Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Painful Diabetic Peripheral Neuropathy: Practical Guidance and Challenges for Clinical Management
Authors Preston FG , Riley DR , Azmi S, Alam U
Received 17 March 2023
Accepted for publication 16 May 2023
Published 2 June 2023 Volume 2023:16 Pages 1595—1612
DOI https://doi.org/10.2147/DMSO.S370050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Frank G Preston,1 David R Riley,1 Shazli Azmi,2 Uazman Alam1,3
1Department of Cardiovascular & Metabolic Medicine, Institute of Life Course and Medical Sciences and the Pain Research Institute, University of Liverpool, Liverpool, UK; 2Institute of Cardiovascular Science, University of Manchester and Manchester Diabetes Centre, Manchester Foundation Trust, Manchester, UK; 3Liverpool Centre for Cardiovascular Science, University of Liverpool and Liverpool University Hospital NHS Foundation Trust, Liverpool, UK
Correspondence: Uazman Alam, Department of Cardiovascular & Metabolic Medicine, Institute of Life Course and Medical Sciences, Clinical Sciences Centre, University Hospital Aintree, Longmoor Lane, Liverpool, L9 7AL, UK, Tel +44 0151 529 5918, Fax +44 0151 529 5888, Email [email protected]
Abstract: Painful diabetic peripheral neuropathy (PDPN) is present in nearly a quarter of people with diabetes. It is estimated to affect over 100 million people worldwide. PDPN is associated with impaired daily functioning, depression, sleep disturbance, financial instability, and a decreased quality of life. Despite its high prevalence and significant health burden, it remains an underdiagnosed and undertreated condition. PDPN is a complex pain phenomenon with the experience of pain associated with and exacerbated by poor sleep and low mood. A holistic approach to patient-centred care alongside the pharmacological therapy is required to maximise benefit. A key treatment challenge is managing patient expectation, as a good outcome from treatment is defined as a reduction in pain of 30– 50%, with a complete pain-free outcome being rare. The future for the treatment of PDPN holds promise, despite a 20-year void in the licensing of new analgesic agents for neuropathic pain. There are over 50 new molecular entities reaching clinical development and several demonstrating benefit in early-stage clinical trials. We review the current approaches to its diagnosis, the tools, and questionnaires available to clinicians, international guidance on PDPN management, and existing pharmacological and non-pharmacological treatment options. We synthesise evidence and the guidance from the American Association of Clinical Endocrinology, American Academy of Neurology, American Diabetes Association, Diabetes Canada, German Diabetes Association, and the International Diabetes Federation into a practical guide to the treatment of PDPN and highlight the need for future research into mechanistic-based treatments in order to prioritise the development of personalised medicine.
Keywords: diabetic peripheral neuropathy, painful diabetic peripheral neuropathy, diabetes complications, pharmacotherapy
Introduction
In 2021, the global prevalence of diabetes mellitus was estimated at 537 million and is expected to rise to 783 million by 2045.1 Diabetic neuropathy affects up to 50% of patients with diabetes2,3 and refers to a heterogenous group of disorders which affect the nervous system leading to a range of clinical presentations.4 The most prevalent form is diabetic peripheral neuropathy (DPN), a symmetrical, length-dependent sensorimotor polyneuropathy.5 DPN typically presents in a “stocking and glove” distribution, beginning distally and moving proximally with disease progression, with lower-limb long axons being most vulnerable to damage.4 DPN may lead to neuropathic pain6 and is the largest initiating risk factor for foot ulceration and amputation.7 Painful DPN (PDPN) affects ~20–24% of patients with diabetes and leads to impaired daily functioning, depression, sleep disturbance, financial instability,8 and decreased quality of life (QoL).9 PDPN is characterised as burning, tingling, and electric shock-like sensation which may be accompanied by negative symptoms (numbness) or positive symptoms (paraesthesia, allodynia [pain sensitisation following normally non-painful stimulation] and hyperalgesia [abnormally increased sensitivity to pain]).4 PDPN is underdiagnosed and undertreated by healthcare professionals.10,11
Several challenges exist in the management of PDPN including lack of timely diagnosis, PDPN refractory to anti-neuropathic therapy, an absence of mechanistic-based treatment in routine clinical practice, and inconsistencies between international guidelines. In this narrative review, we discuss practical guidance and challenges for the clinical management of PDPN.
Screening
The current screening for DPN relies on a combination of history and clinical neurological examination. According to the American Diabetes Association (ADA), individuals diagnosed with type 2 diabetes should have screening at the time of diagnosis, while individuals with type 1 diabetes should have screening 5 years post-diagnosis, followed by annual screening thereafter4 or whenever symptoms arise.12 Additionally, patients with prediabetes with DPN symptoms should be screened.4 Screening for DPN involves a detailed history and assessing small-fibre and large-fibre function through examination of temperature/pinprick sensation and vibration sensation (with a 128-Hz tuning fork), respectively. Examination with 10-g monofilament test should occur annually to assess their risk for foot ulceration and thus subsequent amputation. Additionally, the definition of screening, “a way of identifying apparently healthy people who may have an increased risk of a particular condition”,13 does not reflect the current clinical approach to DPN given the 10-g monofilament and 128-Hz tuning fork only detect established and often advanced disease. In instances where the clinical presentation is atypical, eg, greater motor symptoms/signs, asymmetrical presentation, rapid onset and there is diagnostic uncertainty, and/or alternate causes are suspected, patients should be referred to a neurologist and for neurophysiological testing.4
In general, there is a paucity of screening and discussion for PDPN in routine clinical practice which has been clearly demonstrated in primary care studies.10,14 Current international guidelines provide little guidance on the screening and frequency of screening for PDPN. No recognised, dedicated screening programme exists, unlike with diabetic retinopathy, and PDPN is instead ascertained through opportunistic detection of clinical signs and symptoms or in diabetic foot screening. The latter of which utilises 10-g monofilament and/or 128-Hz tuning fork.
Diagnosis
Diagnosing DPN
Confirming the diagnosis of DPN requires objective measures in addition to clinical features. The Toronto Census criteria set out definitions for the minimum criteria required for DPN diagnoses including “possible DPN”: symptoms or signs of DPN; “probable DPN”: symptoms and signs of DPN, “confirmed DPN”: symptoms or signs of DPN and nerve conduction abnormality or abnormality of another validated measure of small-fibre neuropathy; and “subclinical DPN”: nerve conduction abnormality or abnormality of another validated measure of small-fibre neuropathy without symptoms or signs.5 Nerve conduction studies measure the function of large (β) fibres which are only affected in the latter stages of DPN. For instance, individuals may present with severe pain but normal nerve conduction studies. Skin biopsy has been considered the reference standard method to quantify small nerve fibres by an assessment of intra-epidermal nerve fibres.15 In vivo corneal confocal microscopy (CCM) is a non-invasive imaging technique which evaluates small nerve fibres through quantification of the corneal subbasal nerve plexus.16 The efficacy of CCM in DPN has been thoroughly investigated and has demonstrated good-to-excellent diagnostic ability17–20 particularly in combination with artificial intelligence deep learning techniques.19,21,22 Other sensitive tests for DPN include the Sudoscan test23 to determine electrochemical skin conductance, and the LDI-Flare technique24 which assesses the neurogenic flare response to nociceptive stimuli. However, in clinical practice, most diagnoses are based on only history and clinical neurological examination,4 with objective measures primarily used in patients with atypical presentations, specialist centres or in clinical research.25
Diagnosing PDPN
The IASP defines chronic peripheral neuropathic pain as “chronic pain caused by a lesion or disease of the peripheral somatosensory nervous system”.26 The diagnosis of PDPN is made clinically with symptoms and/or signs of neuropathic pain in a typical distribution. Tools and questionnaires are a valuable resource and facilitate accurate diagnosis of pain, determine the patient’s neuropathic pain phenotype, and assess the effects of pain on a patient’s daily functioning, mood, and QoL.
Tools and Questionnaires
The “Douleur Neuropathique en 4 Questions” (DN4-Interview) is a validated screening tool which can be used in the diagnostic work-up of PDPN,27 consisting of 10 items divided into four questions. Questions 1 and 2 are interview questions, and questions 3 and 4 relate to physical examination. Each positive item scores a point, with the maximum score being 10. A score of 3 has a sensitivity and specificity of 84%, positive predictive value of 71%, and negative predictive value of 92% for diagnosing PDPN.27 The painDETECT questionnaire (PD-Q) can be used to determine the presence of neuropathic pain and has demonstrated a sensitivity of 85%, specificity of 80% and positive predictive value of 83%.28 The McGill Pain Questionnaire allows quantification of a patient’s subjective pain experience through a pain rating index assigned to word descriptors, the number of word descriptors chosen, and an intensity scale of the patient’s current pain.29
On diagnosing PDPN clinicians should also elicit the effect of the neuropathic pain on a patient’s daily functioning, QoL and sleep.12,30 The Brief Pain Inventory for patients with PDPN (BPI-DPN)31 can be used to assess pain interference on daily functioning, QoL and mood. The validated instrument includes a four-item pain severity scale and a seven-item pain interference scale. The Norfolk Quality of Life Questionnaire-Diabetic Neuropathy (QOL-DN) is another tool which can be utilised to determine the effects of pain on a patients’ QoL.32 Additional questionnaires include the Chronic Pain Sleep Inventory (CPSI) which can be utilised to assess the effect of chronic pain on a patient’s quality of sleep33; the inventory for measuring depression created by Beck et al to enable quantitative assessment of the intensity of a patient’s depression34; and the EQ-5D questionnaire which can be employed to evaluate a patient’s level of mobility, self-care, ability to engage in usual activities, as well as their experience of discomfort, pain, anxiety, and depression.35
Autonomic Neuropathy
The relationship between PDPN and autonomic dysfunction has been investigated in several studies, with inconsistent findings reported. While some studies have demonstrated greater autonomic dysfunction in people with PDPN compared to those with painless DPN,36–38 other studies have found no clear association between PDPN and autonomic neuropathy.39,40 Further research is necessary to fully understand the relationship between symptoms in DPN and autonomic neuropathy, with an association providing another potential practical tool for physicians to use in diagnosis.
Differential Diagnoses
The clinical history, examination and biochemical tests are required to exclude other causes of small fibre neuropathy such as vitamin B12 deficiency, alcohol-related neuropathy, genetic neuropathies, hypothyroidism, paraproteinemias, neoplasia, amyloidosis, peripheral arterial disease, and neurotoxic drugs (eg, chemotherapy and HIV treatments).25,41 A recent International Diabetes Federation consensus recommendation advised for the assessment of vitamin B12, serum protein electrophoresis, liver function tests, thyroid function tests, vitamin D, estimated glomerular filtration rate (eGFR), and magnesium levels.41 Patients with either type 1 or type 2 diabetes have a propensity for vitamin B12 deficiency often due to malabsorption and drug effect (metformin), respectively. An inverse correlation between DPN and the plasma level of vitamin B12 has been previously demonstrated.42 B vitamin deficiencies are highly prevalent in low-income countries with deficiencies in B1, B6 and B12 all potentially presenting with neurological manifestations.43 If a deficiency is demonstrated in B1, B6, or B12, replacement should be given to rectify neurological manifestations which may account for the patient’s symptoms.
Additional differentials for PDPN include Morton’s neuroma, radiculopathy, entrapment neuropathy (eg, carpal tunnel syndrome), and chronic inflammatory demyelinating polyneuropathy (CIDP). Rapid onset of symptoms presenting with muscular weakness should instigate nerve conduction studies to evaluate for CIDP. CIDP is potentially treatable with high-dose steroids, immunosuppressive and/or immunoglobulin therapy. Similarly, nerve conduction studies may demonstrate carpal tunnel syndrome in those presenting with greater symptomatology in the hands compared to the feet. Asymmetrical lower limb symptoms or signs may be a feature of (superimposed) lumbar radiculopathy (often accompanied by back pain) with MR spinal imaging helpful in the diagnostic paradigm.
Treatment Goals and Strategies
Counselling the patient and managing expectations are key components in the physician–patient interaction. The treatment goals in managing PDPN include reduction in pain, improvement in daily functioning, QoL, sleep, and mood, with a focus on patient functioning rather than a sole quantitative assessment of pain intensity. Complete resolution in pain is rare, with a good outcome considered a 30–50% reduction in pain.44 Therefore, a treatment goal is to reduce rather than negate pain intensity, given that that complete resolution is rarely achieved.30 This aids in setting patients’ expectations in keeping with the expected efficacy of the available treatments.30 The 0–10 Numerical Rating Scale (NRS) is a scale designed to assist in evaluating a patient’s level of pain and response to treatment. The scores have the ability to classify the level of pain severity, with a score of 0 indicating no pain, 1–3 indicating mild pain, 4–6 indicating moderate pain, and 7–10 indicating severe pain.45 The visual analogue score (VAS) is a validated, subjective tool which can be used to assess pain intensity in patients with PDPN.46 The VAS is a self-reported rating scale that enables patients to rate the intensity of their pain on a continuous line, ranging from 0 (no pain) to 10 (worst possible pain).
Pain, daily functioning, mood, and sleep have several interactions. Pain can lead to impaired daily functioning, mood and sleep,9 meaning modification of pain can lead to improvements in each of these facets.44 In addition, pain perception can be influenced by mood and sleep.47,48 Treatment of mood and sleep interference in concurrence with treatments for pain may also reduce pain and improve QoL.
The treatment strategies for PDPN include prevention in progression of DPN through risk factor reduction and lifestyle modifications and symptomatic treatment through lifestyle modifications, non-pharmacological treatments, and primarily treatment with pharmacotherapy.
Risk Factor Reduction for DPN
Adequate glycaemic control delays the progression of DPN and onset of neuropathy in patients with type 1 diabetes.49–51 However, there is insufficient evidence to demonstrate improved glycaemic control alone delays the progression of DPN in type 2 diabetes,52,53 but remains a key facet of multifactorial risk factor modification as recommended by the ADA.4 Type 2 diabetes is a complex disease, and several factors may contribute to the lack of evidence on the impact of glycaemic control alone on the progression of DPN in this condition. Foremost, type 2 diabetes is a heterogenous condition in which glycaemia is a single facet of the pathogenesis of DPN. In addition, inflammation, hypertension, dyslipidaemia result in multifactorial pathogenesis of DPN and thus impacting on a single pathway, eg, glycaemia, may not alter the natural history of DPN in type 2 diabetes. The ADA recommends optimising glycaemic control in patients with type 1 and type 2 diabetes to delay progression of DPN.4 However, there are no robust evidence for improvement in glycaemic control modifying pain intensity in PDPN.12 Lipid control and lipid lowering therapies have been shown to have associations with the risk of developing DPN54–57 and a reduction in the progression of DPN,58,59 respectively. However, further prospective randomised trials are required to provide more robust data on the effects of lipid control and lipid lowering therapies on nerve fibre regeneration and improvement in neuropathy symptoms.60 Again, there is negligible evidence for lipid control or lipid lowering therapies to be used therapeutically in PDPN.12
Lifestyle Modifications
Regular aerobic and strengthening exercise have demonstrated reductions in neuropathic pain, improvements in small nerve fibres,61 and reductions in pain interference.62 Singleton et al utilized a similar protocol to the Diabetes Prevention Programme (DPP) (5–7% weight loss with diet and exercise) in patients with impaired glucose tolerance, demonstrating improvements in neuropathic pain and small nerve fibre density on skin biopsy.63
Non-Pharmacological Treatments
Several non-pharmacological treatments can be used in the management of PDPN including psychological therapy, acupuncture, dietary supplements, transcutaneous electrical nerve stimulation (TENS), frequency rhythmic electrical modulated system (FREMS), and spinal cord stimulation (SCS).64 Most non-pharmacological treatments have poor strength of evidence, apart from SCS, however may be considered in select patients.
TENS and FREMS
TENS is a non-invasive treatment which applies an electrical current to nerve fibres through electrodes on the skin.65 It is theorised that a reduction in pain may be due to endogenous opioid release, gate control theory, and dilation of blood vessels.66 TENS has shown promise as a treatment in the management of PDPN; however, further large-scale prospective trials are needed.65 FREMS is another non-invasive treatment which applies series of electrical pulses through electrodes attached to a patient’s lower limbs.67 Two RCTs have demonstrated improvements in pain with FREMS,68,69 with a recent pilot RCT study (The FREMSTOP Study) finding that FREMS could be integrated into the treatment algorithm for patients who have inadequate response to two classes of neuropathic pain medications, demonstrating reductions in pain and increased perceived impact of treatment by the patients.67
Spinal Cord Stimulation
SCS involves implantation of a pulse generator into the lower back which is connected to percutaneous leads which are placed in the epidural space.70 SCS can be conducted using low frequencies (LF-SCS, 10–100 Hz) or high frequencies (HF-SCS 1–10 kHz).71 Two RCTs have demonstrated that LF-SCS can significantly reduce pain in patients with PDPN and improve QoL.72–74 LF-SCS can cause paraesthesia which can be uncomfortable for patients.75 HF-SCS does not cause significant paraesthesia and a recent RCT from the US demonstrated significant reductions in pain (≥50% pain relief on VAS) and improvement in health-related QoL in patients with PDPN using 10 kHz SCS.70 6% of the participants experienced study-related adverse events including infection, wound dehiscence, and impaired healing with 2% requiring explantation.70 Another recent systematic review and network meta-analysis of SCS in PDPN concluded that SCS provides pain relief and health-related QoL improvements, with the relative benefits of LF-SCS vs HF-SCS remaining uncertain due to the current lack of head-to-head RCTs in the area.71
Monochromatic Infrared Energy
Monochromatic infrared energy (MIRE) has been studied as a potential treatment for PDPN. MIRE employs light with a wavelength of 890 nm, which is believed to penetrate the skin and promote tissue regeneration. Various studies have evaluated the efficacy of MIRE for PDPN with mixed findings. Two RCTs reported significant improvement in peripheral sensation with MIRE.76,77 However, a double-blind, randomized, sham-controlled trial reported no significant differences in quality of life (QoL), Michigan Neuropathy Screening Instrument (MNSI), vibration perception threshold (VPT), Semmes-Weinstein monofilaments (SWM), or nerve conduction velocities between MIRE and sham therapy for sensory neuropathy in DPN.78 Another randomized, sham-controlled study, specifically examining patients with PDPN, reported that while there was no change in intraepidermal nerve-fibre density with short-term MIRE use, there was a symptomatic benefit and an improvement in QoL.79
Psychological Therapy
In patients with comorbid psychological distress, psychological therapy can be utilised.80 Examples of psychological therapy include cognitive behavioural therapy (CBT), behavioural therapy, and acceptance and commitment therapy (ACT).81 An RCT pilot study assessing the use of CBT in patients with PDPN demonstrated significant decreases in pain severity and intensity in participants who received CBT versus treatment as usual.82 A Cochrane review demonstrated that CBT has a small or very small beneficial effect in the reduction of pain (moderate quality evidence), distress (moderate quality evidence), and disability (low-quality evidence) in patients with chronic pain.81 The evidence for behavioural therapy and ACT was very low-moderate quality, preventing conclusions being drawn on the benefits/lack of benefits of either.81
Pharmacotherapy
Guidelines
Several guidelines exist for pharmacotherapy management in PDPN4,12,30,41,83,84 and neuropathic pain in general.85–87 All guidelines recommend gabapentinoids (gabapentin, pregabalin), tricyclic antidepressants (amitriptyline), and serotonin and norepinephrine reuptake inhibitors (SNRIs) (duloxetine, venlafaxine) as suitable first-line treatments, except for Diabetes Canada (DC) which recommends pregabalin before the other agents.83 The American Academy of Neurology (AAN) recommends sodium channel blockers, specifically carbamazepine, oxcarbazepine, lamotrigine, and lacosamide, as additional first-line agents.30 However, these agents are not considered in all the guidelines. Valproate is recommended as a second-line agent in the Diabetes Canada guidelines83 and third-line in the AAN guidance,30 however both advise against its use in patients of a childbearing age. The SNRI/opioid dual-mechanism agents (tramadol, tapentadol) have varying recommendations within the guidelines. Several guidelines suggest using tramadol and tapentadol as second- or third-line agents.4,83,84 However, more recent guidance from the American Association of Clinical Endocrinology (AACE) and the AAN explicitly advise against the use of SNRI/opioid dual-mechanism agents in the management of PDPN.12,30 Similarly with opioids, the AACE and the AAN advise against their use in the management of PDPN,12,30 whereas the other guidelines recommend their use as second- or third-line agents.4,41,83,84 The capsaicin 8% patch is recommended as a first-line therapy by the AACE, the AAN, and the DDG guidance,12,30,84 third-line by the International Diabetes Federation (IDF) recommendations41 and not considered by ADA and Diabetes Canada guidelines.4,83 Lidocaine 5% infusion which is usually utilized in drug refractory PDPN was not considered by any of the guidelines,4,12,30,41,83,84 with the IDF guidance not recommending the lidocaine 5% patch.41 α-Lipoic acid was either not considered or not recommended by all the guidelines apart from the DDG guidelines in which recommended it as a first-line therapy.84
The pharmacotherapy recommendations from recent guidelines for the pharmacological management of PDPN are displayed in Table 1, including the guidelines from the American Association of Clinical Endocrinology (AACE), American Academy of Neurology (AAN), American Diabetes Association (ADA), Diabetes Canada (DC), German Diabetes Association (DDG), and International Diabetes Federation (IDF).
 |
Table 1 Recent Guidelines for Pharmacotherapy of Painful Diabetic Peripheral Neuropathy |
Pharmacotherapy Treatment Algorithm
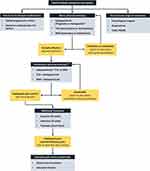
Given the current international guidelines, physicians should offer a gabapentinoid, TCA, or duloxetine as the first-line treatment as a mono-pharmacotherapy (Figure 1). The ANN guidelines state in relation to TCAs, SNRIs, gabapentinoids, and sodium channel blockers that “the best estimates of the effect sizes and the corresponding confidence intervals are comparable for all of these drug classes, which makes recommendations for one over another difficult”.30 Therefore, the choice of first-line treatment depends on the contraindications and comorbidities of the patients. Gabapentinoids may be avoided in patients with peripheral oedema and/or heart failure. TCAs are cautioned or contraindicated in patients with cardiovascular disease including arrhythmias and ischaemic heart disease. Duloxetine is cautioned with co-existing GI symptoms, eg, nausea, bloating and dizziness as these symptoms may be exacerbated. Additional considerations need to be made for potential drug–disease interactions, eg, dose adjustments for renal impairment. Generally, for all therapies, clinicians should start at the lowest dose and titrate to the maximum tolerated dose usually over 2–4 weeks if necessary. For individuals with severe pain at presentation, rapid dose titration of pregabalin (increase in dose by every 3–5 days) with tramadol PRN for breakthrough pain may be considered.
Certain first-line treatments may be preferred in certain sets of patients. For instance, TCAs and SNRIs in patients with depression; gabapentinoids and TCAs with dose adjustments may be preferred in patients with severe renal insufficiency; SNRIs in patients with obesity; gabapentinoids and SNRIs in patients with ischaemic heart disease; and gabapentinoids in patients with liver failure.41
Inadequate Response/Partial Efficacy
In keeping with the AAN, we define 1) a lack of efficacy, when significant pain reduction is not achieved after titration to an effective dose and duration; 2) intolerability, when the adverse effects caused by a medication outweigh the symptomatic benefit; and 3) a failure, when the medication is either ineffective after 12 weeks or intolerable at any duration.30
In the event of failure of mono-pharmacotherapy, the therapy should be discontinued and switched to an alternative treatment of a different class with subsequent adequate dose titration. If the monotherapy has partial efficacy, then an additional first-line treatment should be commenced as combination pharmacotherapy, after considerations of contraindications, comorbidities, and potential drug-disease/drug-drug interactions. Possible combination pharmacotherapy includes the addition of a gabapentinoid to a TCA or an SNRI, or the addition of a TCA or SNRI to a gabapentinoid. The combination of a TCA and an SNRI is usually avoided given the risk of serotonin syndrome, especially at concomitant high doses of each drug.
Tramadol can be used as a second-line analgesic treatment but should only be used in the short term whenever possible.41 Tapentadol may be utilised as an alternative to tramadol, particularly where there is a lack of efficacy or availability.41 If a combination pharmacotherapy is intolerable, it may be switched to an alternative combination therapy. If the combination therapy is found to provide inadequate pain relief, a third-line treatment such as the capsaicin 8% patch, the lidocaine 5% patch, or tramadol may be considered.
Gabapentinoids
Pregabalin and gabapentin belong to the class of α2δ ligands that exhibit high-affinity binding to the α2δ protein subunit of voltage-gated calcium channels.88 The α2δ proteins are predominantly expressed in the central nervous system (brain and spinal cord) and modulation of these channels induces a reduction in the release of excitatory neurotransmitters via a decrease in the exocytosis of synaptic vesicles and inhibition of their diffusion into the synaptic cleft.89 Pregabalin90–96 and gabapentin92–94,97 are a first line in the treatment of PDPN, with pregabalin having FDA regulatory approval. The initial dose of pregabalin is 25–75 mg twice or three times per day, which can be titrated to a maximum dose of 300 mg twice per day (if creatinine clearance [CrCl] is less than 60 mL/min a dose reduction is necessary).88 Common adverse events of pregabalin include weight gain, peripheral oedema, dizziness, somnolence, and headache.41 The initial dose of gabapentin is 100–300 mg three times per day, which can be titrated to a maximum dose of 1200 mg three times per day (if CrCl is less than 60 mL/min a dose reduction is necessary).88 Common adverse events of gabapentin include dizziness, fatigue, somnolence, ataxia, viral infections, and fever.41 Gabapentinoids should be used with caution in patients with peripheral oedema, heart failure, a history of substance misuse and the elderly and are contraindicated in pregnancy.88
Tricyclic Antidepressants
TCAs operate by inhibiting the reuptake of noradrenaline and serotonin from the synaptic cleft of the central descending pain modulatory systems.98 In addition, TCAs exhibit antagonistic effects on opioid and N-methyl-
Serotonin and Norepinephrine Reuptake Inhibitors
Duloxetine and venlafaxine are SNRIs which work through inhibiting serotonin and noradrenaline uptake, enhancing the descending inhibition of centrally sensitized pain. Duloxetine91–96,101,103 and venlafaxine91,92,94,96,101 are used as first-line treatments for PDPN, with only duloxetine having FDA regulatory approval. The initial dose of duloxetine is 30–60 mg as one or two daily divided dosages, which can be titrated to a maximum dose of 120 mg as one or two daily divided dosages.88 Common adverse events include nausea, somnolence, headaches, and dry mouth.41 SNRIs are cautioned in patients with cardiovascular disease, bleeding disorders, mania, seizures, and raised intraocular pressure, and absolutely contraindicated in severe hepatic impairment, renal impairment with a CrCl <30 mL/min, pregnancy, breastfeeding, patients with uncontrolled hypertension, and patients taking monoamine oxidase inhibitors.88
Topical Treatments
The capsaicin 8% patch is a topical analgesic therapy that works through binding to the transient receptor potential vanilloid 1 (TRPV1) receptor, desensitizing and interfering with its function within pain signalling through depletion of substance P.104 The FDA and European Medicines Agency have approved the capsaicin 8% patch for the treatment of PDPN based on the evidence of two large-scale RCTs105,106 and is recommended as a third line treatment by the IDF if combination therapy is found to be inadequate at providing pain control.41 The patch is applied over 30 minutes to the feet and can provide pain relief for weeks-to-months.107,108 Common adverse events include application site pain and erythema, burning sensation, and extremity pain.88 A recent study found that the capsaicin 8% patch can provide pain relief and improvement in function through nerve regeneration in both DPN and PDPN.107
The lidocaine 5% patch decreases pain impulses through antagonising voltage-gated sodium channels and membrane stabilisation of small nerve fibres.98 The lidocaine 5% patch has been studied in several open-label studies in PDPN,109 neuropathic pain,110 and post-herpetic neuralgia,111 demonstrating improvements in pain and QoL. The Cochrane review on the use of topical lidocaine in neuropathic pain concluded that there was a lack of good-quality RCTs to support its use, but that individual studies and clinical experience supported its efficacy and use in certain patients.112 Nitric oxide donors, isosorbide mononitrate spray or glyceryl trinitrate patches have demonstrated efficacy in the treatment of PDPN in small open label or randomisedcontrolled trials. GTN patches may be used in combination with lidocaine 5% patch (12 hour application of each therapy) providing another topical therapy, which may be useful in patients who have had minimal/partial benefit or adverse events from 1st and 2nd line oral pharmacotherpy, or in where oral pharmacotherapy options are limted e.g. in CKD stage 4/5.113–115
SNRI/Opioid Dual Mechanism Agents and Opioids
Tramadol and tapentadol are SNRI/opioid dual-mechanism agents which work through blocking μ opioid receptors and inhibiting the reuptake of serotonin and noradrenaline at the spinal cord.98 Tramadol91,94,96 is recommended as a second-line analgesic treatment in PDPN by the IDF.41 The initial dose of tramadol is 50–100 mg four times per day, which can be titrated to a maximum dose of 400 mg in divided dosages over the day (if renal impairment is present a dose reduction is necessary).88 Nausea, vertigo, dizziness, headache, somnolence, and constipation are common adverse events.41,88 The IDF advises that tramadol should only be used in the short term whenever possible and that tapentadol can be used if tramadol is ineffective or unavailable.41 The initial dose of tapentadol (immediate-release) is 50 mg every 4–6 hours, which can be titrated to a maximum dose of 600 mg in divided dosages over the day.88 The initial dose of tapentadol (modified-release) is 50 mg every 12 hours, with a maximum dose titration of 500 mg in divided over the day. Common adverse effects of tapentadol include nausea, emesis, vertigo, dizziness, headache, and somnolence.41 SNRI/opioid dual-mechanism agents should be used with caution in hypotension, impaired respiratory function, seizure disorders, concomitant use of medications, eg, duloxetine, venlafaxine, which lower the seizure threshold or increase the risk of serotonin syndrome, and the elderly, and are contraindicated in severe hepatic impairment, pregnancy and breastfeeding.88
Oxycodone is a strong opioid that works through blocking μ opioid receptors. Oxycodone92,94,116 can be used in the treatment of PDPN with the IDF advising that strong opioids may be utilised as a third-line treatment for PDPN if combination therapy is found to be inadequate at providing pain control.41 We recommend that opiates use should be avoided unless other first- and second-line treatment therapies have failed. The initial dose of oxycodone is 10–20 mg in divided dosages over the day, which can be titrated to a maintenance dose of 20–50 mg in divided dosages over the day.41 Frequently observed adverse effects of opioids include drowsiness, nausea, emesis, constipation, and pruritus.88 The IDF advises assessing tolerance and the risk of abuse, misuse, and dependence prior to initiating treatment with opioids and regularly during follow-up, with treatment durations lasting over 3 months requiring regular re-evaluation.41
Maximum-Dose Monotherapy versus Standard Dose Combination Therapy
The COMBO-DM study aimed to assess the efficacy of maximum-dose monotherapy versus standard dose combination therapy, specifically assessing the gabapentinoid pregabalin and the SNRI duloxetine.117 The trial consisted of 804 patients randomly assigned into 60 mg/day of duloxetine or 300 mg/day pregabalin. After the 8-week initial period, non-responders to the standard dose monotherapy or were treated with maximum-dose monotherapy (duloxetine 120mg/day or pregabalin 600mg/day) or combination therapy (duloxetine 60 mg/day and pregabalin 300 mg/day). After a further 8 weeks, the results from the study demonstrated clinically relevant pain reduction in both groups but with no significant differences in neuropathic pain between maximum-dose monotherapy and standard dose combination therapy.117 The study demonstrated the feasibility of combination pharmacotherapy in the treatment of PDPN.
Maximum-Dose Monotherapy versus Maximum-Dose Combination Therapy
The recently published OPTION-DM study aimed to assess the benefits of combination therapy and if differences in efficacy exist between mono-pharmacotherapies and combination pharmacotherapies.118 Participants with PDPN were assigned to three treatment pathways: (1) amitriptyline mono-pharmacotherapy supplemented with pregabalin if required, (2) pregabalin mono-pharmacotherapy supplemented with amitriptyline if required, and (3) duloxetine mono-pharmacotherapy supplemented with pregabalin if required. The trial consisted of 130 patients randomly assigned into six groups, with each group receiving the three treatment pathways in different ordered sequences. Mono-pharmacotherapy lasted 6 weeks with titration to the maximum tolerable dose, carried on for a further 10 weeks if effective or supplemented with an additional treatment for 10 weeks if there was suboptimal pain relief, again titrated to the maximum tolerable dose. Following each treatment pathway, patients would commence a wash-out period before commencing the next treatment pathway. The study demonstrated three main findings. (1) The analgesic efficacy of first-line mono-pharmacotherapies (amitriptyline, duloxetine, pregabalin) were similarly efficacious. Pain relief (NRS ≤3) was demonstrated in around a third of patients in the trial. (2) Combination therapy was shown to provide additional pain relief and was well tolerated in patients with suboptimal pain relief on a mono-pharmacotherapy. (3) The analgesic efficacy of the various combination therapies (amitriptyline and pregabalin, pregabalin and amitriptyline, duloxetine and pregabalin) were also similarly efficacious. The study validated using combination therapy in patients who have had suboptimal pain relief on mono-pharmacotherapy. A recent NIHR HTA assessment concluded that the three treatment pathways appear to give comparable patient outcomes at similar costs, suggesting that the optimal treatment may depend on patients’ preference in terms of side effects.119
Refractory PDPN
Refractory PDPN is a common problem with patients failing to respond to first-line mono-pharmacotherapies and combination pharmacotherapies. In these cases, patient should be referred to a pain specialists and pain management services, an endocrinologist with pain expertise, or a neurologist with pain expertise. Further investigation may be indicated if there is doubt regarding the diagnosis in failing to respond to treatment. Several additional treatment options exist which can be utilised by specialist pain services in refractory PDPN such as lidocaine infusions, botulinum toxin, and spinal cord stimulation (SCS).88
The lidocaine infusion involves giving intravenous lidocaine to the patient over an hour, may provide pain relief in with chronic neuropathic pain.120,121 In an RCT of patients with refractory neuropathic pain demonstrated that a lidocaine infusion at 3 mg/kg administered over an hour demonstrated effective short-term pain relief, which became more pronounced following repeated infusions.121 Given the complexities of this therapy, its provision is only recommended in a specialist setting.
Mechanistic-Based Treatment
Understanding and underpinning the neurobiological processes of PDPN is paramount in the future exploitation of mechanism-based therapies to derive maximal analgesic response. Improving patient analgesia and developing a mechanism-based versus a disease-based therapeutic approach is a future requirement. However, there are some data on currently utilised pharmacotherapies in PDPN which demonstrates efficacy based on underlying pain mechanisms. Many of these studies have differentiated patients based on the irritable vs non-irritable nociceptor phenotype. In 1998, Fields et al postulated a mechanism-based therapy in post herpetic neuralgia (PHN) and the irritable nociceptor phenotype is considered to be a functionally abnormal but anatomically intact primary afferent nociceptor.122 Na (Nav) channel hyperexcitability are thought to be involved in irritable primary afferent nociceptors and provide an underpinning mechanism, associating it with symptoms and signs in the patient.
In a double-blind RCT of oxcarbazepine (Na channel antagonist), it was found to demonstrate greater efficacy in patients with the irritable (NNTB: 3.9; 95% CI 2.3–12) vs the non-irritable nociceptor phenotype (NNTB: 13; 95% CI 5.3-∞) for the relief of peripheral neuropathic pain.123 Similarly, by the same group, a sub-analysis of a negative study of Lidocaine 5% patch (Na channel antagonist) in neuropathic pain demonstrated benefit only in the irritable nociceptor group with a reduction in pain paroxysms which are related to aberrant Na channel activity.124 These findings have been corroborated with a study of IV lidocaine.125 Another negative study of topical clonidine in PDPN demonstrated benefit in individuals with functional nociceptors (assessed through burning intensity on a capsaicin patch test) with the degree of nociceptor functionality positively associated with intraepidermal nerve fibre density.126 Hence, the evaluation of cutaneous nociceptor function could aid in discriminating suitable patients for topical therapy in the management of PDPN.126 Similarly, in an randomised crossover trial of pregabalin in prediabetic neuropathic pain, non-responders had lower intraepidermal nerve fibre density.127 Small nerve fibres and their functionality play a potentially important role in understanding drug therapeutic effects.
More recently, there has been a paradigm shift in the understanding of the pathomechanisms of DPN and PDPN with an established association with central nervous system pathology/neuroplasticity.128 Central mechanisms are important for the generation and maintenance of PDPN. Two studies have established that the efficiency of conditioned pain modulation (CPM) paradigm (a measure of diffuse noxious inhibitory control – descending inhibition) could determine response to tapentadol129 and duloxetine.130 Another putative measure of treatment response is the Hoffman’s (H) reflex dependent depression (HRDD). Impaired HRDD has been established in animal models of diabetes, and similarly observed in patients with PDPN vs painless DPN.131 In a recently published study, it was observed that gabapentin can modify the diabetes-induced loss of RDD, indicating that RDD may serve as a useful predictor for the initial efficacy of gabapentin therapy in PDPN.132
Patient stratification according to their sensory phenotype (based on pain mechanisms) are a promising route to implementing personalised treatment in neuropathic pain.133 Classification of patients (retrospectively) based on their sensory phenotype has demonstrated predictive validity and reliability for treatment response in subclasses of individuals with neuropathic pain.133 This has been mirrored by recent prospective studies utilising sensory phenotype-based stratification to confirm this concept.133 We suggest that prospective studies of PDPN and neuropathic pain should undertake detailed sensory phenotyping at baseline to delineate putative subgroups that may benefit from the therapeutic intervention.
Novel Pharmacotherapies
Although various treatment options exist for managing PDPN, limitations of the current therapies often leave patients with no further options once the above treatment options have been exhausted. There is a substantial need for the development of novel pharmacotherapies. Regrettably, no novel analgesic pharmacotherapies have been approved by the FDA in the preceding two decades. Nevertheless, numerous incipient therapies have been developed and subsequently trialled in patients. These novel therapies may serve to transform the current landscape for neuropathic pain management, with at least 50 new molecular entities progressing to clinical development.134
Dextromethorphan, an NMDA receptor antagonist, has been assessed in Phase III clinical trials as a potential treatment for PDPN. However, its use as a monotherapy is limited due to rapid catabolism by hepatic cytochrome P4502D6, leading to restricted bioavailability. Therefore, co-administration of dextromethorphan with a potent P4502D6 inhibitor such as Quinidine is necessary to achieve therapeutic efficacy.
A double-blind, placebo-controlled trial consisting of 379 participants was conducted to evaluate the efficacy and safety profile of two doses of dextromethorphan/quinidine (DMQ) - 45/30 mg and 30/30 mg. The results of the study demonstrated that DMQ was more effective than the placebo and the safety profile of DMQ was deemed to be adequate.135 In a phase III multicentre randomised trial involving 412 participants, the effectiveness of desvenlafaxine, the most potent metabolite of venlafaxine suggested to alleviate pain and improve activity, was investigated. The trial included two doses, namely 200 and 400 mg/day. The results indicated that desvenlafaxine exhibited improved efficacy compared to the placebo.136,137
A multicentre, placebo-controlled, randomized clinical trial was conducted on 183 patients with post-herpetic neuralgia to evaluate the efficacy of EMA401, which is an antagonist of the angiotensin II type 2 receptor (AR2). The trial was conducted over a period of 28 days, and the results demonstrated an improved efficacy of EMA401 compared to the placebo.138 Angiotensin 2 immunostaining has been shown in 75% of the small-to-medium diameter human dorsal root ganglia neurons and this molecule was found to be the primary ligand for AR2.139 Furthermore, it was observed that the signalling pathway mediated by angiotensin 2 and AR2 was effectively inhibited by EM401, thereby establishing a plausible mechanism for the efficacy of EM401 in treating neuropathic pain.139
ARA290, also known as Cibinetide, is a non-hematopoietic peptide of erythropoietin that selectively interacts with the innate repair receptor, thereby mediating tissue protection.140 Additionally, it acts as an antagonist of the TRPV1 receptor, leading to both analgesic and disease-modifying effects.141 Studies have reported analgesic effects in individuals diagnosed with PDPN142 and sarcoid neuropathy.143
A randomized, placebo-controlled proof-of-concept trial evaluating the effectiveness of ISC 17536, a novel inhibitor of the TRPA1 pain receptor, failed to demonstrate significant efficacy in reducing neuropathic pain across the overall patient cohort diagnosed with PDPN.144 However, exploratory analysis identified a subpopulation of patients with preserved small nerve fibre function (defined by quantitative sensory testing) that exhibited statistically significant and clinically meaningful improvements in pain upon treatment with ISC 17536. Consequently, larger confirmatory trials are necessary to validate these observations.
Tanezumab, a monoclonal antibody that is fully humanized and functions as an anti-nerve growth factor (NGF), has been evaluated in a single reported study on DPN. In this study, a subcutaneous injection of 20 mg of tanezumab was administered on day 1 and week 8. The findings of the study demonstrated a reduction in DPN-associated pain.145 However, it should be noted that no statistically significant improvement was observed in patients’ global assessment of pain.145
ATP-gated receptor channels P2X3 and P2X2/3 play a crucial role in pain transmission, by directly sensitizing C-fibres through membrane depolarization and calcium entry. Dysregulation of purinergic signalling, including alterations in the expression and function of these receptors, has been linked to pathological pain such as allodynia.146 A-317491, a P2X3 and P2X2/3 antagonist, and sinomenine, an inhibitor of P2X3 agonist ATP-activated currents, have both been studied in animal models,147,148 but require investigation in human trials.149 Topical agents may also have a role in the management of refractory neuropathic pain, with examples including topical clonidine,126 amitriptyline,150 ketamine151 and gabapentin gel.152
The existing literature provides growing evidence to support the hypothesis that vitamin D may play a role in the pathogenesis of long-term complications of diabetes, and also suggests that a deficiency in vitamin D levels may aggravate the symptoms associated with PDPN.153 Moreover, a meta-analysis comprising 1484 individuals with type 2 diabetes confirmed a statistically significant association between serum levels of vitamin D3 and the incidence of DPN.154,155 In an open-label prospective study carried out in Pakistan, a single intramuscular dose of 600,000 IU of vitamin D3 was found to be efficacious in providing substantial pain relief in individuals with PDPN,156 while also resulting in a significant improvement in their QOL.157 There is a need for extensive and methodologically robust randomized controlled trials to determine the effectiveness of vitamin D supplementation in managing PDPN.
Conclusion
Several challenges exist in the management of PDPN. The condition is highly prevalent and is often underdiagnosed and undertreated. Achieving a complete resolution in pain is rare, with 30–50% reduction considered a good outcome. Additionally, the medications used often have a significant side effect burden requiring careful consideration of comorbidities and contraindications. Many patients do not respond to the primary pharmacotherapy and require a trial-and-error approach to anti-neuropathic pain therapy selection. Further research is required in developing mechanistic-based treatment to facilitate a move towards individualized pain management with a need for future clinical trials incorporating detailed pain phenotyping.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This Review Received No External Funding.
Disclosure
UA has received honoraria from Boehringer Ingelheim, Eli Lilly, Napp, Viatris, Proctor and Gamble and Sanofi for educational meetings and funding for investigator-initiated studies from Proctor and Gamble. FGP, DRR, and SA declare no conflicts of interest in this work.
References
1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
2. Callaghan BC, Price RS, Chen KS, Feldman EL. The importance of rare subtypes in diagnosis and treatment of peripheral neuropathy: a review. JAMA Neurol. 2015;72(12):1510–1518. doi:10.1001/jamaneurol.2015.2347
3. Iqbal Z, Azmi S, Yadav R, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin The. 2018;40(6):828–849. doi:10.1016/j.clinthera.2018.04.001
4. Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. doi:10.2337/dc16-2042
5. Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi:10.2337/dc10-1303
6. Tesfaye S, Vileikyte L, Rayman G, et al. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev. 2011;27(7):629–638. doi:10.1002/dmrr.1225
7. Vinik AI, Nevoret ML, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am. 2013;42(4):747–787. doi:10.1016/j.ecl.2013.06.001
8. Stewart WF, Ricci JA, Chee E, Hirsch AG, Brandenburg NA. Lost productive time and costs due to diabetes and diabetic neuropathic pain in the US workforce. J Occup Environ Med. 2007;49(6):672–679. doi:10.1097/JOM.0b013e318065b83a
9. Alleman CJ, Westerhout KY, Hensen M, et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: a review of the literature. Diabetes Res Clin Pract. 2015;109(2):215–225. doi:10.1016/j.diabres.2015.04.031
10. Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med. 2004;21(9):976–982. doi:10.1111/j.1464-5491.2004.01271.x
11. Sadosky A, Hopper J, Parsons B. Painful diabetic peripheral neuropathy: results of a survey characterizing the perspectives and misperceptions of patients and healthcare practitioners. Patient. 2014;7(1):107–114. doi:10.1007/s40271-013-0038-8
12. Blonde L, Umpierrez GE, Reddy SS, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: developing a Diabetes Mellitus Comprehensive Care Plan-2022 Update. Endocr Pract. 2022;28(10):923–1049. doi:10.1016/j.eprac.2022.08.002
13. UK National Screening Committee. Population screening explained. GOV.UK. Available from: https://www.gov.uk/guidance/population-screening-explained#:~:text=Screening%20is%20the%20process%20of,reduce%20associated%20problems%20or%20complications.
14. Tölle T, Xu X, Sadosky AB. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J Diabetes Complications. 2006;20(1):26–33. doi:10.1016/j.jdiacomp.2005.09.007
15. Sorensen L, Molyneaux L, Yue DK. The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care. 2006;29(4):883–887. doi:10.2337/diacare.29.04.06.dc05-2180
16. Petropoulos IN, Ponirakis G, Khan A, et al. Corneal confocal microscopy: ready for prime time. Clin Exp Optom. 2020;103(3):265–277. doi:10.1111/cxo.12887
17. Petropoulos IN, Bitirgen G, Ferdousi M, et al. Corneal confocal microscopy to image small nerve fiber degeneration: ophthalmology meets neurology. Front Pain Res. 2021;2:725363. doi:10.3389/fpain.2021.725363
18. Alam U, Jeziorska M, Petropoulos IN, et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS One. 2017;12(7):e0180175. doi:10.1371/journal.pone.0180175
19. Preston FG, Meng Y, Burgess J, et al. Artificial intelligence utilising corneal confocal microscopy for the diagnosis of peripheral neuropathy in diabetes mellitus and prediabetes. Diabetologia. 2022;65(3):457–466. doi:10.1007/s00125-021-05617-x
20. Alam U, Ponirakis G, Asghar O, et al. Corneal confocal microscopy identifies people with type 1 diabetes with more rapid corneal nerve fibre loss and progression of neuropathy. J Clin Med. 2022;11(8). doi:10.3390/jcm11082249
21. Meng Y, Preston FG, Ferdousi M, et al. Artificial intelligence based analysis of corneal confocal microscopy images for diagnosing peripheral neuropathy: a binary classification model. J Clin Med. 2023;12(4):1284.
22. Alam U, Anson M, Meng Y, et al. Artificial intelligence and corneal confocal microscopy: the start of a beautiful relationship. J Clin Med. 2022;11(20):6199.
23. Selvarajah D, Cash T, Davies J, et al. SUDOSCAN: a simple, rapid, and objective method with potential for screening for diabetic peripheral neuropathy. PLoS One. 2015;10(10):e0138224. doi:10.1371/journal.pone.0138224
24. Krishnan ST, Rayman G. The LDIflare: a novel test of C-fiber function demonstrates early neuropathy in type 2 diabetes. Diabetes Care. 2004;27(12):2930–2935. doi:10.2337/diacare.27.12.2930
25. Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. doi:10.1038/s41572-019-0092-1
26. Scholz J, Finnerup NB, Attal N, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160(1):53–59. doi:10.1097/j.pain.0000000000001365
27. Spallone V, Morganti R, D’Amato C, Greco C, Cacciotti L, Marfia GA. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med. 2012;29(5):578–585. doi:10.1111/j.1464-5491.2011.03500.x
28. Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. doi:10.1185/030079906x132488
29. Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–299. doi:10.1016/0304-3959(75)90044-5
30. Price R, Smith D, Franklin G, et al. Oral and topical treatment of painful diabetic polyneuropathy: practice guideline update summary: report of the AAN Guideline Subcommittee. Neurology. 2022;98(1):31–43. doi:10.1212/wnl.0000000000013038
31. Zelman DC, Gore M, Dukes E, Tai KS, Brandenburg N. Validation of a modified version of the brief pain inventory for painful diabetic peripheral neuropathy. J Pain Symptom Manage. 2005;29(4):401–410. doi:10.1016/j.jpainsymman.2004.06.018
32. Vinik EJ, Hayes RP, Oglesby A, et al. The development and validation of the Norfolk QOL-DN, a new measure of patients’ perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther. 2005;7(3):497–508. doi:10.1089/dia.2005.7.497
33. Kosinski M, Janagap CC, Gajria K, Schein J. Psychometric testing and validation of the Chronic Pain Sleep Inventory. Clin Ther. 2007;29:2562–2577. doi:10.1016/j.clinthera.2007.12.001
34. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi:10.1001/archpsyc.1961.01710120031004
35. Devlin N, Parkin D, Janssen B. Methods for Analysing and Reporting EQ-5D Data. Springer; 2020. Copyright 2020, The Editor(s) (if applicable) and The Author(s). This book is an open access publication.
36. D’Amato C, Morganti R, Di Gennaro F, Greco C, Marfia GA, Spallone V. A novel association between nondipping and painful diabetic polyneuropathy. Diabetes Care. 2014;37(9):2640–2642. doi:10.2337/dc14-0528
37. Gandhi RA, Marques JL, Selvarajah D, Emery CJ, Tesfaye S. Painful diabetic neuropathy is associated with greater autonomic dysfunction than painless diabetic neuropathy. Diabetes Care. 2010;33(7):1585–1590. doi:10.2337/dc09-2314
38. Spallone V, Morganti R, D’Amato C, et al. Clinical correlates of painful diabetic neuropathy and relationship of neuropathic pain with sensorimotor and autonomic nerve function. Eur J Pain. 2011;15(2):153–160. doi:10.1016/j.ejpain.2010.06.011
39. Hansen CS, Jensen TM, Jensen JS, et al. The role of serum methylglyoxal on diabetic peripheral and cardiovascular autonomic neuropathy: the ADDITION Denmark study. Diabet Med. 2015;32(6):778–785. doi:10.1111/dme.12753
40. Krämer HH, Rolke R, Bickel A, Birklein F. Thermal thresholds predict painfulness of diabetic neuropathies. Diabetes Care. 2004;27(10):2386–2391. doi:10.2337/diacare.27.10.2386
41. Ziegler D, Tesfaye S, Spallone V, et al. Screening, diagnosis and management of diabetic sensorimotor polyneuropathy in clinical practice: international expert consensus recommendations. Diabetes Res Clin Pract. 2022;186:109063. doi:10.1016/j.diabres.2021.109063
42. Alvarez M, Sierra OR, Saavedra G, Moreno S. Vitamin B12 deficiency and diabetic neuropathy in patients taking metformin: a cross-sectional study. Endocrine Connections. 2019;8(10):1324–1329. doi:10.1530/ec-19-0382
43. Ashley JM. Chapter Two - Manifestations and Measurement of Food Insecurity. In: Ashley JM, editor. Food Security in the Developing World. Academic Press; 2016:19–38.
44. Argoff CE, Backonja MM, Belgrade MJ, et al. Consensus guidelines: treatment planning and options. Diabetic peripheral neuropathic pain. Mayo Clin Proc. 2006;81(4Suppl):S12–25. doi:10.1016/s0025-6196(11)61475-4
45. Edwards RR. Chapter 5 - Pain Assessment. In: Benzon HT, Raja SN, Molloy RE, Liu SS, Fishman SM, editors. Essentials of Pain Medicine and Regional Anesthesia (Second Edition). Churchill Livingstone; 2005:29–34.
46. Delgado DA, Lambert BS, Boutris N, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018;2(3):e088. doi:10.5435/JAAOSGlobal-D-17-00088
47. Rosseland R, Pallesen S, Nordhus IH, Matre D, Blågestad T. Effects of sleep fragmentation and induced mood on pain tolerance and pain sensitivity in young healthy adults. Front Psychol. 2018;9:2089. doi:10.3389/fpsyg.2018.02089
48. Tang NKY, Salkovskis PM, Hodges A, Wright KJ, Hanna M, Hester J. Effects of mood on pain responses and pain tolerance: an experimental study in chronic back pain patients. Pain. 2008;138(2):392–401. doi:10.1016/j.pain.2008.01.018
49. Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14(9):528. doi:10.1007/s11892-014-0528-7
50. Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6(6):Cd007543. doi:10.1002/14651858.CD007543.pub2
51. Gouveri E, Papanas N. The emerging role of continuous glucose monitoring in the management of diabetic peripheral neuropathy: a narrative review. Diabetes Ther. 2022;13(5):931–952. doi:10.1007/s13300-022-01257-5
52. Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430. doi:10.1016/s0140-6736(10)60576-4
53. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–117. doi:10.1016/0168-8227(95)01064-k
54. Maser RE, Steenkiste AR, Dorman JS, et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes. 1989;38(11):1456–1461. doi:10.2337/diab.38.11.1456
55. Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55(5):1463–1469. doi:10.2337/db05-1423
56. Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–350. doi:10.1056/NEJMoa032782
57. Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58(7):1634–1640. doi:10.2337/db08-1771
58. Davis TM, Yeap BB, Davis WA, Bruce DG. Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2008;51(4):562–566. doi:10.1007/s00125-007-0919-2
59. Villegas-Rivera G, Román-Pintos LM, Cardona-Muñoz EG, et al. Effects of ezetimibe/simvastatin and rosuvastatin on oxidative stress in diabetic neuropathy: a randomized, double-blind, placebo-controlled clinical trial. Oxid Med Cell Longev. 2015;2015:756294. doi:10.1155/2015/756294
60. Iqbal Z, Bashir B, Ferdousi M, et al. Lipids and peripheral neuropathy. Curr Opin Lipidol. 2021;32(4):249–257. doi:10.1097/mol.0000000000000770
61. Kluding PM, Pasnoor M, Singh R, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26(5):424–429. doi:10.1016/j.jdiacomp.2012.05.007
62. Yoo M, D’Silva LJ, Martin K, et al. Pilot study of exercise therapy on painful diabetic peripheral neuropathy. Pain Med. 2015;16(8):1482–1489. doi:10.1111/pme.12743
63. Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29(6):1294–1299. doi:10.2337/dc06-0224
64. Amato Nesbit S, Sharma R, Waldfogel JM, et al. Non-pharmacologic treatments for symptoms of diabetic peripheral neuropathy: a systematic review. Curr Med Res Opin. 2019;35(1):15–25. doi:10.1080/03007995.2018.1497958
65. Naderi Nabi B, Sedighinejad A, Haghighi M, et al. Comparison of transcutaneous electrical nerve stimulation and pulsed radiofrequency sympathectomy for treating painful diabetic neuropathy. Anesth Pain Med. 2015;5(5):e29280. doi:10.5812/aapm.29280
66. Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain. 2003;4(3):109–121. doi:10.1054/jpai.2003.434
67. Crasto W, Altaf QA, Selvaraj DR, et al. Frequency Rhythmic Electrical Modulation System (FREMS) to alleviate painful diabetic peripheral neuropathy: a pilot, randomised controlled trial (The FREMSTOP study). Diabet Med. 2022;39(3):e14710. doi:10.1111/dme.14710
68. Bosi E, Bax G, Scionti L, et al. Frequency-modulated electromagnetic neural stimulation (FREMS) as a treatment for symptomatic diabetic neuropathy: results from a double-blind, randomised, multicentre, long-term, placebo-controlled clinical trial. Diabetologia. 2013;56(3):467–475. doi:10.1007/s00125-012-2795-7
69. Bosi E, Conti M, Vermigli C, et al. Effectiveness of frequency-modulated electromagnetic neural stimulation in the treatment of painful diabetic neuropathy. Diabetologia. 2005;48(5):817–823. doi:10.1007/s00125-005-1734-2
70. Petersen EA, Stauss TG, Scowcroft JA, et al. Effect of high-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: a randomized clinical trial. JAMA Neurol. 2021;78(6):687–698. doi:10.1001/jamaneurol.2021.0538
71. Duarte RV, Nevitt S, Copley S, et al. Systematic review and network meta-analysis of neurostimulation for painful diabetic neuropathy. Diabetes Care. 2022;45(10):2466–2475. doi:10.2337/dc22-0932
72. de Vos CC, Meier K, Zaalberg PB, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain. 2014;155(11):2426–2431. doi:10.1016/j.pain.2014.08.031
73. Slangen R, Schaper NC, Faber CG, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two-center randomized controlled trial. Diabetes Care. 2014;37(11):3016–3024. doi:10.2337/dc14-0684
74. van Beek M, Slangen R, Schaper NC, et al. Sustained treatment effect of spinal cord stimulation in painful diabetic peripheral neuropathy: 24-month follow-up of a prospective two-center randomized controlled trial. Diabetes Care. 2015;38(9):e132–4. doi:10.2337/dc15-0740
75. Eldabe S, Buchser E, Duarte RV. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literature. Pain Med. 2016;17(2):325–336. doi:10.1093/pm/pnv025
76. Arnall DA, Nelson AG, López L, et al. The restorative effects of pulsed infrared light therapy on significant loss of peripheral protective sensation in patients with long-term type 1 and type 2 diabetes mellitus. Acta Diabetol. 2006;43(1):26–33. doi:10.1007/s00592-006-0207-5
77. Leonard DR, Farooqi MH, Myers S. Restoration of sensation, reduced pain, and improved balance in subjects with diabetic peripheral neuropathy: a double-blind, randomized, placebo-controlled study with monochromatic near-infrared treatment. Diabetes Care. 2004;27(1):168–172. doi:10.2337/diacare.27.1.168
78. Lavery LA, Murdoch DP, Williams J, Lavery DC. Does anodyne light therapy improve peripheral neuropathy in diabetes? A double-blind, sham-controlled, randomized trial to evaluate monochromatic infrared photoenergy. Diabetes Care. 2008;31(2):316–321. doi:10.2337/dc07-1794
79. Rastogi A, Uppula P, Saikia U, Bhansali A. Effect of monochromatic infrared energy on quality of life and intraepidermal nerve fiber density in painful diabetic neuropathy: a randomized, sham control study. Neurol India. 2021;69(5):1331–1337. doi:10.4103/0028-3886.329614
80. Twiddy H, Frank B, Alam U. A Consideration of the psychological aspects to managing patients with painful diabetic neuropathy: an insight into pain management services at a tertiary centre in the UK. Diabetes Ther. 2021;12(2):487–498. doi:10.1007/s13300-020-00983-y
81. Williams ACC, Fisher E, Hearn L, Eccleston C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2020;8(8):Cd007407. doi:10.1002/14651858.CD007407.pub4
82. Otis JD, Sanderson K, Hardway C, Pincus M, Tun C, Soumekh S. A randomized controlled pilot study of a cognitive-behavioral therapy approach for painful diabetic peripheral neuropathy. J Pain. 2013;14(5):475–482. doi:10.1016/j.jpain.2012.12.013
83. Bril V, Breiner A, Perkins BA, Zochodne D. Neuropathy. Can J Diabetes. 2018;42:S217–s221. doi:10.1016/j.jcjd.2017.10.028
84. Ziegler D, Keller J, Maier C, Pannek J. Diabetic neuropathy. Exp Clin Endocrinol Diabetes. 2021;129(S01):S70–s81. doi:10.1055/a-1284-6245
85. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi:10.1016/s1474-4422(14)70251-0
86. Moisset X, Bouhassira D, Avez Couturier J, et al. Pharmacological and non-pharmacological treatments for neuropathic pain: systematic review and French recommendations. Rev Neurol (Paris). 2020;176(5):325–352. doi:10.1016/j.neurol.2020.01.361
87. NICE CfCPa. Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. 2013.
88. Sloan G, Alam U, Selvarajah D, Tesfaye S. The treatment of painful diabetic neuropathy. Curr Diabetes Rev. 2022;18(5):e070721194556. doi:10.2174/1573399817666210707112413
89. Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin--calcium channel alpha2-delta [Cavalpha2-delta] ligands. Pain. 2009;142(1–2):13–16. doi:10.1016/j.pain.2008.11.019
90. Derry S, Bell RF, Straube S, Wiffen PJ, Aldington D, Moore RA. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1(1):Cd007076. doi:10.1002/14651858.CD007076.pub3
91. Dy SM, Bennett WL, Sharma R, et al. AHRQ Comparative Effectiveness Reviews. Preventing Complications and Treating Symptoms of Diabetic Peripheral Neuropathy. Agency for Healthcare Research and Quality (US); 2017.
92. Liampas A, Rekatsina M, Vadalouca A, Paladini A, Varrassi G, Zis P. Pharmacological management of painful peripheral neuropathies: a systematic review. Pain Ther. 2021;10(1):55–68. doi:10.1007/s40122-020-00210-3
93. Quilici S, Chancellor J, Löthgren M, et al. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009;9:6. doi:10.1186/1471-2377-9-6
94. Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Mehta S, Botteman M. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Pract. 2014;14(2):167–184. doi:10.1111/papr.12054
95. Vilar S, Castillo JM, Munuera Martínez PV, Reina M, Pabón M. Therapeutic alternatives in painful diabetic neuropathy: a meta-analysis of randomized controlled trials. Korean J Pain. 2018;31(4):253–260. doi:10.3344/kjp.2018.31.4.253
96. Waldfogel JM, Nesbit SA, Dy SM, et al. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: a systematic review. Neurology. 2017;88(20):1958–1967. doi:10.1212/wnl.0000000000003882
97. Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):Cd007938. doi:10.1002/14651858.CD007938.pub4
98. Alam U, Sloan G, Tesfaye S. Treating pain in diabetic neuropathy: current and developmental drugs. Drugs. 2020;80(4):363–384. doi:10.1007/s40265-020-01259-2
99. Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151(6):737–748. doi:10.1038/sj.bjp.0707253
100. Benbouzid M, Gavériaux-Ruff C, Yalcin I, et al. Delta-opioid receptors are critical for tricyclic antidepressant treatment of neuropathic allodynia. Biol Psychiatry. 2008;63(6):633–636. doi:10.1016/j.biopsych.2007.06.016
101. Griebeler ML, Morey-Vargas OL, Brito JP, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med. 2014;161(9):639–649. doi:10.7326/m14-0511
102. Derry S, Wiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1(1):Cd011209. doi:10.1002/14651858.CD011209.pub2
103. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:Cd007115. doi:10.1002/14651858.CD007115.pub3
104. Markovits E, Gilhar A. Capsaicin--an effective topical treatment in pain. Int J Dermatol. 1997;36(6):401–404. doi:10.1046/j.1365-4362.1997.00102.x
105. Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18(1):42–53. doi:10.1016/j.jpain.2016.09.008
106. Vinik AI, Perrot S, Vinik EJ, et al. Capsaicin 8% patch repeat treatment plus standard of care (SOC) versus SOC alone in painful diabetic peripheral neuropathy: a randomised, 52-week, open-label, safety study. BMC Neurol. 2016;16(1):251. doi:10.1186/s12883-016-0752-7
107. Anand P, Privitera R, Donatien P, et al. Reversing painful and non-painful diabetic neuropathy with the capsaicin 8% patch: clinical evidence for pain relief and restoration of function via nerve fiber regeneration. Front Neurol. 2022;13:998904. doi:10.3389/fneur.2022.998904
108. Martini C, Yassen A, Olofsen E, Passier P, Stoker M, Dahan A. Pharmacodynamic analysis of the analgesic effect of capsaicin 8% patch (Qutenza™) in diabetic neuropathic pain patients: detection of distinct response groups. J Pain Res. 2012;5:51–59. doi:10.2147/jpr.S30406
109. Barbano RL, Herrmann DN, Hart-Gouleau S, Pennella-Vaughan J, Lodewick PA, Dworkin RH. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol. 2004;61(6):914–918. doi:10.1001/archneur.61.6.914
110. Argoff CE, Galer BS, Jensen MP, Oleka N, Gammaitoni AR. Effectiveness of the lidocaine patch 5% on pain qualities in three chronic pain states: assessment with the Neuropathic Pain Scale. Curr Med Res Opin. 2004;20(Suppl 2):S21–8. doi:10.1185/030079904x12960
111. Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. 5% lidocaine medicated plaster versus pregabalin in post-herpetic neuralgia and diabetic polyneuropathy: an open-label, non-inferiority two-stage RCT study. Curr Med Res Opin. 2009;25(7):1663–1676. doi:10.1185/03007990903047880
112. Derry S, Wiffen PJ, Moore RA, Quinlan J. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;2014(7):Cd010958. doi:10.1002/14651858.CD010958.pub2
113. Agrawal R, Goswami J, Jain S and Kochar D. Management of diabetic neuropathy by sodium valproate and glyceryl trinitrate spray: A prospective double-blind randomized placebo-controlled study. Diabetes Res Clin Pr. 2009;83(3):371–378. doi:10.1016/j.diabres.2008.12.018
114. Agrawal R, Choudhary R, Sharma P, et al. Glyceryl trinitrate spray in the management of painful diabetic neuropathy: A randomized double blind placebo controlled cross-over study. Diabetes Res Clin Pr. 2007;77(2):161–167. doi:10.1016/j.diabres.2006.12.003
115. Yuen KC, Baker NR, Rayman G. Treatment of Chronic Painful Diabetic Neuropathy with Isosorbide Dinitrate Spray. Diabetes Care. 2002;25(10):1699–1703. doi:10.2337/diacare.25.10.1699
116. Gaskell H, Moore RA, Derry S, Stannard C. Oxycodone for pain in fibromyalgia in adults. Cochrane Database Syst Rev. 2016;9(9):Cd012329. doi:10.1002/14651858.Cd012329
117. Tesfaye S, Wilhelm S, Lledo A, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study”--a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154(12):2616–2625. doi:10.1016/j.pain.2013.05.043
118. Tesfaye S, Sloan G, Petrie J, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet. 2022;400(10353):680–690. doi:10.1016/s0140-6736(22)01472-6
119. Tesfaye S, Sloan G, Petrie J, et al. Optimal pharmacotherapy pathway in adults with diabetic peripheral neuropathic pain: the OPTION-DM RCT. Health Technol Assess. 2022;26(39):1–100. doi:10.3310/rxuo6757
120. Iacob E, Hagn EE, Sindt J, et al. Tertiary Care Clinical Experience with Intravenous Lidocaine Infusions for the Treatment of Chronic Pain. Pain Med. 2018;19(6):1245–1253. doi:10.1093/pm/pnx167
121. Kim YC, Castañeda AM, Lee CS, Jin HS, Park KS, Moon JY. Efficacy and safety of lidocaine infusion treatment for neuropathic pain: a randomized, double-blind, and placebo-controlled study. Reg Anesth Pain Med. 2018;43(4):415–424. doi:10.1097/aap.0000000000000741
122. Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis. 1998;5(4):209–227. doi:10.1006/nbdi.1998.0204
123. Demant DT, Lund K, Vollert J, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155(11):2263–2273. doi:10.1016/j.pain.2014.08.014
124. Demant DT, Lund K, Finnerup NB, et al. Pain relief with lidocaine 5% patch in localized peripheral neuropathic pain in relation to pain phenotype: a randomised, double-blind, and placebo-controlled, phenotype panel study. Pain. 2015;156(11):2234–2244. doi:10.1097/j.pain.0000000000000266
125. Wilkinson ID, Teh K, Heiberg-Gibbons F, et al. Determinants of treatment response in painful diabetic peripheral neuropathy: a combined deep sensory phenotyping and multimodal brain MRI study. Diabetes. 2020;69(8):1804–1814. doi:10.2337/db20-0029
126. Campbell CM, Kipnes MS, Stouch BC, et al. Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. Pain. 2012;153(9):1815–1823. doi:10.1016/j.pain.2012.04.014
127. González-Duarte A, Lem M, Díaz-Díaz E, Castillo C, Cárdenas-Soto K. The efficacy of pregabalin in the treatment of prediabetic neuropathic pain. Clin J Pain. 2016;32(11):927–932. doi:10.1097/ajp.0000000000000339
128. Selvarajah D, Wilkinson ID, Fang F, et al. Structural and functional abnormalities of the primary somatosensory cortex in diabetic peripheral neuropathy: a multimodal MRI study. Diabetes. 2019;68(4):796–806. doi:10.2337/db18-0509
129. Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth. 2014;113(1):148–156. doi:10.1093/bja/aeu056
130. Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153(6):1193–1198. doi:10.1016/j.pain.2012.02.021
131. Lee-Kubli C, Marshall AG, Malik RA, Calcutt NA. The H-reflex as a biomarker for spinal disinhibition in painful diabetic neuropathy. Curr Diab Rep. 2018;18(1):1. doi:10.1007/s11892-018-0969-5
132. Zhou X, Zhu Y, Wang Z, et al. Rate-dependent depression: a predictor of the therapeutic efficacy in treating painful diabetic peripheral neuropathy. Diabetes. 2022;71(6):1272–1281. doi:10.2337/db21-0960
133. Forstenpointner J, Otto J, Baron R. Individualized neuropathic pain therapy based on phenotyping: are we there yet? Pain. 2018;159(3):569–575. doi:10.1097/j.pain.0000000000001088
134. Gilron I, Coderre TJ. Emerging drugs in neuropathic pain. Expert Opin Emerg Drugs. 2007;12(1):113–126. doi:10.1517/14728214.12.1.113
135. Shaibani AI, Pope LE, Thisted R, Hepner A. Efficacy and safety of dextromethorphan/quinidine at two dosage levels for diabetic neuropathic pain: a double-blind, placebo-controlled, multicenter study. Pain Med. 2012;13(2):243–254. doi:10.1111/j.1526-4637.2011.01316.x
136. Allen R, Sharma U, Barlas S. Clinical experience with desvenlafaxine in treatment of pain associated with diabetic peripheral neuropathy. J Pain Res. 2014;7:339–351. doi:10.2147/jpr.S55682
137. Deecher DC, Beyer CE, Johnston G, et al. Desvenlafaxine succinate: a new serotonin and norepinephrine reuptake inhibitor. J Pharmacol Exp Ther. 2006;318(2):657–665. doi:10.1124/jpet.106.103382
138. Rice ASC, Dworkin RH, McCarthy TD, et al. EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled Phase 2 clinical trial. Lancet. 2014;383(9929):1637–1647. doi:10.1016/s0140-6736(13)62337-5
139. Anand U, Yiangou Y, Sinisi M, et al. Mechanisms underlying clinical efficacy of Angiotensin II type 2 receptor (AT2R) antagonist EMA401 in neuropathic pain: clinical tissue and in vitro studies. Mol Pain. 2015;11:38. doi:10.1186/s12990-015-0038-x
140. Liu Y, Luo B, Han F, et al. Erythropoietin-derived nonerythropoietic peptide ameliorates experimental autoimmune neuritis by inflammation suppression and tissue protection. PLoS One. 2014;9(3):e90942. doi:10.1371/journal.pone.0090942
141. Zhang W, Yu G, Zhang M. ARA 290 relieves pathophysiological pain by targeting TRPV1 channel: integration between immune system and nociception. Peptides. 2016;76:73–79. doi:10.1016/j.peptides.2016.01.003
142. Brines M, Dunne AN, van Velzen M, et al. ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Mol Med. 2015;20(1):658–666. doi:10.2119/molmed.2014.00215
143. Culver DA, Dahan A, Bajorunas D, et al. Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Invest Ophthalmol Vis Sci. 2017;58(6):Bio52–bio60. doi:10.1167/iovs.16-21291
144. Jain SM, Balamurugan R, Tandon M, et al. Randomized, double-blind, placebo-controlled trial of ISC 17536, an oral inhibitor of transient receptor potential ankyrin 1, in patients with painful diabetic peripheral neuropathy: impact of preserved small nerve fiber function. Pain. 2022;163(6):e738–e747. doi:10.1097/j.pain.0000000000002470
145. Bramson C, Herrmann DN, Carey W, et al. Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med. 2015;16(6):1163–1176. doi:10.1111/pme.12677
146. Bernier LP, Ase AR, Séguéla P. P2X receptor channels in chronic pain pathways. Br J Pharmacol. 2018;175(12):2219–2230. doi:10.1111/bph.13957
147. Jarvis MF, Burgard EC, McGaraughty S, et al. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A. 2002;99(26):17179–17184. doi:10.1073/pnas.252537299
148. Rao S, Liu S, Zou L, et al. The effect of sinomenine in diabetic neuropathic pain mediated by the P2X(3) receptor in dorsal root ganglia. Purinergic Signal. 2017;13(2):227–235. doi:10.1007/s11302-016-9554-z
149. Rastogi A, Jude EB. Novel treatment modalities for painful diabetic neuropathy. Diabetes Metab Syndr. 2021;15(1):287–293. doi:10.1016/j.dsx.2021.01.004
150. Kopsky DJ, Hesselink JM. High doses of topical amitriptyline in neuropathic pain: two cases and literature review. Pain Pract. 2012;12(2):148–153. doi:10.1111/j.1533-2500.2011.00477.x
151. Mahoney JM, Vardaxis V, Moore JL, Hall AM, Haffner KE, Peterson MC. Topical ketamine cream in the treatment of painful diabetic neuropathy: a randomized, placebo-controlled, double-blind initial study. J Am Podiatr Med Assoc. 2012;102(3):178–183. doi:10.7547/1020178
152. Ali G, Subhan F, Abbas M, Zeb J, Shahid M, Sewell RD. A streptozotocin-induced diabetic neuropathic pain model for static or dynamic mechanical allodynia and vulvodynia: validation using topical and systemic gabapentin. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(11):1129–1140. doi:10.1007/s00210-015-1145-y
153. Alam U, Arul-Devah V, Javed S, Malik RA. Vitamin D and diabetic complications: true or false prophet? Diabetes Ther. 2016;7(1):11–26. doi:10.1007/s13300-016-0159-x
154. Lv WS, Zhao WJ, Gong SL, et al. Serum 25-hydroxyvitamin D levels and peripheral neuropathy in patients with type 2 diabetes: a systematic review and meta-analysis. J Endocrinol Invest. 2015;38(5):513–518. doi:10.1007/s40618-014-0210-6
155. Alam U, Petropoulos IN, Ponirakis G, et al. Vitamin D deficiency is associated with painful diabetic neuropathy. Diabetes Metab Res Rev. 2021;37(1):e3361. doi:10.1002/dmrr.3361
156. Basit A, Basit KA, Fawwad A, et al. Vitamin D for the treatment of painful diabetic neuropathy. BMJ Open Diabetes Res Care. 2016;4(1):e000148. doi:10.1136/bmjdrc-2015-000148
157. Alam U, Fawwad A, Shaheen F, Tahir B, Basit A, Malik RA. Improvement in neuropathy specific quality of life in patients with diabetes after vitamin D supplementation. J Diabetes Res. 2017;2017:7928083. doi:10.1155/2017/7928083
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.