Back to Journals » Journal of Pain Research » Volume 13
Oxidative Stress Contributes to Hyperalgesia in Osteoporotic Mice
Authors Tu C, Wu DZ, Huang YS, Zhuang JS , Zeng JH, Xu P, Meng TT, Zhong ZM
Received 11 October 2019
Accepted for publication 24 December 2019
Published 15 January 2020 Volume 2020:13 Pages 131—142
DOI https://doi.org/10.2147/JPR.S234334
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Chen Tu, 1 Di-Zheng Wu, 1 Yu-Sheng Huang, 1 Jing-Shen Zhuang, 1 Ji-Huan Zeng, 2 Ping Xu, 1 Ting-Ting Meng, 3 Zhao-Ming Zhong 1
1Department of Spinal Surgery, Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China; 2Department of Orthopaedic Surgery, Jiangxi Province People’s Hospital, Nanchang University, Nanchang, People’s Republic of China; 3Department of Anaesthesia, Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China
Correspondence: Zhao-Ming Zhong; Ting-Ting Meng
Nanfang Hospital, Southern Medical University, 1838 North Guangzhou Avenue, Guangzhou 510515, People’s Republic of China
Email [email protected]; [email protected]
Purpose: Chronic pain is one of the most common complications of postmenopausal osteoporosis. Since oxidative stress is involved in the pathogenesis of postmenopausal osteoporosis, we explored whether oxidative stress contributes to postmenopausal osteoporotic pain.
Methods: Osteoporosis was induced in mice by ovariectomy (OVX). Pain-related behaviours were assessed by measuring sensitivity to mechanical, thermal and cold stimulation. The expression of pain-related transcripts, such acid-sensing ion channel 3 (ASIC3), transient receptor potential vanilloid 1 (TRPV1) and calcitonin gene-related peptide (CGRP), was evaluated. Plasma markers of oxidative stress were also measured. In addition, the effects of the reactive oxygen species scavenger phenyl N-tert-butylnitrone (PBN) on these parameters were assessed.
Results: The OVX mice presented hyperalgesia, as demonstrated by decreased paw withdrawal thresholds to mechanical stimulation and withdrawal latencies to thermal and cold stimulation, along with upregulated expression of ASIC3, TRPV1 and CGRP in the dorsal root ganglia, spinal cord and thalamus tissue. OVX elevated the plasma levels of malondialdehyde (MDA) and advanced oxidation protein products (AOPPs). However, the administration of PBN alleviated these effects.
Conclusion: Our results indicated that oxidative stress contributes to hyperalgesia in OVX mice. Enhanced oxidative stress may be associated with osteoporotic pain. Antioxidant treatment could help alleviate chronic pain in postmenopausal osteoporotic patients.
Keywords: osteoporosis, hyperalgesia, oxidative stress, PBN
Introduction
Postmenopausal osteoporosis is a progressive deteriorative condition characterized by low bone mass and microarchitectural deterioration of the skeleton, leading to enhanced bone fragility and a consequent increased risk of fracture.1 It has been estimated that more than one-third of postmenopausal women suffer from primary osteoporosis.2,3 Postmenopausal osteoporosis may cause not only fractures but also chronic pain. Chronic pain is prevalent in postmenopausal osteoporosis patients with vertebral fractures and in patients with no evidence of fractures.4 Persistent chronic pain potentially results in disability in elderly women.5
The activation of sensory neuron ion channels and the release of neurotransmitters are essential for the development of pain behaviour. Acid-sensing ion channel 3 (ASIC3, a neuronal voltage-independent Na+ channel) and transient receptor potential vanilloid 1 (TRPV1, a nonselective cation channel) play important roles in the process of pain.6–8 In postmenopausal osteoporosis, the enhanced dissolution of minerals by osteoclasts readily creates an acidic extracellular microenvironment, which can activate ASIC3 and TRPV1.9,10 Meanwhile, activation of TRPV1 induces the release of calcitonin gene-related peptide (CGRP), which mediates neurogenic inflammation and hyperalgesia.11
Oxidative stress, a pathological condition characterized by an imbalance between the production and removal of reactive oxygen species (ROS), is closely linked to various human diseases.12–14 Elevated plasma markers of oxidative stress, such as malondialdehyde (MDA) and advanced oxidation protein products (AOPPs), have been demonstrated in postmenopausal women with osteoporosis.15 The involvement of oxidative stress in postmenopausal osteoporosis has been well documented.16,17 However, the effect of oxidative stress on chronic pain in postmenopausal women with osteoporosis remains poorly understood.
In this study, an experimental osteoporotic mouse model was established by ovariectomy (OVX). Changes in pain-related behaviour, pain-related transcripts and plasma markers of oxidative stress were examined in the ovariectomized mice. The purpose of this study was to examine whether oxidative stress is associated with hyperalgesia in an osteoporotic mouse model.
Materials and Methods
Animals
Seven-week-old female C57BL6/129SVJ mice (16–20 g) were obtained from the Animal Center at Southern Medical University (Guangzhou, China). The animals were housed 5 per cage and maintained at a controlled room temperature (22±2°C) on a 12 h/12 h light/dark cycle. Food and water were available ad libitum. All animal experiments were approved by the Laboratory Animal Care and Use Committee of Nanfang Hospital, Southern Medical University (NFYY-2017-107), and conducted according to the regulations for animal experiment at Southern Medical University.
Experimental Protocol
OVX in mice has been demonstrated to cause bone loss, deterioration of bone microstructure and hyperalgesia and is used as an osteoporosis model.18 In the present study, mice were allowed to adapt to their environment for a week and then underwent either bilateral ovariectomy or a sham operation (ovaries exteriorized but not removed) under isoflurane anaesthesia as described previously.18 Twelve weeks after surgery, the mice in the OVX group (n=8) and Sham group (n=8) underwent a series of experiments. Pain-related behaviours were assessed by measuring sensitivity to mechanical, thermal and cold stimulation. After these tests, the mice were sacrificed with an intraperitoneal injection of pentobarbital sodium (0.5 mg/kg). The bilateral hindlimbs were removed to conduct micro-computed tomography (μCT). The bilateral dorsal root ganglia (DRGs) from the L2-L6 segments were collected for immunofluorescence staining, and lumbar spinal cord and thalamus tissue were collected for Western blots. Blood samples were collected to analyse the markers of oxidative stress.
In some experiments, the ROS scavenger phenyl N-tert-butylnitrone (PBN, Sigma, St. Louis, MO) was administered to the ovariectomized mice as previously described.19,20 PBN was dissolved in normal saline, and normal saline was also used as the vehicle treatment. Beginning 12 weeks after OVX, the mice received intraperitoneal (i.p.) injections of PBN (100 mg/kg/day, OVX+PBN group, n = 8) or normal saline (OVX group, n = 8) for 7 consecutive days. Behavioural tests were performed before the first injection to establish baseline values, and the tests were repeated 1 h after treatment with PBN every day.21
μCT Analysis
The left femur was fixed with 4% paraformaldehyde for at least 24 h and then scanned with a micro-CT system (μCT80, SCANCO MEDICAL, Switzerland) at a tube voltage of 50 kV, a tube current of 0.1 mA, and a resolution of 12 mm. The volume of interest was defined as the distalmost 180 slices in the femur. Three-dimensional images were reconstructed from bone trabecular tomograms and analysed with 3D image analysis software (3D software). The trabecular structure parameters, such as bone volume fraction (bone volume divided by tissue volume, BV/VT, %), trabecular number (Tb.N,/mm), trabecular thickness (Tb.Th, um) and trabecular separation (Tb.Sp, μm), were evaluated.
Assessment of Pain-Related Behaviour
Mechanical allodynia was assessed using a digital electronic von Frey anaesthesiometer (IITC Life Science, CA, USA) as previously described.22 This test was performed 5 times with an inter-test period of 15 min. Each mouse was placed in a clear plastic chamber on an elevated wire grid. The animals were acclimated for at least 15 min to the testing environment prior to the experiment. The tip of each polypropylene von Frey filament was applied perpendicularly to the mid-plantar surface of the selected hind paw, and the intensity of stimulation was recorded. A positive response was defined as abrupt paw withdrawal, licking, and shaking.
Thermal or cold sensitivity was assessed by measuring the latency of the hind paw withdrawal response to thermal or cold stimulation with a hot or cold plate as previously described.23 The mouse was placed on a 50°C or 4°C plate that was enclosed with a circular Plexiglas wall to prevent the mouse from escaping. The mice were monitored for behavioural changes such as paw licking, paw lifting, jumping and escaping from the hot plate, and the latency to display such behaviour was recorded. The cut-off time was set at 20 s, and the assay was repeated 5 times at 30 min intervals to avoid cutaneous sensitization.24
Assessment of Oxidative Stress
MDA and AOPPs are extensively used as parameters to evaluate oxidative stress status.25,26 MDA and AOPP levels in plasma were measured with an MDA ELISA Assay Kit (Elabscience, China) and an AOPP ELISA Assay Kit (Cloud-clone, China), respectively. The concentrations of MDA and AOPPs were calculated from the standard curve for each assay. Positive and negative controls were included in each assay. The absorbance of the reaction mixture at 340 nm or 534 nm was immediately read using a microplate reader.
Immunofluorescence Staining
The dorsal root ganglion (DRG) sections were immunofluorescently stained as previously described.27 Briefly, the lumbar DRG (L2-6) sections were fixed in 4% paraformaldehyde overnight at 4°C and then transferred into 30% sucrose solution (in PBS) at 4°C for at least 24 h. The DRGs were placed in Optimal Cutting Temperature compound and cooled with liquid nitrogen. Then, the samples were sectioned at a thickness of 10 μm on a cryostat, blocked for 1 h at room temperature in blocking buffer that consisted of 5% normal goat serum in PBS, and then exposed to the following primary antibodies: rabbit anti-ASIC3 (1:600, Abcam, UK), mouse anti-TRPV1 (1:1000, Abcam, UK), rabbit anti-CGRP (1:1000, Cell Signaling, USA), and rabbit and mouse anti-beta III tubulin (1:200, prointech, China). After being incubated overnight at 4°C, the slides were rinsed 3 times in PBS and incubated for 1 h at room temperature with secondary antibodies conjugated with FITC (1:500, prointech, China) or Cy3 (1:200, prointech, China), then rinsed 3 times in PBS again. Finally, the slides were sealed with neutral rubber and coverslipped. Images were captured with a self-contained confocal laser scanning microscope system (Olympus, Japan).
Western Blots
Mice were anaesthetized with isoflurane, and the spinal cord and thalamus tissue were removed and homogenized in ice-cold RIPA buffer with 1 mM PMSF, protease, and phosphatase inhibitors. The supernatant was extracted by centrifugation (12,000 rpm, 4°C, 20 min). Then, the protein concentration was detected using a bicinchoninic acid (BCA) kit. Samples were separated on 15% or 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel by electrophoresis and then transferred onto nitrocellulose membranes (Bio-Rad, Italy) by electroblotting. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline with 0.1% TWEEN 20 for 1 h at room temperature and then probed overnight with the following primary antibodies: rabbit anti-ASIC3 (1:600, Abcam, UK), mouse anti-TRPV1 (1:1000, Abcam, UK), and rabbit anti-CGRP (1:1000, Cell Signaling, USA). The membrane was washed 3 times with TBST for 10 min, incubated with the appropriate secondary antibody for 2 h, and then washed another 3 times with TBST. Rabbit anti-GAPDH (1:5000, Sigma-Aldrich, Italy) was used as an internal control for tissue samples. The integrated density of all the protein bands was analysed with ImageJ software.
Statistical Analysis
The results were expressed as the means ± S.E.M. and analysed statistically using one-way analysis of variance, two-way analysis of variance, or a two-sample t-test. Data were paired where possible. Values were considered significant when the probability (P) was P < 0.05*. Statistical analysis was conducted with SPSS 20.0 software (SPSS Inc, Chicago, IL).
Results
Body and Uterine Weights
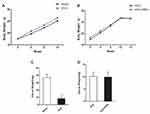
A successful OVX mouse model was confirmed by the absence of ovaries and the presence of uterine atrophy when the mice were killed for the study. Each group of mice exhibited similar initial mean body weights, as shown in Figure 1. Although the average body weight in the OVX group was higher than that in the Sham group, there was no significant difference between the two groups, and PBN treatment did not cause body weight changes in the OVX group. Compared with the uteri of the Sham mice, those of the OVX group were significantly atrophied. As in the case of body weight, PBN had no effect on uterine weight.
OVX-Induced Osteoporosis in Mice
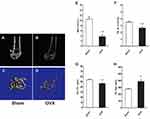
The μCT analysis showed that cancellous bone of distal femoral metaphysis was decreased significantly in the OVX group compared with the Sham group (Figure 2A–D). The cancellous parameters BV/TV, Tb.N, and Tb.Th were significantly lower in the OVX group than in the Sham group, and Tb.Sp was significantly higher in the OVX group than in the Sham group (Figure 2E–H). These results indicated that the OVX-induced osteoporosis mouse model was successfully established.
Hyperalgesia in OVX Mice
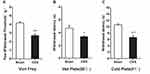
To evaluate pain sensitivity in the OVX-induced osteoporotic mouse model, we measured pain-related behaviour. As shown in Figure 3A, the hind paw withdrawal threshold to mechanical stimulation was significantly lower in the OVX group than in the Sham group. Furthermore, the withdrawal latencies in response to thermal and cold stimulations were significantly lower in the OVX group than in the Sham group (Figure 3B and C). These results showed that OVX mice were hyperalgesic to mechanical, thermal and cold stimulation in OVX mice.
Increased Expression of Pain-Related Transcripts
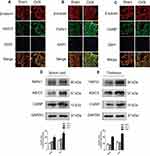
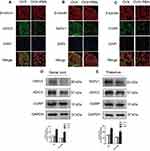
Several pain-related transcripts, namely, TRPV1, CGRP and ASIC3, were measured in the DRG, spinal cord and thalamus tissue. Immunofluorescence staining showed that the expression of TRPV1, CGRP and ASIC3 in the DRGs (L2-L6) was increased in OVX mice compared to Sham mice (Figure 4A–C). Meanwhile, Western blots showed that the expression of TRPV1, CGRP and ASIC3 was significantly higher in the spinal cord and thalamus tissue of the OVX group than in that of the Sham group (Figure 4D and E).
Enhanced Oxidative Stress in OVX Mice
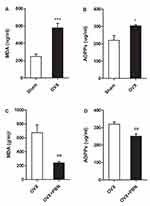
The plasma levels of MDA and AOPPs were tested to evaluate the oxidative stress status in the osteoporotic mouse model. As shown in Figure 5A and B, the plasma levels of MDA and AOPPs were significantly higher in the OVX mice than in the Sham mice. These results indicated that oxidative stress was enhanced in the osteoporotic mouse model.
PBN Treatment Alleviated Oxidative Stress and Hyperalgesia in OVX Mice
To further assess the relationship between oxidative stress and hyperalgesia, we administered the ROS scavenger PBN to OVX mice. As shown in Figure 5C and D, PBN treatment significantly decreased the plasma levels of MDA and AOPPs in OVX mice. As shown in Figure 6A–C, PBN administration changed the pain-related behaviour of OVX mice, as demonstrated by an increased paw withdrawal threshold to mechanical stimulation with von Frey filaments and by increased withdrawal latencies to thermal and cold stimulation. Furthermore, PBN treatment inhibited the expression of TRPV1, CGRP and ASIC3 in the DRGs, spinal cord and thalamus tissue of OVX mice (Figure 7).
Discussion
Chronic pain is one of the most common complaints in postmenopausal osteoporosis patients.28 However, the pathophysiology of pain in postmenopausal osteoporosis remains largely unknown. In this study, OVX mice showed hyperalgesia to mechanical, thermal and cold stimulation. OVX also caused the upregulation of ASIC3, TRPV1 and CGRP expression in the DRG, spinal cord and thalamus and enhanced oxidative stress levels. The administration of PBN, an ROS scavenger, alleviated oxidative stress and hyperalgesia in OVX mice. These data indicate that enhanced oxidative stress may be a common mechanism underlying pain in postmenopausal osteoporosis.
Postmenopausal women with osteoporosis readily develop chronic pain and reduced quality of life.5 Osteoporotic fractures may be the main cause of osteoporotic pain.29 However, postmenopausal osteoporotic patients with no fractures also suffer from chronic pain, which may be related to osteoclastic bone resorption.30 Osteoclasts dissolve bone minerals by releasing protons through the a3 isoform of the vacuolar H+-ATPase (a3V-ATPase) and creating acidic microenvironments, which can activate the ASIC and TRP proteins.31 Previous studies have demonstrated that the activation of ASIC3 and TRPV1 and the release of CGRP play important roles in acute inflammatory, chronic neuropathic and cancer pain.32–35 In our study, OVX caused the increased expression of ASIC3, TRPV1 and CGRP in the DRG, spinal cord and thalamus. Changes in the expression of these pain-related transcripts were associated with decreased thresholds for mechanical, thermal and cold pain in the osteoporotic model mice.
It was well demonstrated that oxidative stress is enhanced in postmenopausal women.36–39 In this study, we found that the plasma levels of MDA and AOPPs significantly increased in OVX mice. To further clarify the relationship between oxidative stress and pain in postmenopausal osteoporosis, we treated the OVX mice with intra-abdominal injection of PBN, which is a potent free radical scavenger and has an analgesic effect in various animal models of pain.40–43 PBN treatment alleviated not only oxidative stress but also hyperalgesia, and inhibited the upregulation of ASIC3, TRPV1 and CGRP in OVX mice, which showed that antioxidants could alleviate osteoporotic hyperalgesia. It is universally acknowledged that the management and prevention of pain are linked to the appropriate treatment of osteoporosis. Importantly, pain management requires a multidimensional strategies to improve quality of life in patients with osteoporosis.44 Our study indicated that antioxidant intervention may be a feasible approach to treat chronic pain in patients with osteoporosis. However, further clinical studies are necessary to determine the therapeutic potential of antioxidants in osteoporotic pain.
This study had certain limitations. First, our study has demonstrated oxidative stress was correlated with hyperalgesia in OVX mice, but we do not know how oxidative stress affects osteoporotic pain. Second, oestrogen deficiency is involved in chronic pain in postmenopausal osteoporosis.24 However, we could not determine whether oxidative stress and oestrogen deficiency had a synergistic effect on hyperalgesia. Third, the administrating time of PBN was short, only 1 week. We did not observed that a short-term PBN intervention have a significant impact on bone microstructure in OVX mice. However, previous studies have demonstrated that antioxidant intervention can reverse bone loss in an osteoporotic animal model.16
Conclusion
Our data show that oxidative stress contributes to hyperalgesia in an OVX-induced osteoporosis mouse model. Enhanced oxidative stress may be associated with osteoporotic pain. Antioxidant treatment could help alleviate chronic pain in postmenopausal osteoporotic patients.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (No.81871819,240 81472135 & 81000785).
Disclosure
The authors report no conflicts of interest in this work.
References
1. LaVallee LA, Scott MA, Hulkower SD. Challenges in the screening and management of osteoporosis. N C Med J. 2016;77(6):416–419. doi:10.18043/ncm.77.6.416
2. Cipriani C, Pepe J, Bertoldo F, et al. The epidemiology of osteoporosis in Italian postmenopausal women according to the National Bone Health Alliance (NBHA) diagnostic criteria: a multicenter cohort study. J Endocrinol Invest. 2018;41(4):431–438. doi:10.1007/s40618-017-0761-4
3. Lin X, Xiong D, Peng Y-Q, et al. Epidemiology and management of osteoporosis in the People’s Republic of China: current perspectives. Clin Interv Aging. 2015;10:1017–1033. doi:10.2147/CIA.S54613
4. Ohtori S, Akazawa T, Murata Y, et al. Risedronate decreases bone resorption and improves low back pain in postmenopausal osteoporosis patients without vertebral fractures. J Clin Neurosci. 2010;17(2):209–213. doi:10.1016/j.jocn.2009.06.013
5. Leidig-Bruckner G, Minne HW, Schlaich C, et al. Clinical grading of spinal osteoporosis: quality of life components and spinal deformity in women with chronic low back pain and women with vertebral osteoporosis. J Bone Miner Res. 1997;12(4):663–675. doi:10.1359/jbmr.1997.12.4.663
6. Palazzo E, Luongo L, de Novellis V, et al. Transient receptor potential vanilloid type 1 and pain development. Curr Opin Pharmacol. 2012;12(1):9–17. doi:10.1016/j.coph.2011.10.022
7. Deval E, Gasull X, Noël J, et al. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther. 2010;128(3):549–558. doi:10.1016/j.pharmthera.2010.08.006
8. Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52(1):77–92. doi:10.1016/j.neuron.2006.09.021
9. Negri L, Lattanzi R, Giannini E, et al. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci. 2006;26(25):6716–6727. doi:10.1523/JNEUROSCI.5403-05.2006
10. Yoneda T, Hata K, Nakanishi M, et al. Involvement of acidic microenvironment in the pathophysiology of cancer-associated bone pain. Bone. 2011;48(1):100–105. doi:10.1016/j.bone.2010.07.009
11. Hou L, Wang X. PKC and PKA, but not PKG mediate LPS-induced CGRP release and [Ca (2+)] (i) elevation in DRG neurons of neonatal rats. J Neurosci Res. 2001;66(4):592–600. doi:10.1002/jnr.1249
12. Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862(4):576–591. doi:10.1016/j.bbadis.2016.01.003
13. Cervellati C, Bonaccorsi G, Cremonini E, et al. Oxidative stress and bone resorption interplay as a possible trigger for postmenopausal osteoporosis. Biomed Res Int. 2014;2014:569563. doi:10.1155/2014/569563
14. Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1005–L1028. doi:10.1152/ajplung.2000.279.6.L1005
15. Wu Q, Zhong ZM, Pan Y et al. Advanced oxidation protein products as a novel marker of oxidative stress in postmenopausal osteoporosis. Med Sci Monit. 2015;21:2428–2432. doi:10.12659/MSM.894347
16. Zhu S-Y, Zhuang J-S, Wu Q, et al. Advanced oxidation protein products induce pre-osteoblast apoptosis through a nicotinamide adenine dinucleotide phosphate oxidase-dependent, mitogen-activated protein kinases-mediated intrinsic apoptosis pathway. Aging Cell. 2018;17:e12764. doi:10.1111/acel.2018.17.issue-4
17. Zhong ZM, Bai L, Chen JT. Advanced oxidation protein products inhibit proliferation and differentiation of rat osteoblast-like cells via NF-kappaB pathway. Cell Physiol Biochem. 2009;24(1–2):105–114. doi:10.1159/000227818
18. Yang L, Zhang B, Liu J, et al. Protective effect of acteoside on ovariectomy-induced bone loss in mice. Int J Mol Sci. 2019;20:12. doi:10.3390/ijms20030569
19. Kim HK, Zhang YP, Gwak YS, et al. Phenyl N-tert-butylnitrone, a free radical scavenger, reduces mechanical allodynia in chemotherapy-induced neuropathic pain in rats. Anesthesiology. 2010;112(2):432–439. doi:10.1097/ALN.0b013e3181ca31bd
20. Zhou Y-Q, Liu D-Q, Chen S-P, et al. Reactive oxygen species scavengers ameliorate mechanical allodynia in a rat model of cancer-induced bone pain. Redox Biol. 2018;14:391–397. doi:10.1016/j.redox.2017.10.011
21. Schwartz ES, Lee I, Chung K, et al. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008;138(3):514–524. doi:10.1016/j.pain.2008.01.029
22. Shibasaki M, Sasaki M, Miura M, et al. Induction of high mobility group box-1 in dorsal root ganglion contributes to pain hypersensitivity after peripheral nerve injury. Pain. 2010;149(3):514–521. doi:10.1016/j.pain.2010.03.023
23. Gunn A, Bobeck EN, Weber C, et al. The influence of non-nociceptive factors on hot-plate latency in rats. J Pain. 2011;12(2):222–227. doi:10.1016/j.jpain.2010.06.011
24. Sanoja R, Cervero F. Estrogen modulation of ovariectomy-induced hyperalgesia in adult mice. Eur J Pain. 2008;12(5):573–581. doi:10.1016/j.ejpain.2007.09.003
25. Ozgocmen S, Kaya H, Fadillioglu E, Aydogan R, Yilmaz Z Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol Cell Biochem. 2007;295(1–2):45–52. doi:10.1007/s11010-006-9270-z
26. Cristani M, Speciale A, Saija A, Gangemi S, Lucia Minciullo P, Cimino F. Circulating advanced oxidation protein products as oxidative stress biomarkers and progression mediators in pathological conditions related to inflammation and immune dysregulation. Curr Med Chem. 2016;23(34):3862–3882. doi:10.2174/0929867323666160902154748
27. Morell M, Camprubí-Robles M, Culler MD, et al. Cortistatin attenuates inflammatory pain via spinal and peripheral actions. Neurobiol Dis. 2014;63:141–154. doi:10.1016/j.nbd.2013.11.022
28. Ryan PJ, Evans P, Gibson T, Fogelman I. Osteoporosis and chronic back pain: a study with single-photon emission computed tomography bone scintigraphy. J Bone Miner Res. 1992;7(12):1455–1460. doi:10.1002/jbmr.5650071213
29. Francis RM, Aspray TJ, Hide G, Sutcliffe AM, Wilkinson P. Back pain in osteoporotic vertebral fractures. Osteoporos Int. 2008;19(7):895–903. doi:10.1007/s00198-007-0530-x
30. Takakuwa M, Iwamoto J. Elcatonin in combination with risedronate is more effective than risedronate alone for relieving back pain in postmenopausal women with osteoporosis. Biol Pharm Bull. 2012;35(7):1159–1165. doi:10.1248/bpb.b12-00200
31. Kanaya K, Iba K, Abe Y, et al. Acid-sensing ion channel 3 or P2X2/3 is involved in the pain-like behavior under a high bone turnover state in ovariectomized mice. J Orthop Res. 2016;34(4):566–573. doi:10.1002/jor.23047
32. Yu L, Yang F, Luo H, et al. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol Pain. 2008;4:61. doi:10.1186/1744-8069-4-61
33. Omori M, Yokoyama M, Matsuoka Y, et al. Effects of selective spinal nerve ligation on acetic acid-induced nociceptive responses and ASIC3 immunoreactivity in the rat dorsal root ganglion. Brain Res. 2008;1219:26–31. doi:10.1016/j.brainres.2008.03.040
34. Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache. 2013;53(8):1230–1244.
35. Sun R-Q, Tu Y-J, Lawand NB, et al. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol. 2004;92(5):2859–2866. doi:10.1152/jn.00339.2004
36. Almeida M, Han L, Martin-Millan M, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282(37):27285–27297. doi:10.1074/jbc.M702810200
37. Smietana MJ, Arruda EM, Faulkner JA, et al. Reactive oxygen species on bone mineral density and mechanics in Cu, Zn superoxide dismutase (Sod1) knockout mice. Biochem Biophys Res Commun. 2010;403(1):149–153. doi:10.1016/j.bbrc.2010.11.006
38. Muthusami S, Ramachandran I, Muthusamy B, et al. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta. 2005;360(1–2):81–86. doi:10.1016/j.cccn.2005.04.014
39. Zeng J-H, Zhong Z-M, Li X-D, et al. Advanced oxidation protein products accelerate bone deterioration in aged rats. Exp Gerontol. 2014;50:64–71. doi:10.1016/j.exger.2013.11.014
40. Kim HK, Park SK, Zhou J-L, et al. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111(1):116–124. doi:10.1016/j.pain.2004.06.008
41. Lee I, Kim HK, Kim JH, et al. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133(1–3):9–17. doi:10.1016/j.pain.2007.01.035
42. Wang J, Cochran V, Abdi S, et al. Phenyl N-t-butylnitrone, a reactive oxygen species scavenger, reduces zymosan-induced visceral pain in rats. Neurosci Lett. 2008;439(2):216–219. doi:10.1016/j.neulet.2008.05.018
43. Hacimuftuoglu A, HANDY C, GOETTL V, et al. Antioxidants attenuate multiple phases of formalin-induced nociceptive response in mice. Behav Brain Res. 2006;173(2):211–216. doi:10.1016/j.bbr.2006.06.030
44. Catalano A, Martino G, Morabito N, et al. Pain in osteoporosis: from pathophysiology to therapeutic approach. Drugs Aging. 2017;34(10):755–765. doi:10.1007/s40266-017-0492-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.