Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Pain Mimicking Trigeminal Neuralgia in Patients with Acute Ischemic Stroke of the Brainstem
Authors Shi Y, Feng Z , Ju Y , Zhang Z
Received 29 March 2022
Accepted for publication 3 June 2022
Published 20 June 2022 Volume 2022:18 Pages 1237—1248
DOI https://doi.org/10.2147/NDT.S368351
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yuzhi Shi, Zhiyuan Feng, Yi Ju, Zaiqiang Zhang
Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Zaiqiang Zhang; Yi Ju, Beijing Tiantan Hospital, Capital Medical University, No. 119 South Fourth Ring West Road, Fengtai District, Beijing, 100070, People’s Republic of China, Tel +86 1059978000, Fax +86 59975570, Email [email protected]; [email protected]
Background and Purpose: Cases of pain mimicking trigeminal neuralgia (TN) induced by ischemic stroke in the brainstem have been sparsely reported. The study was to determine the characteristics of symptomatic TN in patients with acute ischemic stroke in the medulla oblongata and pons, and to determine the location of the ischemic lesion associated with the pain.
Patients and Methods: A total of 6/21 (28.5%) patients with medullary ischemic stroke and 3/34 (8.8%) patients with pontine ischemic stroke who experienced pain mimicking TN between 1 week before and 2 weeks after the stroke onset were enrolled in the study. All patients accepted neuroimaging examinations to determine the location of the ischemic lesion and the etiology of ischemic stroke. The characteristics of pain were recorded and analyzed.
Results: Ischemic lesions of patients who experienced pain mimicking TN were located in the lateral medulla oblongata (n=6), nerve root entry zoo (n=2), and areas involved with the spinal trigeminal tract (n=1) in the pons. Half of the instances of pain induced by medullary ischemic stroke occurred prior to the stroke onset. The branch of V1 was exclusively involved in patients with lateral medullary infarction and the branches of V2 and V3 were typically involved in patients with pontine infarction. The pain was relieved spontaneously (n=4, 44.4%) or was controlled with drugs for neuropathic pain treatment (n=5, 55.5%).
Conclusion: Half of the instances of pain induced by medullary ischemic stroke occurred prior to the stroke onset. Pain mimicking TN might be a premonitory symptom of the medullary ischemic stroke. Pain mimicking TN induced by brainstem infarction has a good prognosis.
Keywords: trigeminal neuralgia, lateral medullary infarction, pontine infarction, brainstem infarction, ischemic stroke
Introduction
Trigeminal neuralgia (TN) is characterized by recurrent, unilateral facial pain restricted to the trigeminal distribution that is precipitated by innocuous stimuli; TN is abrupt in onset and termination, brief (lasting for 1 second to 2 minutes), and is severe in intensity with an electric shock-like, stabbing-like quality.1,2 TN is classified into classical, secondary, and idiopathic subtypes based on the etiology.3 The secondary type, accounting for approximately 15% of cases, is attributable to an identifiable neurological disease, such as cerebellopontine angle tumor, arteriovenous malformation, and multiple sclerosis other than trigeminal NVC.3 TN, as a symptom of the neurological diseases mentioned above, is named as symptomatic TN and is caused by pathology of the peripheral trigeminal nerve and brain stem.3 Lesions centered in the intra-pontine trigeminal primary afferents between the trigeminal nerve root entry zone (NREZ) and the trigeminal nuclei were significantly associated with symptomatic TN in patients with multiple sclerosis (MS).4
Cases of pain mimicking TN induced by ischemic stroke in the brainstem have been sparsely reported since 1996.5,6 Ischemic stroke of the brainstem is another neurological disease underlying secondary TN. Facial pain is considered to be a feature of lateral medullary syndrome and is present in 29–50% of patients with Wallenberg syndrome,7,8 and cases of patients with pain mimicking TN caused by lateral medullary ischemic stroke have been reported in the past 20 years.5,9–13 Cases of pain mimicking TN induced by pontine ischemic stroke were much less frequently reported than cases induced by lateral medullary infarction, and most ischemic lesions in the pons associated with the pain were located in or adjacent to the area of the trigeminal NREZ.14–18
The anatomy of the trigeminal sensory system19 is the basement of the development of pain mimicking TN in patients with pontine or medullary ischemic stroke. The central axons of the neuron within the gasserian ganglion form the dorsal sensory root and extend into the pons through the area of the NREZ. The fibers carry pain and temperature afferents coalescing in the dorsomedial pons and turn caudally to form the spinal trigeminal tract. The spinal trigeminal tract courses just under the lateral surface of the pons and medulla and extends to the upper cervical cord. Along its caudal course through the pons and medulla, the spinal trigeminal tract terminals synapse on neurons of the spinal trigeminal nucleus. The spinal trigeminal nucleus lies just medial to the spinal trigeminal tract throughout its course. We supposed that the involvement of the NREZ, intra-pontine nerve fibers extending from NREZ to the dorsomedial pons adjacent to trigeminal sensory nucleus, spinal trigeminal tract (both pontine and medullary), and spinal trigeminal nucleus resulted in the occurrence of pain mimicking TN.
In most cases of pain associated with ischemic stroke in the medulla oblongata or pons, the pain event started at the onset of or after the indexed stroke. A few cases have been reported as instances of pain starting hours or days before the stroke onset.13,20 A recent study implied that a newly discovered type of headache that occurs during the week before stroke is considered to be a warning symptom of ischemic stroke.21,22 In this study, we consecutively enrolled patients with acute ischemic stroke in medulla oblongata or pons who experienced pain mimicking TN between 1 week before stroke onset and 2 weeks after stroke to investigate the characteristics and courses of symptomatic TN induced by acute ischemic stroke in the brainstem. The location of ischemic lesions associated with the pain was determined in the study.
Materials and Methods
We consecutively screened 62 patients with acute ischemic stroke (within 72 hours to the stroke onset) in the medulla oblongata or pons from the inpatient department of neurology in Beijing Tiantan Hospital between August 2020 and November 2021. The mean screening time to the stroke onset was 45.09±17.85 hours. Patients with concomitant ischemic lesions in the supratentorial cerebral structure or mesencephalon were excluded (n=3). Additional exclusion criteria included a history of facial pain or possible TN 1 week before the stroke onset; other concomitant other neurological disease, such as brain tumor, multiple sclerosis, arteriovenous malformation, epilepsy or antiepileptic drugs treatment, and no consent for follow-up (n=4). The symptom of TN was recognized according to the latest definition provided by the International Headache Society.1 Fifty-five patients (21 patients with medullary ischemic stroke, 34 patients with pontine ischemic stroke) were visited at admission and 14±2 days during the hospital stay to screen for patients who presented pain mimicking TN. At last, nine patients experienced mimicking TN between 1 week before stroke onset and 2 weeks after stroke, and were followed up at 3 months after stroke onset to evaluate the prognosis of the pain (Figure 1).
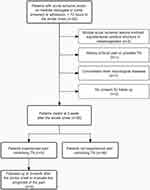 |
Figure 1 Flowchart of patients enrolled in the study. |
Demographic and clinical information associated with ischemic stroke and the symptoms of pain mimicking TN were recorded at admission and during the hospital stay. All patients enrolled in the study underwent magnetic resonance imaging (MRI) and 3D time-of-flight magnetic resonance angiography (MRA) scans within 24 hours of arrival at the emergency department and before the admission. Sequences included T1 weighted, T2 weighted, T2 weighted fluid attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI), and apparent diffusion coefficient (ADC) to locate the ischemic lesion and to detect trigeminal neurovascular compression (NVC) and other possible neurological causes of symptomatic TN. High resolution MRA and computed tomography angiography (CTA) were conducted in patients to determine the angiopathy of the criminal artery in some patients as needed. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committees of Beijing Tiantan Hospital and written informed consents were obtained from all patients.
The onset of stroke was defined as the time of the appearance of the symptoms of neurological impairment. The severity of stroke was rated by the National Institutes of Health Stroke Scale (NIHSS).23 Symptomatic TN was classified into three categories according to the timing of pain mimicking TN beginning: prior to stroke onset, simultaneously with symptoms of neurological impairment, and post-stroke (pain presented after the neurological deficiency to its peak).24 The verbal numerical rating scale (VNRS) was used to assess pain intensity, which was scored from “0 = no pain at all” to “10 = the worst imageable pain”, at visits of admission, 14±2 days, and 3-month after the stroke onset in patients with pain mimicking TN. Termination of pain was defined as pain free and did not require any medications for pain. Time of pain termination was recorded as 90 days after stroke onset in patients who still took medications to achieve pain control or relief at the 3-month follow-up. The duration of pain was recorded as the time between pain initiation and the time of pain termination.
Statistical Analysis
Quantitative data are reported as the mean and standard deviation for normally distributed data and compared by t-tests between patients with and without pain mimicking TN. The nonnormally distributed variables were described as median and quantile, and were compared by non-parametric test between patients with and without pain mimicking TN, as well as between patients with medullary ischemic stroke and patients with pontine ischemic stroke in those who experience pain mimicking TN. Categorical variables are described by number and percentage and compared by crosstabs analyses between patients with and without pain mimicking TN. A two-tailed probability value of P<0.05 was considered statistically significant. Analyses were carried out using SPSS Version 23.
Results
A total of 55 patients with medullary ischemic stroke (n=21, 38.1%) and pontine ischemic stroke (n=34, 61.8%) were visited at admission and 14±2 days after the stroke onset. Six of 21 (28.5%) and three of 34 (8.8%) patients with medullary ischemic stroke and pontine ischemic stroke, respectively, reported suffering from pain mimicking TN between 1 week before stroke and 2 weeks after stroke. The characteristics of patients with pain mimicking TN had no significant difference with those patients without pain referring to age, gender, vascular risk factors, stroke severity, and etiology (Table 1). The mean age of patients with pain mimicking TN was 57.22±14.93 years old, six (66.7%) of them were male (Table 1). The detailed clinical information of the nine patients who presented with pain mimicking TN between 1 week before stroke and 2 weeks after stroke is listed in Table 2.
 |
Table 1 Characteristics of Patients with Acute Ischemic Stroke in the Pons and Medulla Oblongata Presenting with and without Pain Mimicking TN |
 |
Table 2 Characteristics of the Pain Mimicking Trigeminal Neuralgia in Patients with Acute Ischemic Stroke in the Medulla Oblongata and Pons |
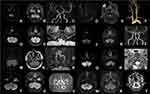
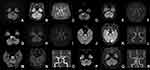
Regions of ischemic lesions in patients with symptomatic TN included the lateral medulla oblongata in which the spinal trigeminal tract and spinal trigeminal nucleus were involved (Figure 2A–S, Table 2), and pons in which intra-pontine nerve fibers extended from NREZ to dorsomedial pons and the NREZ (Figure 3A-I, Table 2). The most common etiology of ischemic stroke in the patients with pain mimicking TN was large artery atherosclerosis (n=5, 55.6%) and the pathogenesis included large artery occlusion, perforating artery occlusion, and arterial-artery embolism. Other etiologies of ischemic stroke were vertebral artery or posterior inferior cerebellar artery dissection (n=3, 33.3%) (Table 2, Figure 2I, P, S) and cardiogenic embolism (n=1, 11.1%) (Table 2, Figures 2J–L). Some representative ischemic lesions in medulla oblongata and pons that did not result in pain mimicking TN are also shown in Figures 2T–Y and 3J–R, respectively. The lesion including ventral and medial medulla oblongata (Figure 2T and U), lateral medulla oblongata without spinal trigeminal tract, and spinal trigeminal nucleus were involved (Figure 2W and X), along with ventral pons (Figure 3J and K) and upper paramedian pons (Figure 3M and N). MR imaging showed an acute ischemic lesion in dorsomedial pons adjacent to sensory nucleus of trigeminal nerve on DWI (Figure 3P) but no marked parenchymal hyperintensity on Flair (Figure 3Q) in a patient with wake-up stroke without presentation of pain mimicking TN.
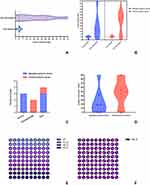
The median of the time window between initiation of a TN event and the stroke onset was 0 (−0.51, 2.0) days. The longest time windows between the appearance of the pain and the onset of the indexed ischemic stroke event were 5 days before the stroke onset and 4 days after the stroke onset (Figure 4A). There was no significant difference in the length of this time window between patients with medullary ischemic stroke and those with pontine ischemic stroke [0.15 (−0.20, 2.0) vs 0 (0–4.0), P=0.435] (Figure 4B). According to the timing of the appearance of the pain mimicking TN relative to the stroke onset, three, two, and four patients were classified as having TN events prior to, simultaneously with, and post stroke symptoms, respectively (Figure 4C). Half of the instances of pain mimicking TN induced by medullary ischemic stroke appeared prior to the stroke onset. The instances of pain mimicking TN started from 5 days to 20 minutes before the stroke onset. The onset of pain in patients with pontine ischemic stroke occurred simultaneously with (n=2, 66.6%) or post (n=1, 33.3%) stroke symptoms (Figure 4C).
The pain could be described as pinprick-like, electric shock-like, stabbing and radiation typical pain, and as spontaneous or induced by touch, chewing, brushing, mouth opening, and wind blowing (Table 2). Four (44.4%) patients had a trigger and 66.7% of patients experienced pain events more than 10 times daily. A duration per event of more than 2 minutes was occurred in only one patient with pontine ischemic stroke. The median of VNRS was 7.0 (6.5–8.0). The majority of patients with symptomatic TN (n=7, 77.8%) exhibited involvement of more than one branch of the trigeminal nerve (Table 3). The branch of V1 was exclusively involved in patients with medullary ischemic stroke (Figure 4E), and the branches of V2 and V3 were typically involved in patients with pontine ischemic stroke (Figure 4F). All patients enrolled in the study had sensory abnormalities in the face on the side ipsilateral to the pain mimicking TN. Symptomatic mimic TN was accompanied by decreased acupuncture sensation (n=6, 66.7%) or hyperesthesia (n=3, 33.3%) in the area in which the affected branches of the trigeminal nerve are distributed.
 |
Table 3 Characteristics of the Pain Mimicking TN in Patients with Acute Ischemic Stroke in the Pons and Medulla Oblongata |
Regarding the prognosis of the symptomatic TN in patients with ischemic stroke in the brainstem, four (44.4%) patients experienced spontaneous relief of pain lasting from minutes to 2 weeks, and one patient among them even experienced pain resolution before stroke onset. Five (55.5%) patients had pain controlled or relieved by treatment with pregabalin, oxcarbazepine, or gabapentin. Among patients who accepted drug therapy, one patient took two kinds of medications for pain, and another four patients took one kind of medication to achieve pain control or acceptable improvement. Only one patient continued taking medication to achieve pain control at the 3-month follow-up after the stroke onset. The median number of days to pain termination after stroke onset in all patients enrolled in the study was 21.0 (0.1–52.5) (Figure 4A). Although patients with medullary ischemic stroke tended to experience pain termination earlier than those with pontine ischemic stroke, the difference was not significant [20.5 (0.01–54.0) vs 45.0 (0.13–60.0), P=0.439] (Figure 4B). The median of duration of the pain for all patients enrolled in the study was 19.0 (0.48–49.50) days. The patients with medullary ischemic stroke had a tendency to experience a shorter duration of pain than that experienced by patients with pontine infarction [18.5 (0.65–55.0) vs 45.0 (0.13–54.0), P=0.606] (Figure 4D). There was no significant difference among patients categorized according to the timing of the appearance of mimic TN relative to the stroke onset.
Discussion
In this study, we investigated nine of 55 patients with acute ischemic stroke in the medulla oblongata or pons who presented with pain mimicking TN between 1 week before stroke onset and 2 weeks after stroke. Pain presented as paroxysmal attack in different areas in which the V1, V2, or V3 are distributed, occurred with or without a trigger, and accompanied a decreased or increased acupuncture sensation. The symptoms of pain mimicking TN could appear prior to, simultaneously with, or post stroke symptoms. The pain was relieved spontaneously or was controlled with drugs for neuropathic pain treatment. The majority of patients experienced pain termination without pharmacological intervention at the 3-month follow-up after stroke onset.
Facial pain was reported in 50% (6 in 12 cases) of patients with Wallenberg syndrome along with a decrease in pain and temperature sensation in the ipsilateral side of the face in a previous study.8 Symptomatic TN associated with medullary infarction mainly involves the V1 branch of the trigeminal nerve, which is different from classical TN with the branches of V2 and V3 commonly involved.8,25,26 In our study, six patients with acute ischemic stroke in the lateral medulla reported events of pain mimicking TN. Vulnerability of the branch of V1 was also discovered in patients with medullary ischemic stroke, 80% of them experienced neuralgia involved in the regions of V1 distributed and 20% of them exhibited the V1 distribution solely affected. The frequency of V1 involvement in patients with acute medullary ischemic stroke is much higher than that of classic TN (80% vs 28% for V1 involvement and 20% vs 4% for solely V1 involvement).26 The vulnerability of V1 distribution could be explained by the precise arrangement of fibers in the descending course of spinal trigeminal tract: fibers originating from the V1 division are located most ventrally and laterally in the tract, V2 division axons are intermediate, and V3 division axons are positioned posteriorly and medially in the tract.19 Lateral medullary ischemic stroke is more likely to involve the lateral spinal trigeminal tract in which V1 division axons are located. The scope of pain mimicking TN in patients with pontine ischemic stroke was restricted to the distribution of the branches V2 and V3, which is in line with the involvement observed in patients with classical TN.26 The ischemic lesions in or adjacent to the trigeminal NREZ might explain the vulnerability of the branches of V2 and V3.
The instance of pain started prior to, simultaneously with, or post stroke symptoms. Half of the instances of pain mimicking TN induced by ischemic stroke in the medulla oblongata occurred prior to the onset of ischemic stroke symptoms. The first-ever facial pain mimicking TN might be a warning symptom of medullary ischemic stroke, especially in individuals with a high risk of cerebral vascular diseases. Pain induced by pontine ischemic stroke appeared simultaneously with or post the stroke symptoms.
For a small number of facial, glossopharyngeal, and vagal nerve fibers, along with the V3 division axons which join in the trigeminal spinal tract,19 it has been reported that facial autonomic symptoms12,20 and glossopharyngeal neuralgia13 are concurrent with pain mimicking TN in patients with medullary infarction. In our study, four patients presented with Horner syndrome, and no other facial autonomic symptoms or glossopharyngeal neuralgia were detected in patients with acute medullary ischemic stroke.
Unlike four patients whose pain was relieved spontaneously, five patients experienced pain relief or control at 3-months after stroke by treatment with oxcarbazepine, gabapentin, or pregabalin. All these medications used are the common pharmacological treatment for patients with classical TN.27 Patients with pain mimicking TN lasting for more than 3 months could be classified as exhibiting central post-stroke pain.2 Amitriptyline and lamotrigine were considered are the first choice for treatment of patients with central post-stroke pain.28 The effectiveness of amitriptyline or lamotrigine in treating patients with pain mimicking TN induced by brainstem ischemic stroke needed to be further studied. For those unable to tolerate medication side-effects or interactions, surgical intervention may be considered.6
There are some limitations to this study. Pain mimicking TN is a rare symptom in patients with acute ischemic stroke in the brainstem. The number of patients enrolled in the study was small. The small sample size of the study limited the statistical analysis and might result in bias in the results. A multicenter study is necessary to investigate the mechanisms of and optimal therapy for symptomatic TN in patients with ischemic stroke in the brainstem.
Conclusion
Symptomatic TN may appear prior to, simultaneously with, or post stroke symptom in patients with medullary ischemic stroke and pontine ischemic stroke. Half of pain mimicking TN induced by medullary ischemic stroke appeared prior to the stroke onset. Pain mimicking TN might be a premonitory symptom of the medullary ischemic stroke. Patients who without a history of facial pain and at high risk of cerebrovascular disease, presenting with pain mimicking TN should be treated with caution. Pain mimicking TN induced by ischemic stroke in the brainstem has a good prognosis, and most of the symptoms can be relieved spontaneously or by drug treatment.
Acknowledgments
Thanks to all the patients who participated for their involvement in the study.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Headache Classification Subcommittee of The International Headache Society. The international classification of headache disorders. 3rd edn. Cephalalgia. 2018;38(1):1–211. doi:10.1177/0333102417738202
2. Scholz J, Finnerup NB, Attal N, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160(1):53–59. doi:10.1097/j.pain.0000000000001365
3. Lambru G, Zakrzewska J, Matharu M. Trigeminal neuralgia: a practical guide. Pract Neurol. 2021;21:1–12. doi:10.1136/practneurol-2020-002782
4. Cruccu G, Biasiotta A, Di Rezze S, et al. Trigeminal neuralgia and pain related to multiple sclerosis. Pain. 2009;143(3):186–191. doi:10.1016/j.pain.2008.12.026
5. Nakamura K, Yamashita T, Yamashita M. Small medullary infarction presenting as painful trigeminal sensory neuropathy. J Neurol Neurosurg Psychiat. 1996;61(2):138. doi:10.1136/jnnp.61.2.138
6. Shanker RM, Kim M, Verducci C, et al. Surgical management of trigeminal neuralgia induced by brainstem infarct: a systematic review of the literature. World Neurosurg. 2021;151:209–217. doi:10.1016/j.wneu.2021.04.099
7. Vestergaard K, Andersen G, Nielsen MI, Jensen TS. Headache in stroke. Stroke. 1993;24(11):1621–1624. doi:10.1161/01.STR.24.11.1621
8. Fitzek S, Baumgärtner U, Fitzek C, et al. Mechanisms and predictors of chronic facial pain in lateral medullary infarction. Ann Neurol. 2001;49(4):493–500. doi:10.1002/ana.99
9. Ravichandran A, Elsayed KS, Yacoub HA. Central pain mimicking trigeminal neuralgia as a result of lateral medullary ischemic stroke. Cas Rep Neurol Med. 2019;2019:423574.
10. Huang C-T, Lo C-P, Chen Y-C, Tu M-C, Rare A. Case of painful trigeminal neuropathy secondary to lateral medullary infarct: neuroimaging and electrophysiological studies. Acta Neurol Taiwanica. 2015;24(2):63–68.
11. John AA, Abbas MM, Javali M, Mahale R, Mehta A, Srinivasa R. A rare presentation of trigeminal neuralgia in lateral medullary syndrome. Neurol India. 2017;65(3):638–640. doi:10.4103/neuroindia.NI_1323_15
12. Lambru G, Trimboli M, Tan SV, Al-Kaisy A. Medullary infarction causing coexistent SUNCT and trigeminal neuralgia. Cephalalgia. 2017;37(5):486–490. doi:10.1177/0333102416652093
13. Warren HG, Kotsenas AL, Czervionke LF. Trigeminal and concurrent glossopharyngeal neuralgia secondary to lateral medullary infarction. Am J Neuroradio. 2006;27(3):705–707.
14. Kim JB, Yu S. Trigeminal neuralgia after pontine infarction affecting the ipsilateral trigeminal nerve. J Neurol Neurosurg Psychiat. 2013;84(8):881–882. doi:10.1136/jnnp-2013-305024
15. Goel R, Kumar S, Panwar A, Singh AB. Pontine infarct presenting with atypical dental pain: a case report. Open Dent J. 2015;9(Suppl 2):337–339. doi:10.2174/1874210601509010337
16. Katsuno M, Teramoto A. Secondary trigeminal neuropathy and neuralgia resulting from pontine infarction. J Stroke Cerebrovasc Dis. 2010;19(3):251–252. doi:10.1016/j.jstrokecerebrovasdis.2009.04.005
17. Ferroli P, Farina L, Franzini A. Linear pontine and trigeminal root lesions and trigeminal neuralgia. Arch Neurol. 2001;58(8):1311–1312. doi:10.1001/archneur.58.8.1311
18. Peker S, Akansel G, Sun I, Pamir NM. Trigeminal neuralgia due to pontine infarction. Headache. 2004;44(10):1043–1055. doi:10.1111/j.1526-4610.2004.4200_1.x
19. Traurig HH. The trigeminal system. In: Conn PM, editor. Neuroscience in Medicine. Totowa, NJ: Humana Press; 2008:287–299.
20. Galende AV, Camacho A, Gomez-Escalonilla C, et al. Lateral medullary infarction secondary to vertebral artery dissection presenting as a trigeminal autonomic cephalalgia. Headache. 2004;44(1):70–74. doi:10.1111/j.1526-4610.2004.04012.x
21. Lebedeva ER, Ushenin AV, Gurary NM, Gilev DV, Olesen J. Sentinel headache as a warning symptom of ischemic stroke. J Headache Pain. 2020;21(1):70. doi:10.1186/s10194-020-01140-3
22. Lebedeva ER, Ushenin AV, Gurary NM, et al. Headache at onset of first-ever ischemic stroke: clinical characteristics and predictors. Eur J Neurol. 2021;28(3):852–860. doi:10.1111/ene.14684
23. Lyden P. Using the National Institutes of Health Stroke Scale. Stroke. 2017;48(2):513–519. doi:10.1161/STROKEAHA.116.015434
24. Harriott AM, Karakaya F, Ayata C. Headache after ischemic stroke. Neurology. 2020;94(1):e25–e28. doi:10.1212/WNL.0000000000008591
25. Ordás CM, Cuadrado ML, Simal P, et al. Wallenberg’s syndrome and symptomatic trigeminal neuralgia. J Headache Pain. 2011;12(3):377–380. doi:10.1007/s10194-011-0305-9
26. Maarbjerg S, Gozalov A, Olesen J, Bendtsen L. Trigeminal neuralgia-a prospective systematic study of clinical characteristics in 158 patients. Headache. 2014;54(10):1574–1582. doi:10.1111/head.12441
27. Gambeta E, Chichorro JG, Zamponi WG. Trigeminal neuralgia: an overview from pathophysiology to pharmacological treatments. Mol Pain. 2020;16(1):1–18. doi:10.1177/1744806920901890
28. Choi HR, Aktas A, Bottros MM. Pharmacotherapy to manage central post-stroke pain. CNS Drugs. 2021;35(2):151–160. doi:10.1007/s40263-021-00791-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.