Back to Journals » Cancer Management and Research » Volume 10
Overexpression of RALY promotes migration and predicts poor prognosis in hepatocellular carcinoma
Authors Zhu Z , Zhang Y, Huang CS, Tang Y, Sun C, Ju W, He X
Received 7 August 2018
Accepted for publication 26 September 2018
Published 9 November 2018 Volume 2018:10 Pages 5559—5572
DOI https://doi.org/10.2147/CMAR.S182996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Beicheng Sun
Zebin Zhu,1–3,* Yixi Zhang,1–3,* Chensong Huang,4 Yunhua Tang,1–3 Chengjun Sun,1–3 Weiqiang Ju,1–3 Xiaoshun He1–3
1Organ Transplant Center, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou 510080, Guangdong, China; 2Guangdong Provincial Key Laboratory of Organ Donation and Transplant Immunology, Guangzhou 510080, Guangdong, China; 3Guangdong Provincial International Cooperation Base of Science and Technology, Guangzhou 510080, Guangdong, China; 4Department of Pancreato-Biliary Surgery, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou 510080, Guangdong, China
*These authors contributed equally to this work
Introduction: RALY plays a critical role in promoting invasiveness and is associated with poor prognosis in different types of cancers. However, the prognostic value of RALY and its precise role in hepatocellular carcinoma (HCC) remain unknown.
Materials and methods: We detected the expression of RALY in 127 clinical HCC tissue samples and seven HCC cell lines by immunohistochemical staining and Western blotting. The prognostic value of RALY expression was assessed using the Kaplan–Meier method. The expression and prognostic value of RALY were also studied by bioinformatics analysis of data from the Gene Expression Omnibus and The Cancer Genome Atlas. The biological influence of RALY on HCC cell lines was studied using proliferation, transwell migration, and invasion assays in vitro.
Results: The expression of RALY in HCC tissues was significantly higher than that in adjacent normal liver tissues. Abnormally high expression of RALY was associated with tumor size (P=0.031), TNM stage (P=0.026), presurgical serum AFP levels (P=0.025), and vascular invasion (P=0.001). Kaplan–Meier analysis demonstrated that higher expression of RALY correlated with poorer overall survival and disease-free survival in HCC patients. High RALY expression was an independent adverse prognostic factor for overall survival (HR =2.559, 95% CI: 1.710–3.827, P<0.001) and disease-free survival (HR =2.053, 95% CI: 1.384–3.047, P<0.001) in HCC. Moreover, knockdown of RALY expression using a specific shRNA suppressed the proliferation, migration, and invasion capabilities of HCC cells in vitro. Knockdown of RALY expression in HCC cell lines resulted in upregulation of E-cadherin and downregulation of N-cadherin, vimentin, and snail.
Conclusion: Taken together, our results indicate that RALY represents a biomarker for the prognosis of patients with HCC and highlight the importance of RALY as an oncogene in HCC.
Keywords: RALY, hepatocellular carcinoma, migration, invasion
Introduction
Hepatocellular carcinoma (HCC), which accounts for approximately 91.5% of all malignant hepatic tumors, is characterized by a poor prognosis.1,2 As the majority of HCC patients are diagnosed late, the 5-year survival rate is low. Even for patients who undergo surgical resection, the 5-year overall survival rate is only 30%–50%.3,4 The main reasons are the high invasion, metastasis, and recurrence rates of HCC. Hence, the treatment of HCC progression and metastasis has become a worldwide challenge. New insights into the biologic processes associated with HCC and the identification of novel biomarkers are urgently required for HCC management. Similar to other solid tumors, the development and progression of HCC are associated with accumulation of genetic and epigenetic alterations.5 Although some recent studies have identified genes that contribute to the tumorigenesis and progression of HCC, the detailed molecular mechanism underlying the malignant characteristics of HCC remains to be elucidated.
RALY was identified as an RNA-binding protein and plays a critical role in promoting invasiveness in cancer cells.6 Previous bioinformatics data demonstrated that the overexpression of RALY is associated with poor survival in ovarian, lung, bladder, brain, and breast cancers, as well as in multiple myelomas and melanomas.7 In addition, based on microarray studies of cell lines, the RALY protein is overexpressed in ovarian cancer cells that are resistant to oxaliplatin.8 However, the prognostic value of RALY and its precise role in modulating tumor metastasis in HCC have not been fully elucidated.
In this study, we investigated the clinical significance of RALY expression and the effect of RALY on tumor metastasis in HCC. First, the expression of RALY was compared between HCC tissues and adjacent normal liver tissues. Next, the correlations between RALY expression and the malignant characteristics of HCC were studied. Univariate and multivariate Cox regression analyses were used to identify the independent prognostic factors for overall survival and disease-free survival in HCC patients. Furthermore, specific shRNA was used to knock down RALY expression to investigate the function of RALY in the proliferation, migration, and invasion of different HCC cell lines. We also found that the expression level of RALY correlated with epithelial-to-mesenchymal transition (EMT) markers.
Materials and methods
Patient specimens
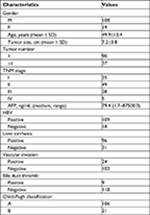
One hundred and twenty-seven histologically proven HCC patients who underwent hepatectomy between January 2009 and December 2012 at the First Affiliated Hospital of Sun Yat-Sen University were recruited for clinicopathological and prognostic analysis. The entry criteria included patients who 1) were undergoing curative resection; 2) were receiving no preoperative therapies for tumors; 3) had no distant metastasis; 4) survived longer than 30 days after hepatectomy; and 5) had integrated clinicopathological and follow-up data. In this study, TNM staging was evaluated based on the seventh edition of the Cancer Staging Manual of the American Joint Committee on Cancer. The clinicopathological characteristics of the 127 HCC patients included are presented in Table S1. This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University. The patients whose tissues were used in this research have provided written informed consent. All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethical committee of the First Affiliated Hospital of Sun Yat-Sen University and with the 1964 Helsinki Declaration and its later amendments or comparable guidelines.
Extraction and processing of Gene Expression Omnibus (GEO) data
Two gene expression profiles (GSE25097 and GSE57958) were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/gds/). The array data for GSE579589 included 39 HCC tissue samples and 39 adjacent normal liver tissue samples, and the array data for GSE2509710 included 268 HCC tissue samples and 243 adjacent normal liver tissue samples. GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r/) is an interactive web tool for comparing two groups of data that can analyze any GEO series.11 In this study, GEO2R was applied to screen for the differential expression of RALY mRNA between HCC tissues and adjacent normal liver tissues.
Extraction and processing of The Cancer Genome Atlas (TCGA) data
TCGA data for RALY expression in HCC tissues and clinicopathological data from the patients were downloaded from the TCGA database (https://gdc.cancer.gov/). Only patients with operations of R0 resection and with follow-up time greater than 1 month were included. A total of 318 HCC tissues from the corresponding patients were obtained from the TCGA database. RALY expression in HCC and its correlations with clinical parameters of HCC were analyzed. We stratified HCC patients into high vs low RALY expression according to the statistical cutoff value, which was defined by the highest Youden index (specificity+ sensitivity–1) obtained from the receiver operating characteristic (ROC) curves. The mRNA expression of RALY >18.6 was used to define HCC with high RALY expression, and the mRNA expression of RALY ≤18.6 was defined as low RALY expression.
Immunohistochemical staining
First, paraffin-embedded tissue sections were deparaffinized and rehydrated before antigen retrieval. Then, tissue sections were blocked in 3% H2O2 and subjected to a staining protocol using an anti-RALY antibody (1:200, Abcam, Cambridge, UK). The tissue sections were developed with the DAB Horseradish Peroxidase Color Development Kit (Maixin Co., Fuzhou, China) at room temperature, followed by counterstaining with hematoxylin. All slides were documented by light microscopy and photography. Two experienced pathologists independently scored the magnitude of the staining. The area positive for staining was scored according to the following standard:12 0 points indicate that no tumor cells were positively stained; 1 point indicates that <30% of the area was positively stained tumor cells; 2 points indicates that 30%–60% of the area was positively stained tumor cells; and 3 points indicates that >60% of the area was positively stained tumor cells. The intensity of the positive staining was scored according to the following standard: 0 points indicate no positive staining; 1 point indicates that the color was light yellow (weak staining); 2 points indicate that the color was yellow-brown (moderate staining); and 3 points indicate that the color was brown (strong staining). The final immunohistochemistry staining score was calculated by multiplying the staining intensity score by the score for the positively stained area. Ultimately, the expression of RALY in the HCC tissues and adjacent normal liver samples was assessed according to the final immunohistochemistry staining score. Thus, the results could be classified into scores of 0, 1, 2, 3, 4, 6, and 9. The statistical cutoff value for the immunohistochemistry staining scores was defined by the highest Youden index obtained from the ROC curves. Therefore, a final immunohistochemistry staining score >4 was used to define tissues with high RALY expression, and a score ≤4 was defined as low RALY expression.
Cell culture and transfection
Human hepatocarcinoma cell lines (HepG2, HepB3, Huh7, SMMC-7721, MHCC-97H, MHCC-97L, and Bel-7402) and an immortalized hepatocyte cell line (LO2) were purchased from the Institute of Cell Biology of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM (Sigma-Aldrich Co., St Louis, MO, USA) or RPMI-1640 supplemented with 10% FBS (HyClone, Shanghai, China), 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in a humidified chamber containing 5% CO2. The cells were transfected with RALY shRNAs or a negative control (NC) shRNA using the Lipofectamine 2000 reagent (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. The sequences of the shRNA oligos that targeted RALY were as follows: 5′-AAG CAA UGU AAC CAA CAA G-3′ (shRNA-1), 5′-CUC AGC CAA GAU CAA GUU A-3′ (shRNA-2), and 5′-CGG GCA GAC CCU GGA CAU C-3′ (shRNA-3). Three candidate RALY shRNA sequences were used, and the two most efficient interference sequences were ultimately chosen. The sense NC shRNA sequence was 5′- GCG CGC TTT GTA GGA TTC G-3′.
Western blot analysis
Total proteins were extracted from the cell lines and resolved by 10% SDS-PAGE. Next, the protein samples were electrotransferred onto PVDF membranes. Western blotting was performed using antibodies directed against RALY (1:500, Abcam), E-cadherin (1:1,000, Abcam), N-cadherin (1:1,000, Abcam), vimentin (1:1,000, Abcam), and snail (1:500, Abcam). The primary antibodies were incubated at 4°C overnight, followed by the secondary antibodies. Then, the protein-antibody complexes were detected by chemiluminescence (Pierce ECL Western Blotting Substrate, Thermo Fisher Scientific), according to the manufacturer’s protocol (Applied Biosystems, Thermo Fisher Scientific). β-Actin (1:2,000, Abcam) was used as an internal control. All the band intensities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Cell proliferation, migration, and invasion assays
The cell proliferative potential was measured at 48 hours by Cell Counting Kit-8 (DOJINDO, Kumamoto, Japan) according to the manufacturer’s instructions. Absorbance values at 450 nm were measured to represent the cell proliferative potential.
Tumor cell migration and invasion abilities were assessed by cell migration and invasion arrays using transwell chambers with or without Matrigel (BD Biosciences, San Jose, CA, USA). Approximately 5×104 cells in medium without FBS were seeded in transwell chambers with or without Matrigel. Medium containing 10% FBS in the lower chamber served as the chemoattractant. After 24 hours’ culture in an incubator, the migratory and invasive cells attached to the lower surface of the membrane insert were fixed with 4% formaldehyde, stained with crystal violet, and counted under a microscope. Five random fields of view at a 200× magnification were selected and captured. Each experiment was repeated three times under the same conditions.
Statistical analysis
Differences in continuous variables between two groups were analyzed using Student’s t-test, and differences among multiple groups were analyzed using ANOVA. The Mann–Whitney U rank-sum test was used to compare the differences of non-normally distributed continuous variables. The differences of categorical variables were compared using the chi-squared test. Spearman correlation analysis was used to determine the correlations between clinicopathologic characteristics and RALY expression. Kaplan–Meier analysis was used to calculate the overall survival and disease-free survival rates, and the survival rate curves were analyzed by the log-rank test. A Cox proportional hazards regression model was used for multivariate analysis of independent prognostic factors. All analyses were performed using SPSS for Windows, version 22.0 (IBM Corporation, Armonk, NY, USA). In all the statistical analyses, two-sided P-values less than 0.05 were considered statistically significant.
Results
RALY expression is abnormally upregulated in HCC
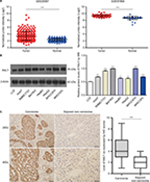
To analyze the expression of RALY in HCC, we first used two gene expression profiles (GSE25097 and GSE57958) obtained from the GEO database. The data showed that RALY mRNA expression was significantly increased in HCC tissues compared with that in adjacent normal liver tissues (P<0.001 for each dataset, Figure 1A). Then, we detected RALY protein expression in HCC cell lines (Huh7, SMMC-7721, Bel-7402, HepB3, HepG2, MHCC-97H, and MHCC-97L) and an immortal hepatocellular cell line (LO2) by Western blotting. As demonstrated in Figure 1B, the protein levels of RALY in LO2 cells were significantly lower than those in HCC cells. In particular, RALY was most highly overexpressed in highly metastatic cancer cell lines, including Bel-7402 and MHCC-97H. Furthermore, RALY protein expression was detected by immunohistochemistry in 127 pairs of HCC tissues and corresponding adjacent normal liver tissues. Positivity for RALY protein expression was distinctly located in the nucleus, with slight expression in the cytoplasm of cells in the HCC tissues (Figure 1C), while low or scarce RALY expression was found in adjacent noncancerous tissues. Immunostaining scores for RALY expression in HCC tissues were significantly higher than those for adjacent noncancerous tissues (P<0.001, Figure 1C).
Correlations between RALY expression and clinicopathological features in patients with HCC
To further investigate the role of RALY expression in the aggressive progression of HCC, the correlations between RALY expression and the clinicopathologic characteristics of HCC patients were analyzed in Table 1. Our results indicate that abnormally high expression of RALY was associated with tumor size (P=0.031), TNM stage (P=0.026), presurgical serum AFP levels (P=0.025), and vascular invasion (P=0.001). In contrast, there was no obvious correlation between RALY expression and gender (P=0.139), age (P=0.416), tumor number (P=0.825), hepatitis B virus infection (P=0.801), liver cirrhosis (P=0.199), bile duct thrombi (P=0.864) or Child-Pugh classification (P=0.181). Moreover, Spearman correlation analysis demonstrated that there was a good positive correlation between high RALY expression and later TNM stage (r=0.612, P<0.001) and vascular invasion (r=0.646, P<0.001), but there were weak positive correlations between high RALY expression and bigger tumor size (r=0.315, P=0.012) and higher AFP levels (r=0.413, P=0.003). Similarly, by analyzing the 318 HCC patients from TCGA, we also found that high expression of RALY was associated with tumor stage (P=0.024), TNM stage (P=0.035), and AFP levels (P=0.048) (Table S2).
Additionally, we compared the RALY immunohistochemistry staining scores between HCC tissues with different tumor sizes, TNM stages, AFP levels, and conditions of vascular invasion, as depicted in Figure 2. The expression of RALY in HCC tissues with later TNM stage (III + IV), or higher AFP levels (>200 ng/mL) was significantly higher than that in HCC tissues with earlier TNM stage (I+II) (Figure 2B, P=0.002), or lower AFP levels (≤200 ng/mL) (Figure 2C, P=0.007), respectively. In addition, the expression of RALY in HCC tissues without vascular invasion was significantly lower than that in HCC tissues with vascular invasion (Figure 2D, P=0.006). However, the difference in RALY expression in HCC tissues with tumor size >5 cm and in HCC tissues with tumor size ≤5 cm was not significant (Figure 2A, P=0.092).
High RALY expression predicts poor outcome in HCC patients
To clarify the prognostic value of RALY in HCC patients, the relationship between RALY expression and survival time was further investigated in 127 HCC patients. Figure 3C, D shows a significant difference in overall survival and disease-free survival between the high RALY expression (immunohistochemistry staining score of RALY >4) group and the low RALY expression (immunohistochemistry staining score of RALY ≤4) group (P<0.001 and P<0.001, respectively). Similarly, the RALY level was found to be correlated with survival time in HCC patients recruited from the TCGA database (Figure 3A, B; P=0.003 and P=0.012, respectively).
Furthermore, to identify the independent prognostic factors for overall survival and disease-free survival in HCC patients, univariate and multivariate Cox proportional hazards regression analyses were used. The results demonstrated that later TNM stage (HR, 1.784; 95% CI, 1.052–3.026 for III + IV vs I, P=0.032) and higher RALY expression (HR, 2.559; 95% CI, 1.710–3.827, P<0.001) were significant adverse prognostic factors for overall survival (Table 2). Additionally, the results showed that later TNM stage (HR, 2.126; 95% CI, 1.227–3.540, P=0.004), vascular invasion (HR, 1.842; 95% CI, 1.106–3.068, P=0.019), and higher RALY expression (HR, 2.053; 95% CI, 1.384–3.047, P<0.001) were significant adverse prognostic factors for disease-free survival in HCC patients (Table 3).
Suppression of RALY inhibits HCC cell proliferation, migration, and invasion capabilities in vitro
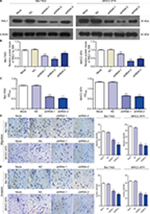
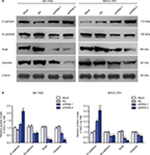
To further study the effect of RALY on the promotion of proliferation, migration, and invasion in HCC cells, the expression of RALY in the Bel-7402 and MHCC-97H cell lines was knocked down by transfection with RALY shRNA. In this assay, three different sequences of RALY shRNAs (shRNA-1, shRNA-2, and shRNA-3) were used. Ultimately, shRNA-1 and shRNA-2 were chosen as the two best shRNAs. The protein level of RALY was significantly decreased in the Bel-7402 and MHCC-97H cells after RALY shRNA interference (Figure 4A, B). Bel-7402 and MHCC-97H cells transfected with RALY shRNAs displayed significantly decreased proliferative abilities (Figure 4C). Next, we examined the effect of RALY on cell migration and invasion abilities. Following gene silencing of RALY, the Bel-7402 and MHCC-97H cells displayed significantly decreased migration (Figure 4D) and invasion (Figure 4E) abilities.
RALY induces EMT in HCC cells
To study how RALY affects the migratory and invasive phenotype of HCC cells, we detected markers of the EMT by Western blotting. After knockdown of RALY, the epithelial marker E-cadherin was significantly upregulated, whereas the mesenchymal markers N-cadherin, snail, and vimentin were inhibited in Bel-7402 and MHCC-97H cells (Figure 5). These results imply that RALY might play an important role in inducing the EMT process in HCC cells.
Discussion
In the present study, we found that RALY was abnormally overexpressed in HCC tissues and cells. HCC-related microarray data from GEO were first analyzed, and the results showed that HCC tumor tissues present higher RALY levels than adjacent normal liver tissues. Then, immunohistochemistry was used to detect the expression of the RALY protein in 127 HCC samples, and the findings were consistent with the results mentioned previously. Then, clinical significance analysis indicated that the upregulation of RALY was significantly associated with poorer overall survival and disease-free survival rates in patients with HCC, suggesting that RALY might play important oncogenic roles in HCC metastasis. Furthermore, based on in vitro assays, we demonstrated that increased levels of RALY promoted the proliferation, migration, and invasion abilities of HCC cells. This study provided new insights into the promotive roles of RALY in HCC progression.
HCC is ranked as the sixth most common neoplasm and the third leading cause of cancer death,13 and the prognosis of HCC is poor. The poor prognosis of HCC is mainly caused by its high potential for metastasis and recurrence after surgical resection.14 It has been proven that a later TNM stage is an important independent predictor of poor outcome in HCC.15–18 Similarly, in our study, Cox proportional hazards analyses indicated that later TNM stages and high RALY expression were independent prognostic factors for overall survival and disease-free survival in HCC. In addition, Kaplan–Meier analysis showed that patients with high RALY expression had a poorer prognosis than patients with low RALY expression. Higher RALY expression was also found to be correlated with malignant behaviors of HCC, including larger tumor size, later TNM stages, higher serum AFP levels, and vascular invasion, which are similar to the results obtained after analyzing the data from TCGA. These findings indicate that high expression of RALY is associated with the degree of tumor progression in HCC.
RALY is a member of the heterogeneous nuclear ribonucleoprotein family that binds poly-U-rich elements within several RNAs and regulates the expression of specific transcripts. Previous studies have shown that RALY is upregulated in different types of cancer,6–8,19 and its downregulation impairs cell cycle progression.19 A recent study demonstrated that RALY can promote invasiveness in breast cancer cells; additionally, RALY expression is increased in breast tumors, which correlates with decreased patient survival.6 Rossi et al20 studied gene expression in HeLa cells in response to RALY silencing to better characterize the impairment in cell cycle progression, and they provided evidence for the accelerative function of RALY in tumorigenesis in mammalian cells. Interestingly, the FOXI, ANXA1, and CST6 mRNAs were upregulated and the CXCR4, KNSTRN, and H1FX mRNAs were downregulated in cells lacking RALY expression. In breast cancer cells, RALY has been identified as an indirect regulator of transcription and cell cycle progression through the regulation of specific mRNA targets.19 Knocking down RALY significantly counteracts oxaliplatin resistance in colorectal cancer cells7 overexpressing YB-1, which is related to secondary resistance to cisplatin in different cancers.21–23 Although the roles of RALY in regulating migration and invasion in other tumors have been identified, the roles of RALY in HCC development and progression have not been discussed. In the present study, the oncogenic effects of RALY on HCC pathogenesis were demonstrated by in vitro functional assays. Our findings indicated that RALY was able to promote proliferation, migration, and invasion in HCC cells.
EMT endows cells with migratory and invasive properties and is thought to play fundamental roles in tumor progression and metastasis formation.24 Previous studies25–27 have reported that EMT is an important step in HCC migration and invasion. Therefore, we evaluated whether RALY could induce EMT in HCC cells. In the present study, we detected the markers of EMT and found that decreased RALY levels reduced the expression levels of mesenchymal markers while enhancing those of epithelial markers in HCC cells. These findings suggest that RALY enhances migration and invasion capabilities, most likely through the induction of an EMT transition in HCC.
In the current study, we first revealed the exceptional overexpression of RALY in both HCC tissues and cell lines. In addition, high expression of RALY was found to be closely associated with the malignant characteristics of HCC. We noted that RALY could be used as an independent prognostic factor for patients with HCC. Moreover, we demonstrated that RALY increases the proliferative, migratory, and invasive abilities of HCC cells. Nevertheless, there are some limitations in our study. First, the present study was a single-center retrospective study, and the sample size was limited. Therefore, we also analyzed data from a larger sample size from TCGA (the sample size was 318) to demonstrate that higher RALY expression was associated with malignant characteristics and prognosis in HCC. To overcome this limitation, a prospective and randomized multicenter study should be conducted in the future. Second, the effect of RALY on promoting the migration and invasion of HCC cells was studied only in vitro; animal experiments were not conducted. Moreover, the upstream or downstream molecules of RALY were not investigated in the present study. A previous study showed that mammalian cells lacking RALY expression exhibit opposing changes in the levels of the HIFX and ANXA1 mRNAs and proteins.20 Additional studies are required to clarify the molecular mechanisms through which RALY affects other molecules or signaling pathways in the future.
Conclusion
Our data demonstrated that RALY plays a significant role in the progression and metastasis of HCC. High RALY expression is predictive of a poor prognosis in HCC, and RALY can serve as an independent prognostic factor for overall survival and disease-free survival in patients with HCC. Moreover, the overexpression of RALY promotes the proliferation, migration, and invasion of HCC cells. RALY may thus provide a theoretical basis for targeted therapy for HCC patients.
Acknowledgments
This study was supported by the Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (2015B050501002), the Guangdong Provincial Natural Science Funds for Distinguished Young Scholars (2015A030306025), the Special Support Program for Training High-Level Talent in Guangdong Province (2015TQ01R168), and the Pearl River Nova Program of Guangzhou (201506010014).
Author contributions
ZZ and WJ contributed to the conception and design of the study; YZ and CSH acquired the data; YT and CS performed the experiments; ZZ and YZ contributed to data analysis; ZZ, WJ, and XH contributed to drafting the article or revising it critically for important content and provided final approval of the version to be published. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
de Souza JA, Hunt B, Asirwa FC, Adebamowo C, Lopes G. Global health equity: Cancer care outcome disparities in high-, middle-, and low-income countries. J Clin Oncol. 2016;34(1):6–13. | ||
Torre LA, Sauer AM, Chen MS Jr, Kagawa-Singer M, Jemal A, Siegel RL. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging incidence in males and females. CA Cancer J Clin. 2016;66(3):182–202. | ||
Fan ST, Mau Lo C, Poon RT, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253(4):745–758. | ||
Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151(4):356–363. | ||
Jiang L, Yan Q, Fang S, et al. Calcium-binding protein 39 promotes hepatocellular carcinoma growth and metastasis by activating extracellular signal-regulated kinase signaling pathway. Hepatology. 2017;66(5):1529–1545. | ||
Bondy-Chorney E, Baldwin RM, Didillon A, Chabot B, Jasmin BJ, Côté J. RNA binding protein RALY promotes Protein Arginine Methyltransferase 1 alternatively spliced isoform v2 relative expression and metastatic potential in breast cancer cells. Int J Biochem Cell Biol. 2017;91(Pt B):124–135. | ||
Tsofack SP, Garand C, Sereduk C, et al. NONO and RALY proteins are required for YB-1 oxaliplatin induced resistance in colon adenocarcinoma cell lines. Mol Cancer. 2011;10:145. | ||
Samimi G, Manorek G, Castel R, et al. cDNA microarray-based identification of genes and pathways associated with oxaliplatin resistance. Cancer Chemother Pharmacol. 2005;55(1):1–11. | ||
Mah WC, Thurnherr T, Chow PK, et al. Methylation profiles reveal distinct subgroup of hepatocellular carcinoma patients with poor prognosis. PLoS One. 2014;9(8):e104158. | ||
Sung WK, Zheng H, Li S, et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet. 2012;44(7):765–769. | ||
Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41(Database issue):D991–D995. | ||
Huang CS, Zhai JM, Zhu XX, et al. BTG2 is down-regulated and inhibits cancer stem cell-Like features of side population cells in hepatocellular carcinoma. Dig Dis Sci. 2017;62(12):3501–3510. | ||
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. | ||
Badvie S. Hepatocellular carcinoma. Postgrad Med J. 2000;76(891):4–11. | ||
Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909–922. | ||
Cheng CH, Lee CF, Wu TH, et al. Evaluation of the new AJCC staging system for resectable hepatocellular carcinoma. World J Surg Oncol. 2011;9:114. | ||
Liu PH, Hsu CY, Hsia CY, et al. Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. J Hepatol. 2016;64(3):601–608. | ||
Kee KM, Wang JH, Lin CY, Wang CC, Cheng YF, Lu SN. Validation of the 7th edition TNM staging system for hepatocellular carcinoma: an analysis of 8,828 patients in a single medical center. Dig Dis Sci. 2013;58(9):2721–2728. | ||
Cornella N, Tebaldi T, Gasperini L, et al. The hnRNP RALY regulates transcription and cell proliferation by modulating the expression of specific factors including the proliferation marker E2F1. J Biol Chem. 2017;292(48):19674–19692. | ||
Rossi A, Moro A, Tebaldi T, et al. Identification and dynamic changes of RNAs isolated from RALY-containing ribonucleoprotein complexes. Nucleic Acids Res. 2017;45(11):6775–6792. | ||
Schittek B, Psenner K, Sauer B, Meier F, Iftner T, Garbe C. The increased expression of Y box-binding protein 1 in melanoma stimulates proliferation and tumor invasion, antagonizes apoptosis and enhances chemoresistance. Int J Cancer. 2007;120(10):2110–2118. | ||
Shiota M, Yokomizo A, Itsumi M, et al. Twist1 and Y-box-binding protein-1 promote malignant potential in bladder cancer cells. BJU Int. 2011;108(2 Pt 2):E142–E149. | ||
Yahata H, Kobayashi H, Kamura T, et al. Increased nuclear localization of transcription factor YB-1 in acquired cisplatin-resistant ovarian cancer. J Cancer Res Clin Oncol. 2002;128(11):621–626. | ||
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. | ||
Han J, Wang F, Lan Y, et al. KIFC1 regulated by miR-532-3p promotes epithelial-to-mesenchymal transition and metastasis of hepatocellular carcinoma via gankyrin/AKT signaling. Oncogene. Epub 2018. Aug 16. | ||
Steinway SN, Zañudo JG, Ding W, et al. Network modeling of TGFβ signaling in hepatocellular carcinoma epithelial-to-mesenchymal transition reveals joint sonic hedgehog and Wnt pathway activation. Cancer Res. 2014;74(21):5963–5977. | ||
Zhu K, Dai Z, Pan Q, et al. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2011;17(23):7294–7302. |
Supplementary materials
  | Table S1 Clinicopathological characteristics of the 127 HCC patients Abbreviations: HCC, hepatocellular carcinoma; HBV, hepatitis B virus. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.