DN.39 In vitro study demonstrated that high glucose increased expression of β-catenin and snail and promoted EMT.40 Mounting evidence indicated that Wnt/β-catenin signaling might be a novel therapeutic target for proteinuria kidney disease.41 In the present study, we found that increased protein expression of wnt1, β-catenin, snail, desmin, and α-SMA, as well as decreased protein expression of nephrin, were detected in diabetic rats. All of these abnormalities were restored by PN. These results indicated that the regulatory effect of PN on Wnt/β-catenin signaling pathway might be accountable for its protective effects against podocyte EMT.
Moreover, a systematic review and meta-analysis provided evidence that PN was beneficial to patients with unstable angina pectoris and did not affect blood routine, urine routine, stool routine, as well as liver and renal function.42 In our study, PN had no significant effect on blood glucose, which indicated that the protective effects of PN on albuminuria were independent of lowering blood glucose (data not shown). Furthermore, there was no significant difference in the level of serum creatinine between PN treated and untreated diabetic rats, which indicated that PN did not cause apparent toxicity to the kidney.
STZ-model is one of the widely used animal models of early DN, due to their brief experimental procedure and absence of advanced pathological lesions. But the effects of STZ-induced diabetes are closely dependent on mouse strain and gender, with male mice being more susceptible to STZ-induced diabetes and obvious renal injury compared to their female counterparts.43 STZ selectively leads to damage to the insulin-producing β-cells in the pancreas. Destruction to these cells causes insulin deficiency and impairs normal glucose metabolism,44 which similar to human type 1 diabetes progresses in DN. We will use the type 2 diabetes model to further investigate the protective effects of PN in future studies.
In conclusion, our study demonstrated that PN attenuated albuminuria and podocyte EMT in diabetic rats partly through inhibiting Wnt/β-catenin signaling pathway (Figure 9). These findings highlight a promising novel treatment for DN and other renal diseases that affect podocytes.
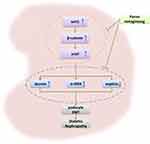 |
Figure 9 Schematic mechanism of PN of ameliorating podocyte EMT by Wnt/β-catenin signaling pathway in STZ-induced diabetic rats. |
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81774052 and 81573738), the Pudong New Area Science and Technology Commission (PKJ2015-Y10). This work was also sponsored by the Shanghai Pujiang Program (17PJD031) and Three-year action plan for Shanghai further accelerating the development of traditional Chinese medicine (ZY (2018-2020)-CCCX-4007).
Disclosure
The authors report no conflicts of interest in this work.
References
1. USRDS: the United States Renal Data System. Am J Kidney Dis. 2003;42(6 Suppl 5):1–230.
2. Gnudi L, Coward RJM, Long DA. Diabetic nephropathy: perspective on novel molecular mechanisms. Trends Endocrinol Metab. 2016;27(11):820–830. doi:10.1016/j.tem.2016.07.002
3. Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56(8):2155–2160. doi:10.2337/db07-0019
4. Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99(2):342–348. doi:10.1172/JCI119163
5. Maezawa Y, Takemoto M, Yokote K. Cell biology of diabetic nephropathy: roles of endothelial cells, tubulointerstitial cells and podocytes. J Diabetes Investig. 2015;6(1):3–15. doi:10.1111/jdi.12255
6. Andeen NK, Nguyen TQ, Steegh F, Hudkins KL, Najafian B, Alpers CE. The phenotypes of podocytes and parietal epithelial cells may overlap in diabetic nephropathy. Kidney Int. 2015;88(5):1099–1107. doi:10.1038/ki.2015.273
7. Eid S, Boutary S, Braych K, et al. mTORC2 signaling regulates Nox4-induced podocyte depletion in diabetes. Antioxid Redox Signal. 2016;25(13):703–719. doi:10.1089/ars.2015.6562
8. Sweetwyne MT, Gruenwald A, Niranjan T, Nishinakamura R, Strobl LJ, Susztak K. Notch1 and Notch2 in podocytes play differential roles during diabetic nephropathy development. Diabetes. 2015;64(12):4099–4111. doi:10.2337/db15-0260
9. MS M. The podocyte a potential therapeutic target in diabetic nephropathy. Curr Pharm Des. 2007;13(26):2713–2720. doi:10.2174/138161207781662957
10. Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15(1):1–12. doi:10.1097/01.ASN.0000106015.29070.E7
11. Dai H, Liu Q, Liu B. Research progress on mechanism of podocyte depletion in diabetic nephropathy. J Diabetes Res. 2017;2017:2615286. doi:10.1155/2017/2615286
12. Anil Kumar P, Welsh GI, Saleem MA, Menon RK. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol (Lausanne). 2014;5:151. doi:10.3389/fendo.2014.00151
13. K RA S, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–233. doi:10.2337/diabetes.55.01.06.db05-0894
14. Gui D, Wei L, Jian G, Guo Y, Yang J, Wang N. Notoginsenoside R1 ameliorates podocyte adhesion under diabetic condition through alpha3beta1 integrin upregulation in vitro and in vivo. Cell Physiol Biochem. 2014;34(6):1849–1862. doi:10.1159/000366384
15. Liu WJ, Tang HT, Jia YT, et al. Notoginsenoside R1 attenuates renal ischemia-reperfusion injury in rats. Shock. 2010;34(3):314–320. doi:10.1097/SHK.0b013e3181ceede4
16. Du YG, Wang LP, Qian JW, Zhang KN, Chai KF. Panax notoginseng saponins protect kidney from diabetes by up-regulating silent information regulator 1 and activating antioxidant proteins in rats. Chin J Integr Med. 2016;22(12):910–917. doi:10.1007/s11655-015-2446-1
17. Kang YS, Lee MH, Song HK, et al. CCR2 antagonism improves insulin resistance, lipid metabolism, and diabetic nephropathy in type 2 diabetic mice. Kidney Int. 2010;78(9):883–894. doi:10.1038/ki.2010.263
18. Varghese F, Bukhari AB, Malhotra R, De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9(5):e96801. doi:10.1371/journal.pone.0096801
19. G D, Finucane MM, Lu Y, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980 systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 27 million participants. Lancet. 2011;378:31–40. doi:10.1016/S0140-6736(11)60679-X
20. Forbes JM, Fukami K, Cooper ME. Diabetic nephropathy: where hemodynamics meets metabolism. Exp Clin Endocrinol Diabetes. 2007;115(2):69–84. doi:10.1055/s-2007-949721
21. Jerums G, Panagiotopoulos S, Premaratne E, MacIsaac RJ. Integrating albuminuria and GFR in the assessment of diabetic nephropathy. Nat Rev Nephrol. 2009;5(7):397–406. doi:10.1038/nrneph.2009.91
22. Zhu WW, Chen HP, Ge YC, et al. Ultrastructural changes in the glomerular filtration barrier and occurrence of proteinuria in Chinese patients with type 2 diabetic nephropathy. Diabetes Res Clin Pract. 2009;86(3):199–207. doi:10.1016/j.diabres.2009.09.009
23. Su J, Li SJ, Chen ZH, et al. Evaluation of podocyte lesion in patients with diabetic nephropathy: wilms’ tumor-1 protein used as a podocyte marker. Diabetes Res Clin Pract. 2010;87(2):167–175. doi:10.1016/j.diabres.2009.10.022
24. Peng L, Li J, Xu Y, et al. The protective effect of beraprost sodium on diabetic nephropathy by inhibiting inflammation and p38 MAPK signaling pathway in High-Fat Diet/Streptozotocin-induced diabetic rats. Int J Endocrinol. 2016;2016:1690474. doi:10.1155/2016/1690474
25. Watanabe M, Nakashima H, Mochizuki S, et al. Amelioration of diabetic nephropathy in OLETF rats by prostaglandin I(2) analog, beraprost sodium. Am J Nephrol. 2009;30(1):1–11. doi:10.1159/000195722
26. Sato N, Kaneko M, Tamura M, Kurumatani H. The prostacyclin analog beraprost sodium ameliorates characteristics of metabolic syndrome in obese Zucker (fatty) rats. Diabetes. 2010;59(4):1092–1100. doi:10.2337/db09-1432
27. Owada A, Suda S, Hata T. Effect of long-term administration of prostaglandin I(2) in incipient diabetic nephropathy. Nephron. 2002;92(4):788–796. doi:10.1159/000065445
28. Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol (Berl). 1995;192(5):385–397. doi:10.1007/BF00240371
29. Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7(4):255–259. doi:10.1007/s10157-003-0259-6
30. Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 1998;54(3):687–697. doi:10.1046/j.1523-1755.1998.00044.x
31. Reidy K, Susztak K. Epithelial-mesenchymal transition and podocyte loss in diabetic kidney disease. Am J Kidney Dis. 2009;54(4):590–593. doi:10.1053/j.ajkd.2009.07.003
32. Yamaguchi Y, Iwano M, Suzuki D, et al. Epithelial-mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis. 2009;54(4):653–664. doi:10.1053/j.ajkd.2009.05.009
33. Ying Q, Wu G. Molecular mechanisms involved in podocyte EMT and concomitant diabetic kidney diseases: an update. Ren Fail. 2017;39(1):474–483. doi:10.1080/0886022X.2017.1313164
34. Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172(2):299–308. doi:10.2353/ajpath.2008.070057
35. Chang YP, Sun B, Han Z, et al. Saxagliptin Attenuates Albuminuria by Inhibiting Podocyte Epithelial- to-Mesenchymal Transition via SDF-1alpha in Diabetic Nephropathy. Front Pharmacol. 2017;8:780. doi:10.3389/fphar.2017.00780
36. Wu X, Gao Y, Xu L, et al. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci Rep. 2017;7(1):9371. doi:10.1038/s41598-017-09907-6
37. Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi:10.1016/j.cell.2012.05.012
38. Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20(9):1997–2008. doi:10.1681/ASN.2009010019
39. Bose M, Almas S, Prabhakar S. Wnt signaling and podocyte dysfunction in diabetic nephropathy. J Investig Med. 2017;65(8):1093–1101. doi:10.1136/jim-2017-000456
40. Guo J, Xia N, Yang L, et al. GSK-3beta and vitamin D receptor are involved in beta-catenin and snail signaling in high glucose-induced epithelial-mesenchymal transition of mouse podocytes. Cell Physiol Biochem. 2014;33(4):1087–1096. doi:10.1159/000358678
41. Zhou L, Liu Y. Wnt/beta-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol. 2015;11(9):535–545. doi:10.1038/nrneph.2015.88
42. Song H, Wang P, Liu J, Wang C. Panax notoginseng Preparations for Unstable Angina Pectoris: a systematic review and meta-analysis. Phytother Res. 2017;31(8):1162–1172. doi:10.1002/ptr.v31.8
43. Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol. 2006;290(1):F214–F222. doi:10.1152/ajprenal.00204.2005
44. Chow BSM, Allen TJ. Mouse models for studying diabetic nephropathy. Curr Protoc Mouse Biol. 2015;5(2):85–94. doi:10.1002/9780470942390.mo140192
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.