Back to Journals » Journal of Inflammation Research » Volume 16
Oxidative Stress Contributes to Inflammatory and Cellular Damage in Systemic Lupus Erythematosus: Cellular Markers and Molecular Mechanism
Received 27 November 2022
Accepted for publication 18 January 2023
Published 4 February 2023 Volume 2023:16 Pages 453—465
DOI https://doi.org/10.2147/JIR.S399284
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Zhu Yan, Qin Chen, Yumin Xia
Department of Dermatology, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710004, People’s Republic of China
Correspondence: Yumin Xia, Department of Dermatology, The Second Affiliated Hospital of Xi’an Jiaotong University, 157 Xiwu Road, Xi’an, 710004, People’s Republic of China, Tel/Fax +86-29-87679969, Email [email protected]
Abstract: Systemic lupus erythematosus (SLE) is a multifactorial autoimmune disease with complex pathogenesis, the treatment of which relies exclusively on the use of immunosuppressants. Increased oxidative stress is involved in causing inflammatory and cellular defects in the pathogenesis of SLE. Various inflammatory and cellular markers including oxidative modifications of proteins, lipids, and DNA contribute to immune system dysregulation and trigger an aggressive autoimmune attack through molecular mechanisms like enhanced NETosis, mTOR pathway activation, and imbalanced T-cell differentiation. Accordingly, the detection of inflammatory and cellular markers is important for providing an accurate assessment of the extent of oxidative stress. Oxidative stress also reduces DNA methylation, thus allowing the increased expression of affected genes. As a result, pharmacological approaches targeting oxidative stress yield promising results in treating patients with SLE. The purpose of this review is to examine the involvement of oxidative stress in the pathogenesis and management of SLE.
Keywords: oxidative stress, systemic lupus erythematosus, inflammation, reactive oxygen species, molecular mechanism
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease primarily afflicting women of childbearing age. It is characterized by a flare-up of autoantibodies, particularly against nuclear components. There is a broad spectrum of its clinical manifestations involving multiple organ systems including the genitourinary, cardiopulmonary, neuromuscular-psychiatric, endocrine, integumentary, and hematologic systems.1,2 The molecular mechanisms underlying this systemic autoimmune response remain largely unknown, and any further advancement in therapeutic approaches requires assessments in preclinical studies.
In broad terms, molecular mimicry, aberrant exposure, or alteration of endogenous antigens, in combination with autoantibodies production, could promote the creation of pathogenic immune complexes, and thus mediate tissue damage in SLE and other systemic autoimmune diseases.3 Recently, overwhelming evidence has been discovered to demonstrate that elevated oxidative stress correlates with autoimmune disease activity and the facilitation of tissue damage.4–6 The effect of free radicals in the pathogenesis of systemic autoimmune diseases including SLE, rheumatoid arthritis (RA), Sjögren’s syndrome (SS) and multiple sclerosis (MS) is shown in Table 1. Reactive oxygen species (ROS) are generated by incomplete oxygen reduction during the redox process, where immoderate liberation and insufficient clearance are found to be implicated in the development of SLE.7 Oxidative stress; defined as a disequilibrium between the synthesis and neutralization of ROS; is deemed to partially explain the abnormal exposure of endogenous antigens and the response to cell-death signals characterizing the pathology SLE.8 This review will address how oxidative stress triggers deranged immunological aberrations and disturbed tissue functions in SLE.
 |
Table 1 Effects of Free Radicals in the Pathogenesis of Autoimmune Diseases |
The Basic Functions of Responses to Oxidative Stress
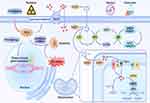
The sources of ROS can be exogenous and endogenous to the physiological system affected. Exogenous ROS mainly derives from external predisposing factors such as ultraviolet radiation, chemical exposure, and viral or bacterial infection, whereas endogenous sources include excessive ROS formation in mitochondria and extra-mitochondrial organelles like the endoplasmic reticulum. The transfer of electrons to molecular oxygen in the processes of the electron transport chain (ETC) creates ROS during oxidative phosphorylation in the mitochondria. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), nitric oxide synthase (NOS), and xanthine oxidase (XO) are the main causes of extramitochondrial oxidative stress.9 Reactive oxygen species; such as superoxide anion radical (O2•−) and hydrogen peroxide (H2O2); can interact with NO, the expression of which is regularly accompanied by pro-inflammatory signals, thereby accounting for the emergence of reactive nitrogen species (RNS), including nitrosonium cation (NO+), nitroxyl anion (NO−), and peroxynitrite (ONOO−).10 Multiple intracellular antioxidant defense systems that are principally dependent on antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione reductase (GR) impose constraints on ROS,11 as shown in Figure 1, yet non-enzymatic antioxidants also play a crucial role, and these include vitamin C, carotenoids, flavonoids, and glutathione.
ROS are widely acknowledged for having a dual function in various cellular processes. These short-lived molecules also serve as lethal weapons for the host defense system at low to modest concentrations. When confronted with an excess of ROS/RNS or a deficiency of enzymatic and non-enzymatic antioxidants, noxious effects will occur, thereby causing oxidative damage to intracellular biomolecules including lipids, proteins, and DNA.12 Any disruption to the oxidant/antioxidant equilibrium in autoimmune diseases by either endogenous or exogenous toxic substances incites an aberrant innate and adaptative immune response with a continuing supply of autoantigens.13 Some translational investigations1,14,15 have associated oxidative stress with the pathogenesis of SLE. High levels of oxidative and nitrosative stress have been detected in individuals with active SLE. Increased levels of NO, malondialdehyde (MDA), 4-hydroxy-2-nonenal (HNE), anti-SOD, and anti-CAT antibodies in the serum of SLE patients as well as diminished levels of reduced glutathione in patients with lupus nephritis (LN) were discovered to be correlated with disease activity.
Oxidative Stress Dysregulates Immune Cells in Lupus Erythematosus
The development of SLE is attributed to disorders with adaptive immunity that cause a lack of tolerance of self-antigens. Indeed, the onset and progression of SLE require immune cells, which underscores the crucial role of autoimmune reactivity in this disease. Ongoing studies in both human and mouse models have revealed oxidative stress to occur alongside both T cell dysfunction and SLE progression, revealing that mitochondrial dysfunction in T cells involves mitochondrial hyperpolarization (MHP), increased ROS synthesis, and high consumption of glutathione (the primary intracellular-acting antioxidant).16,17 Oxidative stress leads to MHP as manifested by an elevated mitochondrial transmembrane potential and a failure to couple with sufficient ATP, resulting in obstructed electron transfer and greater electron leakage, thus exacerbating the generation of ROS.18 The majority of lymphocytes in SLE undergo apoptosis during the generation of naive lymphocytes, particularly in the process of positive and negative selection in the central and peripheral lymphoid organs, which leads to lymphocytopenia in SLE. Lymphocytes from healthy subjects have been shown to undergo apoptosis when exposed to exogenous ROS in the form of H2O2 in vitro,19 insinuating that the persistent elevation in mitochondrial ROS was noteworthy in the apoptosis and necrosis of SLE lymphocytes, and might be a spur to the inflammation cascade in SLE patients.
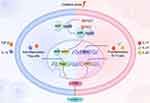
As a ubiquitous serine/threonine kinase, the mammalian target of rapamycin (mTOR) is a pertinent regulator in the activation, differentiation, and homeostasis of T cells.20,21 Researchers have recognized excessive levels of NO and prolonged MHP to be fundamental upstream checkpoints of mTOR activation in T cells, which effectively promote effector CD4+ T cell activation and subsequent differentiation.22 In addition, mTOR makes up the catalytic subunit of two protein complexes: mTOR complex 1 (mTORC1) and 2 (mTORC2).23 Previous research demonstrated that mTORC1 activity was enhanced in lupus patients, which restrained interleukin (IL) −2 and/or T cell receptor (TCR)-induced regulatory T (Treg) cell expansion.24 Conversely, the depletion of Treg cells; deemed as a hallmark of SLE; could be prevented by therapeutic intervention with rapamycin,25 as circulating Treg cells would decrease during SLE flares and their immune suppressive function in lupus patients was also weakened.26 Moreover, accumulating data supports the concept that both mTORC1 and mTORC2 suppress the differentiation of CD4+CD25+Foxp3+ Treg cells.27 In addition, mTORC1 facilitates the expansion of pro-inflammatory lymphocyte subpopulations in T helper 1 (Th1) and T helper 17 (Th17) cells through the regulation of hypoxia-inducible factor (HIF) −1, phosphorylation of signal transduction and transcription activator 3 (STAT3), and nuclear translocation of the transcription factor RORγt.28 T follicular helper (Tfh) cells and Th2 cells; which drive B cell activation and autoantibody synthesis; are favored by mTORC2.29 The transcriptional regulator FOXP3 is required for CD4+CD25+ Treg cell activation. Oxidative stress can refrain DNA methyltransferase 1 (DNMT1) activity and demethylate FOXP3 promoter, thereby inhibiting FOXP3 transcription and CD4+CD25+Treg cell differentiation. The protein IL-6 is capable of inhibiting FOXP3 expression during Treg cell differentiation. Oxidative damage has been observed to give rise to increased levels of IL-6. Concordantly, serum IL-6 level was doubled in lupus patients and pristane-induced lupus mouse models,1,30 reflecting that oxidative stress might have an appreciable effect on Treg cell differentiation by regulating IL-6. Adipokines were found to have been overproduced and strongly correlated to oxidative stress in SLE patients.31,32 Leptin, an adipocyte-derived hormone, has close relevance to adipokines and has been designated as another T cell biology integrator derived from oxidative stress.33 Its signaling seeks to modulate T cell proliferation and preferential differentiation of Th1 cells over Th2 cells while restricting Treg cell expansion, as the neutralization of leptin normally helps boost TCR and/or IL-2-induced Treg cell proliferation.
A burgeoning number of studies have demonstrated that the expansion of Th17 cells; as reflected by the production of IL-17; was embroiled in the pathogenesis of autoimmune disorders, including SLE.34 The aryl hydrocarbon receptor (AHR) is documented to be a transcription factor manipulating Treg cell and Th17 cell differentiation and typically exists in the cytoplasm in a complex with Hsp90.35 Augmented UV exposure, which has been the most studied among the environmental factors in association with SLE, has been shown to directly accelerate the emergence of 6-formylindolo [3,2-b]carbazole (FICZ); a natural high-affinity ligand of AHR; whose activation yields an exchange of Hsp90 for the nuclear translocation component ARNT and triggers expression of the CYP1A1 gene, subsequently resulting in Th17 cell expansion as well as IL-17, IL-21, and IL-22 production, but interfering with Treg cell development.36 As aforementioned, oxidative stress also activates mTORC1, which then favors Th17 cell differentiation and the generation of certain cytokines, while its blockade by rapamycin holds potential to augment Treg cell expansion, as shown in Figure 2. Overall, Th17/Treg cells are capable of attaining a delicate immune balance in normal circumstances, which can otherwise be disequilibrated by the superabundance of oxidative stress. Lineage specification in T cells becomes skewed by oxidative stress, which curtails anti-inflammatory Treg cell differentiation while extending proinflammatory Th17 cells, thereby deteriorating autoimmune derangement and resulting in tissular damage.
The oxidative metabolism of B lymphocytes in SLE is represented by heightened glycolysis and downregulation in lipid peroxidation. The activation of B cells is contingent on the initiation of glycolysis via the B-cell receptor (BCR) and the B cell-activating factor of the TNF family (BAFF); a cytokine aligned to SLE.37 Prolonged exposure to BAFF stimulation comprises a cascade of aerobic glycolysis and miscellaneous metabolic pathways that are instrumental in cell proliferation and antibody manufacturing, suggesting that severe impairments in controlling B cell metabolic reconfiguration may exert an influence on SLE progression.38 Glycolytic activity was found to be comparatively higher in transgenic mice that yielded lupus-like antibodies compared to controls.39 Since SLE patients had high BAFF levels, their glycolysis status might appear noticeably higher than the controls, which warrants more definitive investigation. Depressed lipid oxidation in B cells of SLE patients is a potential consequence of the condition that the lupus allele potentiates the expression of the metabolic gene encoding the protein fatty acid amide hydrolase (FAAH), whose level is explicitly raised in plasma cells.40 On top of that, as signal transducers and substance transport regions of the cell membrane, the lipid rafts on the B cell membranes of SLE patients possess structural and distributional anomalies, and their metabolism performs a prominent role in the regulation of BCR signaling. Dong et al41 have observed that treating SLE patients with leflunomide was able to modify the structure and distribution of the lipid rafts on B cell membranes, thereby aiding in the gradual decline of disease activity, ultimately suggesting that oxidative stress could interfere with lipid metabolism in B lymphocytes to alter the pathogenesis of SLE. Apart from lymphocytes acting as the main motivators of oxidative stress, neutrophils have also emerged in other studies to be detrimentally impaired by SLE.
As an integral component of the innate immune system in the first line of defense against invading micro-organisms, the process of neutrophil-induced elimination of microbes occurs through multiple accesses, such as through the release of microbicidal substances from cytoplasmic granules, phagocytosis, and the formation of neutrophil extracellular traps (NETs) by cellular residues, nucleic acids, and nuclear proteins. This so-called NETosis; referring to the process of cell death engendered by NETs; is distinguished from cell necrosis or apoptosis, existing as a specialized form of cell death whose lethality hinges primarily on NOX2-dependent ROS generation via the respiratory burst, which, intriguingly, brings about spontaneous NETosis.42 Previous data43 has shown that the enhancement in NET formation observed in SLE might hold a significant position in the pathogenesis of autoimmunity. Neutrophils extrude foreign DNA into NETs, which then excite B cells via Toll-like receptors (TLRs), while the proteins in NETs operate as auto-antigens and enable plasmacytoid dendritic cells to release interferon (IFN)-α, which terminates autoimmunity. Further supporting evidence has been found to show that the deficiency of Nox2 could clearly exacerbate lupus flares in MRL/lpr mice.44 The reduction of oxidative stress generation results in the inability to clear bacteria whose remaining DNA could persistently stimulate the innate immune system and expedite the development of lupus damage by blocking NETs formation and NETosis.45 In addition, mTOR signaling in neutrophil chemotaxis has been reported to necessitate large amounts of ATP,46 thus, it’s worth investigating whether a lack of ATP supply during the mTORC1-based activation of T cells and neutrophils is relevant to the pathogenesis of SLE.
ROS Affect Interactions Between Biological Macromolecules
Free radicals and other reactive species can interact with all biomolecules present in patients with SLE, potentially creating deleterious cascade products that disrupt immune regulation and trigger autoimmunity.47 Their abundance is correlated with disease activity and their corresponding deleterious cascade is held in check only by the existence of multiple antioxidant and repair systems as well as the replacement of damaged nucleic acids, proteins, and lipids.
Lipids are susceptible targets of oxidation. The double bonds of unsaturated fatty acids in mitochondria, lysosomes, and cell membranes serve as the principal sites of oxidative stress, allowing for enhanced lipid peroxidation and potential indicators for oxidative status in SLE.12 Lipid peroxidation cascades delivered a plethora of decomposition end products, including reactive aldehydes such as MDA, MDA-modified proteins, HNE, HNE-modified proteins, and F2 isoprostane; the levels of which, according to numerous independent investigations,48 were markedly higher in biological samples obtained from SLE patients. Furthermore, F2 isoprostane is generated by a free radical-mediated peroxidation of arachidonic acid that is independent of cyclooxygenase activity. Its process of formation, distinct structural properties, and relative chemical stability distinguish it from other radical-generated products, for which the measurement of plasma F2 isoprostane by MS/GC is contemplated to be the most reliable indicator of oxidative stress in vivo.49 Elevated levels of F2 isoprostane have been attributed to tissue oxidation in SLE and therefore can be utilized as a marker to assess the relationship between mitochondrial function and oxidative stress in fatigued patients.50 These metabolites establish covalent bonds to proteins, thereby enhancing their immunogenicity and prompting pathogenic autoantibodies to form. Taken together, current evidence suggests a positive correlation between elevated levels of lipid peroxidation and disease activity in SLE patients. Identically, oxidative stress mediates autoimmune involvement in the pathogenesis of SLE through lipid peroxidation metabolites.
In addition to the oxidative modification of lipids, oxygen radicals can alter the structure and function of proteins using any of the following: protein-peptide chain breakage, cross-linking and polymerization, the oxidative deamination of amino acids, attacking protein reducing groups, or causing secondary reactions with lipid peroxidation products.51 Formed as chemically stable protein oxidation products, protein carbonyls and the protein nitrotyrosine are extensively employed as biomarkers in SLE, with the former being more addressed these years owing to its longer circulation durations in the blood. Protein nitrotyrosine is delivered on tyrosine residues by RNS. There have been independent studies indicating that the levels of protein carbonyls were substantially higher in SLE patients than in controls, exhibiting varying correlations with disease activity, whereas antioxidant enzyme activities like catalase and superoxide dismutase were decreased.52 Levels of 3-nitrotyrosine were also documented to be increased, which has been referenced to cardiac, renal, and arthritis progression in SLE patients.53 Antibody-based ELISA can be applied to determine 3-nitrotyrosine levels, with GC-MS and LC-MS incorporating relatively high measurement accuracy. Nonetheless, additional efforts are still required in order to optimize the measurement of 3-nitrotyrosine, particularly at very low concentrations, and to reassess the relevance of circulating nitrotyrosine to the cardiac and renal complications observed in SLE patients. Moreover, high-density lipoprotein (HDL); which is prone to oxidation in patients with lupus lacks periodic vascular protective capabilities; instead fosters a pro-inflammatory response.54 Levels of the oxidized β2GPI antigen are proportional to the extent of antiphospholipid syndrome (APS) and thrombotic risk.55 However, the molecular etiology by which oxidized modified proteins lead to lupus flares remains largely unknown, and as many have surmised, might be affiliated with their heightened immunogenicity in vivo.
Similarly, ROS can destabilize DNA integrity by altering individual nucleotide bases, thereby fueling both single-strand breaks and cross-linking events. Continuous oxidative damage to DNA is an irreplaceable contributor to the development of age-related illnesses, and in particular, those major malignancies of the colon, breast, rectum, and prostate.56 DNA response and repair mechanisms are purposed to identify genetic flaws and ensure appropriate DNA replacement during the cell cycle. The cell transfers the mutant genome to its descendants in the presence of unrepaired lesions; otherwise, it will be counteracted by either apoptosis or senescence. The synthesis of 8-hydroxydeoxyguanosine (8-OHdG); as involved in the instance of oxidative DNA damage; is a consistent predictor of oxidative stress. This biomarker 8-OHdG is created and amplified physiologically by chemical carcinogens, and if not adequately disposed of, will accumulate in tissues and induce genomic instability and cellular dysfunction.57 Levels of 8-OHdG, which is detectable in a variety of biological samples (serum, plasma, urine, and tissues), have been alluded to in the disease activity of SLE patients in several studies.56,58 These denote 8-OHdG to be perceived as a sensitive predictor for examining oxidative DNA damage in the occurrence of lupus flares. Urinary 8-OHdG measurement has already been amply demonstrated to reflect the oxidative status of the entire body, which is negligibly affected by dietary factors. Further scientific reports have demonstrated that 8-oxoguanine-DNA-glycosylase (OGG1) is vicariously liable for the clean-up of 8-OHdG, with its overexpression lowering DNA lesions by 8-OHdG reparation in conditions of oxidative stress in vitro.59 These findings suggest that OGG1 performs preventive action in inflammatory responses by reconfiguring 8-OHdG levels and that its polymorphism offers genetic susceptibility to LN.
Oxidative Stress Modulates the Transcription of Susceptibility Genes
Despite the undefined etiology of lupus, familial clustering and the identification of genetic susceptibility loci indeed recommend a hereditary component of disease progression. DNA methylation is well accepted to be an epigenetic mechanism regulating gene expression, hence the hypomethylation of DNA in T cells has been extensively explored and found to engage in SLE pathogenesis.
Previous work exploiting CD4+T cells from patients with active SLE uncovered oxidative stress to be a promoter of both hypomethylation and the upregulation of certain immune genes. The replication of DNA methylation patterns during mitosis requires S-adenosylmethionine (SAM) and DNA methyltransferase 1 (DNMT1). Treatment of CD4+T cells with the DNMT1 inhibitor 5-azacytidine (5-azaC) provoked hypomethylation and the overexpression of immune genes such as CD11a, CD70, KIR, and perforin, culminating in the transformation of T cells from antigen-specific “helper” cells to autoreactive cells, and ultimately facilitating the advancement of lupus-like autoimmunity in animal models.60,61 Synthesis of ONOO− has been shown to bolster protein kinase C delta (PKCδ) nitration while having impeded ERK-dependent activation of DNMT1.62 Further evidence has also emerged to show that CD4+ T cells treated with ERK pathway inhibitors and an oxidizing agent could hypomethylate DNA and were thus prone to cause lupus flares in murine models.63 Glutathione deficiency translated into a limited availability of SAM, which constitutes an alternative pathway of hypomethylation as a methyl donor for DNA methylation. From the above viewpoint, we assume that T cell DNA hypermethylation, howsoever caused, tends to promote inflammation and give rise to the onset in SLE patients and lupus-susceptible mice.
Oxidative Stress Contributes to End-Organ Injuries in Lupus Erythematosus
Oxidative stress is associated with multiorgan involvement in SLE via stimulating undetermined immune complex deposition in the kidney, cardiovascular system, and other organs.64 Moreover, lipid peroxidation exerts an unfavorable impact on the progression of end-organ injuries in SLE.
LN; one of the most perplexing manifestations of SLE; is a leading cause of morbidity and mortality which occurs in the majority of patients at some point in the course of the disease. The deposition of immune complexes in the glomerulus and the activation of the complement system are engaged in its pathogenesis. Over time, cumulative data have strongly implicated oxidative stress to be a valuable addition to the renal impairment of SLE.15 Two biomarkers reflected by elevated MDA concentrations are that of diminished vitamin C levels in the plasma of LN as well as a lower glutathione:GSSG ratio in the kidneys of female B/W mice model of SLE.65 Decades of research have unearthed that either hydrogen peroxide or ONOO− is sufficient to evoke anti-DNA antibodies and glomerulonephritis in genetically susceptible mice.66 Patients with active LN have an imbalanced redox state, a condition that causes lipid peroxidation of the glomerular basement membrane to alter its integrity, thereby impacting tubular function in affected patients. Oxidative stress may potentially inflict renal damage through the production of tumor necrosis factor (TNF) by infiltrating macrophages, excessive Th17 cell differentiation, secretion of the inflammatory cytokine IL-17, and activation of neutrophils to form NETs. By using TNF blockade, researchers could alleviate oxidative stress and renal lesion in NZB/NZW mice with IFNα-induced LN.67 Nestin, on the other hand, has been shown to shield podocytes from injury by mediating severe mitochondrial division or convergence impairment, thereby resulting in impaired oxidative phosphorylation and reduced mitochondrial ROS (mROS), which in turn governs the expression and phosphorylation of nephrogenic proteins.66 There’s reason to believe that downregulated nestin expression and the consequently elevated levels of ROS may facilitate podocyte dysfunction, which alters glomerular basement membrane permeability and causes proteinuria in LN patients.
Since renal injury acts as a noteworthy risk factor for cardiovascular disease (CVD), oxidative stress is deemed to underlie CVD pathogenesis. A higher risk of atherosclerosis and CVD is observed in SLE patients compared to healthy individuals, and the disruption of lipid and lipoprotein metabolism induced by oxidative stress is of critical significance.68 Malondialdehyde (MDA)-modified oxidized low-density lipoprotein (OxLDL), autoantibodies against endothelial cells, and phospholipids can all contribute to endothelial damage at the onset of lupus together with NETs, either directly, or through activation of the type I IFN pathway.69 Oxidised LDL is phagocytosed by monocytes, which then differentiate into foam cells and release inflammatory substances, ultimately predisposing one to atherosclerosis. Neutrophil extracellular traps, previously described as core components of neutrophils involved in oxidative stress, can damage endothelial cells, activate macrophages, and release myeloperoxidase; an NADPH oxidase capable of oxidizing HDL; ultimately decreasing the levels of the antioxidant HDL.70 Furthermore, oxidative stress is an essential agent involved in anti-β2GPI antibody formation and thrombotic events in SLE patients with APS. The OxLDL/β2GPI/anti-2GPI complex was reported to induce macrophage differentiation into foam cells, thus furthering the development of atherosclerosis possibly via the mechanism that HNE oxidizes the β2GPI antigen, thereby potentiating its immunogenicity and response to the increase in anti-β2GPI antibody titers. Antiphospholipid antibodies (APLs) have been reported to cross-react with OxLDL, thereby optimizing their influx into macrophages and promoting the progression of atherosclerosis.71 In fact, most atherosclerosis risk factors have been reported to augment oxidative stress and hasten disease progression. Hypertension is associated with oxidative stress and its presence in individuals with SLE involves a dysregulated Th1/Th2 cytokine ratio, increased IL-17 production, and enhanced insulin resistance.72 By increasing the susceptibility of tiny renal inlet arteries to angiotensin II and the expression of sodium-chloride cotransporters, oxidative stress may intensify the renal tubular reabsorption of both water and sodium, thereby precipitating the development of hypertension.
Likewise, the role of oxidative stress appears to underly the occurrence of cutaneous disease, another recurrent complication in patients with SLE. Ultraviolet light exposure to keratinocytes permits the liberation of autoantigenic Ro antibodies in apoptotic blebs by evoking oxidative stress, thereby triggering a morbific autoimmune response and propelling the development of photosensitive dermatitis.73 In relation to liver damage, and despite the fact that sub-clinical liver disease is commonplace in SLE, dramatically high levels of liver enzymes are rarely accomplished. Oxidative stress may perform a specific role in liver function alterations, as liver enzymes are closely interconnected to increases in serum NO metabolites to a certain extent.74 Glutamyl transferase; a nonspecific marker of liver injury; is liable to have its expression induced by oxidative stress, and the resultant abnormal liver enzyme profile implies a possible correlation between oxidative stress and liver injury. This supports the hypothesis of drug-induced oxidative stress with thereafter liver injury. In conclusion, oxidative stress contributes to specific damage of certain end-organs/systems in SLE, which is shown in Table 2.
 |
Table 2 Oxidative Stress Contributes to Organ/System Specific Damage in SLE |
Therapeutic Strategies Targeting Oxidative Stress Pathways
Currently approved therapies for SLE are inadequate, relying exclusively on the use of either anti-proliferative or anti-metabolite drugs. The imbalanced redox observed in patients with active SLE as previously described signifies that the therapeutic interventions targeting oxidative stress are those which hold high potential to be efficacious in the treatment of SLE.
Among the antioxidants, N-acetyl cysteine (NAC) has been stated to confer therapeutic benefits in SLE patients and murine lupus. As a precursor to supplement glutathione, NAC treatment has been demonstrated to prevent the generation of autoantibodies, restrain LN development, and prolong survival in (NZB × NZW) F1 lupus-prone mice.75 This well-tolerated medication could decline anti-DNA production and lupus activity via the suppression of mTOR, which was also accompanied by increasing FoxP3 expression in CD4+CD25+ T cells and reversing the expansion of CD4−CD8− T cells in a trial of SLE.76 Consistent with these results, further scientific research has demonstrated NAC’s ability to decrease lipid peroxidation, alleviate complications of the central nervous system (CNS), and improve endothelial function in patients with cerebrovascular involvement.77 Moreover, improvements in the lupus condition have also been seen to result from the action of conjugated linoleic acid (CLA) promoting glutathione synthesis and reversing oxidative stress by the upregulation of glutamate-cysteine ligase in MRL/lpr mice.78,79 Because mTOR is a sensor of oxidative stress in lupus T cells, its blockade by rapamycin is promising to be a viable therapeutic option.20,25,80 Rapamycin has been acknowledged by ongoing studies76,81 to diminish disease activity by modulating mitochondrial transmembrane potential and Ca2+ flux, thereby augmenting Treg cell expansion in lupus-prone mice and SLE patients who are either resistant or intolerant to conventional immunosuppressants.
Former studies in both humans and mouse models of lupus have provided evidence of elevated mROS in T cells, hinting at a plausible therapeutic strategy with respect to inhibiting mROS and oxidative stress.16 MitoQ; a mitochondria-targeted antioxidant; has been observed to mitigate renal injury by suppressing NET formation and serum IFN-I in lupus-prone MRL/lpr mice without altering T cell homeostatic proliferation and the inflammatory state. Therefore, MitoQ must be used in conjunction with traditional immunosuppression for a comprehensively effective anti-inflammatory antioxidant therapy.16,82,83 Similarly, coenzyme Q10 (CoQ10); an indispensable component of the mitochondrial respiratory chain; has been highlighted as an antioxidant possessing properties which prevent mitochondrial dysfunction, oxidative stress, and suppress atherosclerosis progression. These features are relevant to the pathophysiology of APS and SLE through the mechanisms of lowered LDL oxidation levels and proinflammatory cytokine production.71,83,84 From the perspective of oxidative stress in Th17/Treg imbalance, we can deduce that the antioxidative drugs which target the Th17/Treg balance might serve as potential remedies for SLE. Baicalin is a recognized antioxidant isolated from a Chinese herb that has been shown to endorse Treg cell differentiation and shield MRL/lpr mice from LN, possibly via antioxidative stress effects, according to related studies.85,86 In a pristane-induced lupus mouse model, another robust antioxidant known as resveratrol has been shown to play a protective role, probably via the inhibition of mTOR-mediated Th17 cell expansion.87 The antioxidant and free radical scavenging compounds antroquinonol and epigallocatechin-3-gallate have been proposed by correlational research88,89 to prevent LN aggravation. This prophylactic effect is owed to reduced ROS/NO generation and enhanced Treg cell suppression via activating the Nrf2 antioxidant signaling pathway.
Dietary antioxidant nutrient consumption, such as those of vitamin A, C, E, zeaxanthin, lycopene, and carotene, was evaluated in epidemiological studies and was subsequently found to cause a reduction in the plasma levels of several oxidative indicators while having no effect on disease activity of SLE, presumably due to their inability to mediate intracellular signaling with respect to the immune system.90,91 A series of investigations indicated that a methyl-poor micronutrient diet synergized with oxidative stress-related low T cell Dnmt1 levels could increase the severity of lupus flares. As a result, “methylation diet” supplementation may help to attenuate the impairment by oxidative stress.60 Melatonin supplementation was discovered to reduce cytokine production and dampen oxidative stress without downregulating disease activity in SLE patients, probably via affecting the NF-kβ signaling pathway.14 Reputed to be effective agents against oxidative stress-induced tissular injuries, synthetic triterpenoids are able to block selected signaling pathways like STAT-3 in immune cells and promote Nrf2 pathway activation, thereby inhibiting oxidative stress and the formation of LN in mice.75,92 Though the precise therapeutic mechanisms have not yet been exhaustively addressed, antioxidant drugs are well-positioned to become an auxiliary therapy for the treatment of SLE, which are shown in Table 3. However, antioxidant therapy cannot be generalized, as the condition of patients with SLE is extremely complex, immunosuppressant therapy must be given depending on the organs involved in most cases.
 |
Table 3 Antioxidant Therapeutic Strategies for SLE |
Conclusions and Prospective Views
Collectively, through molecular mechanisms like enhanced NETosis, mTOR pathway activation, imbalanced T-cell differentiation, and altered related gene expression, oxidative stress elicited by the overproduction of ROS and diminished antioxidants potentiates immunological derangements and expedites the onset of SLE. Various inflammatory and cellular markers especially oxidative modifications of lipids, proteins, and DNA are intimately and proportionally relevant to disease activity in patients with SLE and other autoimmune diseases. A diligent effort has been underway to explore whether the administration of antioxidants to restore redox homeostasis attenuates the severity of the disease. Though there has been some progress, the precise effects and underlying molecular mechanisms still require further laboratory and clinical data to expand upon the current understanding of them.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82173445) and the Key Research and Development Plan of Shaanxi Province (No. 2020ZDLSF02-08).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pannu N, Bhatnagar A. Oxidative stress and immune complexes: pathogenic mechanisms in pristane induced murine model of lupus. Immunobiology. 2020;225(1):151871. doi:10.1016/j.imbio.2019.11.006
2. Smallwood MJ, Nissim A, Knight AR, Whiteman M, Haigh R, Winyard PG. Oxidative stress in autoimmune rheumatic diseases. Free Radic Biol Med. 2018;125:3–14. doi:10.1016/j.freeradbiomed.2018.05.086
3. Fujii J, Kurahashi T, Konno T, Homma T, Iuchi Y. Oxidative stress as a potential causal factor for autoimmune hemolytic anemia and systemic lupus erythematosus. World J Nephrol. 2015;4(2):213–222. doi:10.5527/wjn.v4.i2.213
4. Duarte-Delgado NP, Cala MP, Barreto A, Rodriguez CL. Metabolites and metabolic pathways associated with rheumatoid arthritis and systemic lupus erythematosus. J Transl Autoimmun. 2022;5:100150. doi:10.1016/j.jtauto.2022.100150
5. Ahsan H, Ali A, Ali R. Oxygen free radicals and systemic autoimmunity. Clin Exp Immunol. 2003;131(3):398–404. doi:10.1046/j.1365-2249.2003.02104.x
6. Wojcik P, Gegotek A, Zarkovic N, Skrzydlewska E. Oxidative stress and lipid mediators modulate immune cell functions in autoimmune diseases. Int J Mol Sci. 2021;22(2):723. doi:10.3390/ijms22020723
7. Ahmad R, Ahsan H. Singlet oxygen species and systemic lupus erythematosus: a brief review. J Immunoassay Immunochem. 2019;40(4):343–349. doi:10.1080/15321819.2019.1616555
8. Shruthi S, Thabah MM, Zachariah B, Negi VS. Association of oxidative stress with disease activity and damage in systemic lupus erythematosus: a cross sectional study from a tertiary care centre in Southern India. Indian J Clin Biochem. 2021;36(2):185–193. doi:10.1007/s12291-020-00879-5
9. Wang S, Liu Y, Liu J, et al. Mitochondria-derived methylmalonic acid, a surrogate biomarker of mitochondrial dysfunction and oxidative stress, predicts all-cause and cardiovascular mortality in the general population. Redox Biol. 2020;37:101741. doi:10.1016/j.redox.2020.101741
10. Costa JH, Mohanapriya G, Bharadwaj R, et al. ROS/RNS balancing, aerobic fermentation regulation and cell cycle control - a complex early trait (‘CoV-MAC-TED’) for combating SARS-CoV-2-induced cell reprogramming. Front Immunol. 2021;12:673692. doi:10.3389/fimmu.2021.673692
11. Zhang VX, Sze KM, Chan LK, et al. Antioxidant supplements promote tumor formation and growth and confer drug resistance in hepatocellular carcinoma by reducing intracellular ROS and induction of TMBIM1. Cell Biosci. 2021;11(1):217. doi:10.1186/s13578-021-00731-0
12. Hu C, Zhang J, Hong S, et al. Oxidative stress-induced aberrant lipid metabolism is an important causal factor for dysfunction of immunocytes from patients with systemic lupus erythematosus. Free Radic Biol Med. 2021;163:210–219. doi:10.1016/j.freeradbiomed.2020.12.006
13. Wang L, Law HKW. Immune complexes impaired glomerular endothelial cell functions in lupus nephritis. Int J Mol Sci. 2019;20(21):5281. doi:10.3390/ijms20215281
14. Nabatian-Asl M, Ghorbanihaghjo A, Malek Mahdavi A, Khabbazi A, Hajialilo M, Ghojazadeh M. Effects of melatonin supplementation on serum oxidative stress markers and disease activity in systemic lupus erythematosus patients: a randomised, double-blind, placebo-controlled trial. Int J Clin Pract. 2021;75(7):e14246. doi:10.1111/ijcp.14246
15. Ene CD, Georgescu SR, Tampa M, et al. Cellular response against oxidative stress, a novel insight into lupus nephritis pathogenesis. J Pers Med. 2021;11(8):693. doi:10.3390/jpm11080693
16. Fortner KA, Blanco LP, Buskiewicz I, et al. Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci Med. 2020;7(1):e000387. doi:10.1136/lupus-2020-000387
17. Ramalingam A, Budin SB, Mohd Fauzi N, Ritchie RH, Zainalabidin S. Targeting mitochondrial reactive oxygen species-mediated oxidative stress attenuates nicotine-induced cardiac remodeling and dysfunction. Sci Rep. 2021;11(1):13845. doi:10.1038/s41598-021-93234-4
18. Nazim UM, Yin H, Park SY. Autophagy flux inhibition mediated by celastrol sensitized lung cancer cells to TRAILinduced apoptosis via regulation of mitochondrial transmembrane potential and reactive oxygen species. Mol Med Rep. 2019;19(2):984–993. doi:10.3892/mmr.2018.9757
19. Lee HT, Wu TH, Lin CS, et al. The pathogenesis of systemic lupus erythematosus - From the viewpoint of oxidative stress and mitochondrial dysfunction. Mitochondrion. 2016;30:1–7. doi:10.1016/j.mito.2016.05.007
20. Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol. 2016;12(3):169–182. doi:10.1038/nrrheum.2015.172
21. Liu J, Ming S, Song W, et al. B and T lymphocyte attenuator regulates autophagy in mycobacterial infection via the AKT/mTOR signal pathway. Int Immunopharmacol. 2021;91:107215. doi:10.1016/j.intimp.2020.107215
22. Zhang JC, Chen G, Chen L, et al. TGF-beta/BAMBI pathway dysfunction contributes to peripheral Th17/Treg imbalance in chronic obstructive pulmonary disease. Sci Rep. 2016;6:31911. doi:10.1038/srep31911
23. Szwed A, Kim E, Jacinto E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol Rev. 2021;101(3):1371–1426. doi:10.1152/physrev.00026.2020
24. Shi H, Chapman NM, Wen J, et al. Amino acids license kinase mTORC1 activity and Treg cell function via small G proteins Rag and Rheb. Immunity. 2019;51(6):1012–27e7. doi:10.1016/j.immuni.2019.10.001
25. Oaks Z, Winans T, Huang N, Banki K, Perl A. Activation of the mechanistic target of rapamycin in SLE: explosion of evidence in the last five years. Curr Rheumatol Rep. 2016;18(12):73. doi:10.1007/s11926-016-0622-8
26. Peixoto TV, Carrasco S, Botte DAC, et al. CD4(+)CD69(+) T cells and CD4(+)CD25(+)FoxP3(+) Treg cells imbalance in peripheral blood, spleen and peritoneal lavage from pristane-induced systemic lupus erythematosus (SLE) mice. Adv Rheumatol. 2019;59(1):30. doi:10.1186/s42358-019-0072-x
27. Sahni A, Narra HP, Sahni SK. Activation of mechanistic target of rapamycin (mTOR) in human endothelial cells infected with pathogenic spotted fever group rickettsiae. Int J Mol Sci. 2020;21(19):7179. doi:10.3390/ijms21197179
28. Braga A, Neves E, Guimaraes J, Braga J, Vasconcelos C. The dynamics of Th17 / Treg ratio in SLE patients during pregnancy. J Reprod Immunol. 2022;151:103622. doi:10.1016/j.jri.2022.103622
29. He J, Ma J, Ren B, Liu A. Advances in systemic lupus erythematosus pathogenesis via mTOR signaling pathway. Semin Arthritis Rheum. 2020;50(2):314–320. doi:10.1016/j.semarthrit.2019.09.022
30. He S, Xue M, Cai G. IL-6 alters migration capacity of CD4(+)Foxp3(+) regulatory T cells in systemic lupus erythematosus. Scand J Immunol. 2021;94(5):e13099. doi:10.1111/sji.13099
31. Zhang X, Lindwall E, Gauthier C, et al. Circulating CXCR5+CD4+helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus. 2015;24(9):909–917. doi:10.1177/0961203314567750
32. Gigante A, Iannazzo F, Navarini L, et al. Metabolic syndrome and adipokine levels in systemic lupus erythematosus and systemic sclerosis. Clin Rheumatol. 2021;40(10):4253–4258. doi:10.1007/s10067-021-05731-6
33. Yang J, Yang X, Zou H, Li M. Oxidative stress and Treg and Th17 dysfunction in systemic lupus erythematosus. Oxid Med Cell Longev. 2016;2016:2526174. doi:10.1155/2016/2526174
34. Shan J, Jin H, Xu Y. T Cell Metabolism: a new perspective on Th17/Treg cell imbalance in systemic lupus erythematosus. Front Immunol. 2020;11:1027. doi:10.3389/fimmu.2020.01027
35. Yu H, Jiang L, Liu R, et al. Association between the ratio of aryl hydrocarbon receptor (AhR) in Th17 cells to AhR in Treg cells and SLE skin lesions. Int Immunopharmacol. 2019;69:257–262. doi:10.1016/j.intimp.2019.01.039
36. de Araujo EF, Feriotti C, Galdino NAL, Preite NW, Calich VLG, Loures FV. The IDO-AhR axis controls Th17/Treg immunity in a pulmonary model of fungal infection. Front Immunol. 2017;8:880. doi:10.3389/fimmu.2017.00880
37. Mockel T, Basta F, Weinmann-Menke J, Schwarting A. B cell activating factor (BAFF): structure, functions, autoimmunity and clinical implications in systemic lupus erythematosus (SLE). Autoimmun Rev. 2021;20(2):102736. doi:10.1016/j.autrev.2020.102736
38. Stohl W, Yu N, Chalmers S, Putterman C, Jacob CO. Development of murine systemic lupus erythematosus in the absence of BAFF. Arthritis Rheumatol. 2020;72(2):292–302. doi:10.1002/art.41097
39. Wilson CS, Stocks BT, Hoopes EM, et al. Metabolic preconditioning in CD4+ T cells restores inducible immune tolerance in lupus-prone mice. JCI Insight. 2021;6(19):e143245. doi:10.1172/jci.insight.143245
40. Pathak S, Kumar KR, Kanta H, et al. Fatty acid amide hydrolase regulates peripheral B cell receptor revision, polyreactivity, and B1 cells in lupus. J Immunol. 2016;196(4):1507–1516. doi:10.4049/jimmunol.1500291
41. Dong GF, Zhang X, He DN, Li L, Zhang GF. Effect of leflunomide on the abnormal expression of lipid rafts and F-actin in B lymphocytes from patients with systemic lupus erythematosus. J Immunol Res. 2015;2015:832916. doi:10.1155/2015/832916
42. Azzouz D, Khan MA, Palaniyar N. ROS induces NETosis by oxidizing DNA and initiating DNA repair. Cell Death Discov. 2021;7(1):113. doi:10.1038/s41420-021-00491-3
43. Breitenstein MK, Hu VJ, Bhatnagar R, Ratnagiri M. Approaching neural net feature interpretation using stacked autoencoders: gene expression profiling of systemic lupus erythematosus patients. AMIA Jt Summits Transl Sci Proc. 2019;2019:435–442.
44. Campbell AM, Kashgarian M, Shlomchik MJ. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci Transl Med. 2012;4(157):157ra41. doi:10.1126/scitranslmed.3004801
45. El-Ghoneimy DH, Hesham M, Hasan R, Tarif M, Gouda S. The behavior of neutrophil extracellular traps and NADPH oxidative activity in pediatric systemic lupus erythematosus: relation to disease activity and lupus nephritis. Clin Rheumatol. 2019;38(9):2585–2593. doi:10.1007/s10067-019-04547-9
46. Bao Y, Ledderose C, Graf AF, et al. mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J Cell Biol. 2015;210(7):1153–1164. doi:10.1083/jcb.201503066
47. Lightfoot YL, Blanco LP, Kaplan MJ. Metabolic abnormalities and oxidative stress in lupus. Curr Opin Rheumatol. 2017;29(5):442–449. doi:10.1097/BOR.0000000000000413
48. Bona N, Pezzarini E, Balbi B, et al. Oxidative stress, inflammation and disease activity biomarkers in lupus nephropathy. Lupus. 2020;29(3):311–323. doi:10.1177/0961203320904784
49. Granick M, Leuin AS, Trepanier LA. Plasma and urinary F2-isoprostane markers of oxidative stress are increased in cats with early (stage 1) chronic kidney disease. J Feline Med Surg. 2021;23(8):692–699. doi:10.1177/1098612X20969358
50. Krata N, Foroncewicz B, Zagozdzon R, et al. Peroxiredoxins as markers of oxidative stress in IgA nephropathy, membranous nephropathy and lupus nephritis. Arch Immunol Ther Exp (Warsz). 2021;70(1):3. doi:10.1007/s00005-021-00638-1
51. Zavadskiy S, Sologova S, Moldogazieva N. Oxidative distress in aging and age-related diseases: spatiotemporal dysregulation of protein oxidation and degradation. Biochimie. 2022;195:114–134. doi:10.1016/j.biochi.2021.12.002
52. Song YR, Kim JK, Lee HS, Kim SG, Choi EK. Serum levels of protein carbonyl, a marker of oxidative stress, are associated with overhydration, sarcopenia and mortality in hemodialysis patients. BMC Nephrol. 2020;21(1):281. doi:10.1186/s12882-020-01937-z
53. Ahsan H. 3-Nitrotyrosine: a biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum Immunol. 2013;74(10):1392–1399. doi:10.1016/j.humimm.2013.06.009
54. Ren W, Liu G, Yin J, et al. Amino-acid transporters in T-cell activation and differentiation. Cell Death Dis. 2017;8(3):e2655. doi:10.1038/cddis.2016.222
55. Liu T, Han J, Zhang R, et al. Characteristics of purified anti-beta2GPI IgG N-glycosylation associate with thrombotic, obstetric and catastrophic antiphospholipid syndrome. Rheumatology (Oxford). 2022;61(3):1243–1254. doi:10.1093/rheumatology/keab416
56. Peluso M, Russo V, Mello T, Galli A. Oxidative stress and DNA damage in chronic disease and environmental studies. Int J Mol Sci. 2020;21(18):6936. doi:10.3390/ijms21186936
57. Ntouros PA, Vlachogiannis NI, Pappa M, et al. Effective DNA damage response after acute but not chronic immune challenge: SARS-CoV-2 vaccine versus systemic lupus erythematosus. Clin Immunol. 2021;229:108765. doi:10.1016/j.clim.2021.108765
58. Tumurkhuu G, Chen S, Montano EN, et al. Oxidative DNA damage accelerates skin inflammation in pristane-induced lupus model. Front Immunol. 2020;11:554725. doi:10.3389/fimmu.2020.554725
59. Ene CD, Penescu MN, Georgescu SR, Tampa M, Nicolae I. Posttranslational modifications pattern in clear cell renal cell carcinoma. Metabolites. 2020;11(1):10. doi:10.3390/metabo11010010
60. Ray D, Strickland FM, Richardson BC. Oxidative stress and dietary micronutrient deficiencies contribute to overexpression of epigenetically regulated genes by lupus T cells. Clin Immunol. 2018;196:97–102. doi:10.1016/j.clim.2018.04.003
61. Gorelik GJ, Yarlagadda S, Patel DR, Richardson BC. Protein kinase Cdelta oxidation contributes to ERK inactivation in lupus T cells. Arthritis Rheum. 2012;64(9):2964–2974. doi:10.1002/art.34503
62. Richardson B, Strickland FM, Sawalha AH, Gorelik G. Protein kinase Cdelta mutations may contribute to lupus through effects on T cells: comment on the article by Belot et al. Arthritis Rheumatol. 2014;66(1):228–229. doi:10.1002/art.38235
63. He XJ, Ding Y, Xiang W, Dang XQ. Roles of 1,25(OH)2D3 and vitamin D receptor in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus by regulating the activation of CD4+ T cells and the PKCdelta/ERK signaling pathway. Cell Physiol Biochem. 2016;40(3–4):743–756. doi:10.1159/000453135
64. Zhang CX, Wang HY, Yin L, Mao YY, Zhou W. Immunometabolism in the pathogenesis of systemic lupus erythematosus. J Transl Autoimmun. 2020;3:100046. doi:10.1016/j.jtauto.2020.100046
65. Bethunaickan R, Sahu R, Liu Z, et al. Anti-tumor necrosis factor alpha treatment of interferon-alpha-induced murine lupus nephritis reduces the renal macrophage response but does not alter glomerular immune complex formation. Arthritis Rheum. 2012;64(10):3399–3408. doi:10.1002/art.34553
66. Tian Y, Guo H, Miao X, et al. Nestin protects podocyte from injury in lupus nephritis by mitophagy and oxidative stress. Cell Death Dis. 2020;11(5):319. doi:10.1038/s41419-020-2547-4
67. Perl A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat Rev Rheumatol. 2013;9(11):674–686. doi:10.1038/nrrheum.2013.147
68. Akiyama M, Kaneko Y, Takeuchi T. Lupus aortitis: a fatal, inflammatory cardiovascular complication in systemic lupus erythematosus. Lupus. 2020;29(12):1652–1654. doi:10.1177/0961203320950017
69. Martin N, Tu X, Egan AJ, Stover C. Complement activation on endothelial cell-derived microparticles-A key determinant for cardiovascular risk in patients with systemic lupus erythematosus? Medicina. 2020;56(10):533. doi:10.3390/medicina56100533
70. Giannelou M, Mavragani CP. Cardiovascular disease in systemic lupus erythematosus: a comprehensive update. J Autoimmun. 2017;82:1–12. doi:10.1016/j.jaut.2017.05.008
71. Lopez-Pedrera C, Barbarroja N, Jimenez-Gomez Y, Collantes-Estevez E, Aguirre MA, Cuadrado MJ. Oxidative stress in the pathogenesis of atherothrombosis associated with anti-phospholipid syndrome and systemic lupus erythematosus: new therapeutic approaches. Rheumatology. 2016;55(12):2096–2108. doi:10.1093/rheumatology/kew054
72. Lozovoy MA, Simao AN, Morimoto HK, et al. Hypertension is associated with serologically active disease in patients with systemic lupus erythematosus: role of increased Th1/Th2 ratio and oxidative stress. Scand J Rheumatol. 2014;43(1):59–62. doi:10.3109/03009742.2013.834963
73. Russell-Goldman E, Nazarian RM. Subacute cutaneous lupus erythematosus with positive anti-Ro antibodies following palbociclib and letrozole treatment: a case report and literature review. J Cutan Pathol. 2020;47(7):654–658. doi:10.1111/cup.13673
74. Lozovoy MA, Simao AN, Panis C, et al. Oxidative stress is associated with liver damage, inflammatory status, and corticosteroid therapy in patients with systemic lupus erythematosus. Lupus. 2011;20(12):1250–1259. doi:10.1177/0961203311411350
75. Wu T, Ye Y, Min SY, et al. Prevention of murine lupus nephritis by targeting multiple signaling axes and oxidative stress using a synthetic triterpenoid. Arthritis Rheumatol. 2014;66(11):3129–3139. doi:10.1002/art.38782
76. Lai ZW, Hanczko R, Bonilla E, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64(9):2937–2946. doi:10.1002/art.34502
77. Tan MKX, Heng TYJ, Mak A. The potential use of metformin, dipyridamole, N-Acetylcysteine and statins as adjunctive therapy for systemic lupus erythematosus. Cells. 2019;8(4):323. doi:10.3390/cells8040323
78. Monaco A, Ferrandino I, Boscaino F, et al. Conjugated linoleic acid prevents age-dependent neurodegeneration in a mouse model of neuropsychiatric lupus via the activation of an adaptive response. J Lipid Res. 2018;59(1):48–57. doi:10.1194/jlr.M079400
79. Bergamo P, Maurano F, Rossi M. Phase 2 enzyme induction by conjugated linoleic acid improves lupus-associated oxidative stress. Free Radic Biol Med. 2007;43(1):71–79. doi:10.1016/j.freeradbiomed.2007.03.023
80. Oaks Z, Winans T, Caza T, et al. Mitochondrial dysfunction in the liver and antiphospholipid antibody production precede disease onset and respond to rapamycin in lupus-prone mice. Arthritis Rheumatol. 2016;68(11):2728–2739. doi:10.1002/art.39791
81. Lai ZW, Borsuk R, Shadakshari A, et al. Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J Immunol. 2013;191(5):2236–2246. doi:10.4049/jimmunol.1301005
82. Buskiewicz IA, Montgomery T, Yasewicz EC, et al. Reactive oxygen species induce virus-independent MAVS oligomerization in systemic lupus erythematosus. Sci Signal. 2016;9(456):ra115. doi:10.1126/scisignal.aaf1933
83. Lopez-Pedrera C, Villalba JM, Patino-Trives AM, et al. Therapeutic potential and immunomodulatory role of coenzyme Q(10) and its analogues in systemic autoimmune diseases. Antioxidants. 2021;10(4):600. doi:10.3390/antiox10040600
84. Blanco LP, Pedersen HL, Wang X, et al. Improved mitochondrial metabolism and reduced inflammation following attenuation of murine lupus with coenzyme Q10 analog idebenone. Arthritis Rheumatol. 2020;72(3):454–464. doi:10.1002/art.41128
85. Lin M, Li L, Zhang Y, et al. Baicalin ameliorates H2O2 induced cytotoxicity in HK-2 cells through the inhibition of ER stress and the activation of Nrf2 signaling. Int J Mol Sci. 2014;15(7):12507–12522. doi:10.3390/ijms150712507
86. Ding H, Wang H, Zhao Y, Sun D, Zhai X. Protective effects of baicalin on Abeta(1)(-)(4)(2)-induced learning and memory deficit, oxidative stress, and apoptosis in rat. Cell Mol Neurobiol. 2015;35(5):623–632. doi:10.1007/s10571-015-0156-z
87. Park D, Jeong H, Lee MN, et al. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci Rep. 2016;6:21772. doi:10.1038/srep21772
88. Tsai PY, Ka SM, Chang JM, et al. Antroquinonol differentially modulates T cell activity and reduces interleukin-18 production, but enhances Nrf2 activation, in murine accelerated severe lupus nephritis. Arthritis Rheum. 2012;64(1):232–242. doi:10.1002/art.33328
89. Tsai PY, Ka SM, Chang JM, et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic Biol Med. 2011;51(3):744–754. doi:10.1016/j.freeradbiomed.2011.05.016
90. Fouad H, Yahia S, Elsaid A, et al. Oxidative stress and vitamin D receptor BsmI gene polymorphism in Egyptian children with systemic lupus erythematosus: a single center study. Lupus. 2019;28(6):771–777. doi:10.1177/0961203319846380
91. Costenbader KH, Kang JH, Karlson EW. Antioxidant intake and risks of rheumatoid arthritis and systemic lupus erythematosus in women. Am J Epidemiol. 2010;172(2):205–216. doi:10.1093/aje/kwq089
92. Sporn MB, Liby KT, Yore MM, Fu L, Lopchuk JM, Gribble GW. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod. 2011;74(3):537–545. doi:10.1021/np100826q
93. Pagano G, Castello G, Pallardo FV. Sjogren’s syndrome-associated oxidative stress and mitochondrial dysfunction: prospects for chemoprevention trials. Free Radic Res. 2013;47(2):71–73. doi:10.3109/10715762.2012.748904
94. Tobore TO. Oxidative/nitroxidative stress and multiple sclerosis. J Mol Neurosci. 2021;71(3):506–514. doi:10.1007/s12031-020-01672-y
95. Yevgi R, Demir R. Oxidative stress activity of fingolimod in multiple sclerosis. Clin Neurol Neurosurg. 2021;202:106500. doi:10.1016/j.clineuro.2021.106500
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.