Back to Journals » International Journal of General Medicine » Volume 16
Overexpression of NRP1 is Associated with Poor Prognosis via Accelerating Immunosuppression in Head and Neck Squamous Cell Carcinoma
Authors Yang X, Xu T, Song X , Wu Y
Received 18 March 2023
Accepted for publication 27 June 2023
Published 4 July 2023 Volume 2023:16 Pages 2819—2829
DOI https://doi.org/10.2147/IJGM.S409336
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Xueming Yang,1– 4,* Teng Xu,1,2,4,* Xiaomeng Song,1,2,4 Yunong Wu1,2,4
1Jiangsu Key Laboratory of Oral Diseases, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 2Jiangsu Province Engineering Research Center of Stomatological Translational Medicine, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 3Department of Stomatology, the Affiliated People’s Hospital of Jiangsu University, Zhenjiang, Jiangsu, People’s Republic of China; 4Department of Oral and Maxillofacial Surgery, Affiliated Hospital of Stomatology, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yunong Wu, Department of Oral and Maxillofacial Surgery, Affiliated Hospital of Stomatology, Nanjing Medical University, No. 1, Shanghai Road, Nanjing, Jiangsu, 210029, People’s Republic of China, Tel +86-25-69593100, Email [email protected]
Background: Neuropilin-1 (NRP1) is a significant molecular structure that participates in many diseases progress including malignant tumors. However, its role in head and neck squamous cell carcinoma (HNSCC) remains to be uncovered. In this study, we determined the function of NRP1 as a crucial biomarker in proliferation, metastasis and immunosuppression in HNSCC.
Methods: We collected samples of normal tissues (n = 18) and HNSCC tissues (n = 202) for immunohistochemical staining of NRP1 and evaluated its correlation to clinical prognostic characteristics. Furthermore, we enrolled 37 HNSCC patients received immune checkpoint blockade (ICB) treatment with defined therapeutic effects records. The biological process, signal pathways, and immune infiltration relevance to NRP1 were analyzed utilized transcriptome data from The Cancer Genome Atlas (TCGA).
Results: The NRP1 protein expression was significantly upregulated in HNSCC tissue and was associated with T stage, N stage, histological differentiation, recurrence and NRP1 expression. The high expression of NRP1 indicated poor survival rate and was found to be an independent prognosis factor. Enrichment analysis showed that NRP1 was associated with cell adhesion, extracellular matrix organization, homophilic cell adhesion via plasma membrane in biological process and neuroactive ligand–receptor interaction, protein digestion and absorption, calcium signal pathways. Moreover, NRP1 mRNA level was found positively correlated to cancer associated fibroblast cells, Treg cells and macrophage/monocyte cells.
Conclusion: NRP1 might be likely to develop into a potential immunoregulation target as well as a predictive biomarker in HNSCC immune treatment.
Keywords: NRP1, head and neck squamous cell carcinoma, immunosuppression, prognosis, bioinformatics
Introduction
Head and neck cancer is identified as the 6th most common cancer worldwide and squamous cell carcinoma is the most common subtype accounting for approximately 95% in head and neck malignant lesions.1 Though radical excision combined with postoperative adjuvant radiotherapy, the overall survival does not improve obviously over the past three decades. Apart from the traditional risk factors including smoking, alcohol2,3 and human papillomavirus (HPV) infection,4–6 immunosuppressive status and immune escape have been the hot research topics in recent years.7,8 Although some drugs of immune checkpoint inhibitors have been approved by Food and Drug Administration (FDA), the responsivity is still at a low level. Therefore, the mechanisms of immunosuppressive status and immune escape are urgently to be elucidated.
Neuropilin-1 (NRP1) is recognized as an important membrane-spanning protein that participates in many different signaling pathways.9,10 It has been reported to involve in metastasis and radiation resistance in tumor progression.11–13 Generally, NRP1 serves as the ligands or co-receptors including semaphorin family members and vascular endothelial growth factor (VEGF).10,14 In breast cancer, up regulation of the VEGF/NRP1 is associated with the enhancement of epithelial-mesenchymal transition (EMT) process and the NF-κB, β-catenin signaling.15,16 Recently, NRP1 was found to be an entrance of SARS-CoV-2 virus except ACE2 and simultaneously blocked NRP1/ACE2 and viral spike protein integration might be a valuable treatment option.17
Tumor-infiltrating immune cell is an important component of tumor associated microenvironment (TAM). In normal tissue, the relative balance is maintained to keep the homeostasis of microenvironment, whereas such circumstance is destroyed in the tumor, which in turn results in tumor progression and failure of anti-cancer therapies.18–20 Thus, exploring the crosstalk pattern between tumor cell and TAM may help to improve the sensitive of immunotherapy. In this research, immunohistochemical (IHC) analysis was performed to determine the expression level of NRP1 in normal and HNSCC tissue. The association between NRP1 expression and the clinicopathological characteristics of HNSCC was also investigated. Transcriptome data extracted from TCGA were analyzed to determine the role of NRP1 in HNSCC including biological functions, signal pathways and correlation of infiltrating immune cells using bioinformatics methods.
Materials and Methods
Clinical Samples
This study was approved by the Ethical Committee of the Affiliated Stomatology hospital of Nanjing Medical University (No. PJ2018-051-001). Patients were excluded if they accepted chemotherapy, radiotherapy, immunological/targeted therapy before surgery. Finally, a total of 202 HNSCC tissues who received surgery at the Affiliated Stomatology Hospital of Nanjing Medical University between 2009 and 2014 were included in this study. Besides, 18 normal oral mucosa tissues were derived from 18 patients who received gingivectomy or blind pouch resection. Furthermore, we enrolled 37 HNSCC patients received immune checkpoint blockade (ICB) treatment with defined therapeutic effects records. All patients signed informed consents in this study. Clinicopathological characteristics of HNSCC patients were collected and analyzed simultaneously. The clinical grading criteria was according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system. The 4th edition of the head and neck tumors World Health Organization classification of adopted to determine histopathological classification. The details of 202 primary HNSCC patients and 37 ICB treatment HNSCC patients are provided in the Supplementary Table 1 and 2.
IHC and Scoring System
Paraffin-embedded HNSCC and normal tissues were dewaxed, rehydrated and antigen-retrieval by in 10 mmol/L citric acid buffer (pH 6.0) for 15 minutes in microwave oven. After cooling to room temperature, endogenous peroxides activity was eliminated using 3% hydrogen peroxide. Fifteen minutes later, the sides were washed 3 times with phosphate buffer saline (PBS) and blocked with 5% bovine albumin for 1 h at room temperature. Whereafter, the primary antibody of Neuropilin-1(ab81321; Abcam) was incubated at 4°C overnight. Next day, blots were washed 3 times with PBS and incubated with the Horseradish peroxidase (HRP) for 1 h at room temperature. Finally, slides were developed color, counterstained with hematoxylin, dehydrated, and sealed tablet using neutral gum. Three senior pathologists evaluated the staining reaction separately according to the staining intensity and extent. The intensity was scored to 0–3 based on the color degree. The staining extent classification was divided into 4 categories: 0 to 1 (0–25%), 1 to 2 (26–50%), 2 to 3 (51–75%), and 3 to 4 (76–100%). The final score equal to above two scores multiplied (range, 1–12). According to the mean score, NRP1 expression was stratified high and low.
TCGA Data Acquisition and Analysis
HNSCC-related gene counts data was extracted from TCGA database (https://portal.gdc.cancer.gov/). Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed using R software, version 4.1.0, and related packages (org. Hs. eg db, limma, clusterProfiler, ggplot2 and enrichplot). Gene set enrichment analysis (GSEA) was performed to analyze gene function. The function and related signal pathways of NRP1 in HNSCC were explored based on the above results.
Immune Infiltration Degree Analysis
TIMER2.0 online tool (http://timer.cistr ome.org/) was utilized to explore the relationship between NRP1 expression level and immune infiltration degree. Six subtypes of tumor infiltrating immune cell, including B cells, CD8+ T cells, NK cell, cancer associated fibroblast, Treg cell and macrophages were evaluated. CellMarker online database (http://biocc.hrbmu.edu.cn/CellMarker/) was adopted to determine the biomarkers of immune cells include in our research and that were used to further explore the association between NRP1 and immune cells.
Statistical Analysis
The relationship between NRP1 abundance and clinicopathological characteristics was analyzed using chi-square test. The survival rate of HNSCC patient was evaluated by Kaplan–Meier method and compared by Log rank test. Univariate and multivariate Cox regression models were performed to further explore whether NRP1 expression level was an independent prognosis factor. All statistical tests were two-tailed Student’s t-test and values of P < 0.05 were considered statistically significant.
The SPSS 26, GraphPad Prism v8 software and R 4.1.0 software were used in data analysis and mapping.
Results
NRP1 Protein Abundance Analysis
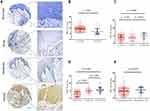
There were 18 normal and 202 HNSCC tissues incorporated in this study. The expression localization of NRP1 is shown in Figure 1A. We found that NRP1 protein was mainly expressed on the cytomembrane and weak or negative staining was always examined in normal tissues (Figure 1B).
Based on the expression score of NRP1 protein, HNSCC samples were divided into low (n = 105) and high (n = 97) subgroups. We then analyzed the correlation between NRP1 expression level and clinicopathological characteristics (Table 1). It was shown that patients with higher T stage (Figure 1C) and lymph node metastasis (Figure 1D) always exhibited significantly high expression of NRP1. Moreover, higher NRP1 expression implied more recurrence possibility (Figure 1E). In summary, our results indicated that overexpression of NRP1 might accelerate tumor progression and metastasis.
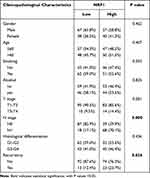 |
Table 1 Expression of NRP1 and its associations with clinicopathological characteristics in 202 patients with HNSCC |
NRP1 Abundance is Associated to HNSCC Patients’ Poor Prognosis
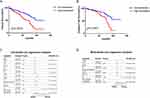
To determine the impact of NRP1 expression on HNSCC patients’ prognosis, we conducted Kaplan–Meier analysis of overall survival and disease-free survival. As shown in Figure 2A and B, high expression of NRP1 obviously reduced overall survival (P = 0.0003) and disease-free survival (P = 0.0043). We wondered whether the expression of NRP1 in HNSCC patients could be an independent prognosis predicted marker and univariate and multivariate Cox proportional hazard regression were utilized subsequently. As shown in Figure 2C and D, NRP1 expression level (HR = 1.76, 95% CI 1.12–2.77, P = 0.015) as well as tumor size (T stage) (HR = 2.08, 95% CI 1.18–3.68, P = 0.012), lymph nodes metastasis (N stage) (HR = 1.83, 95% CI 1.16–2.88, P = 0.008), histological differentiation (HR = 2.18, 95% CI 1.38–3.45, P = 0.001) and recurrence (HR = 7.93, 95% CI 4.93–12.76, P < 0.001) were significantly associated with overall survival in the univariate survival analysis. After eliminated confounding factors, NRP1 expression level was confirmed as the independent prognostic biomarker in HNSCC patients (HR = 1.65, 95% CI 1.04–2.60, P = 0.033).
Expression Level of NRP1 mRNA and Enrichment Analysis in HNSCC Based on TCGA
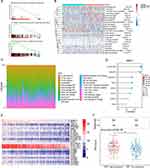
In this section, TCGA database was utilized to explore the transcriptome level and related functions of NRP1 in HNSCC. Compared to normal tissue, the mRNA expression level of NRP1 was significantly higher in four subtypes of HNSCC (Figure 3A). According to the NRP1 mRNA mean value, we categorized HNSCC patients into high-expression group (n = 204) and low-expression group (n = 296). There were 1272 genes significantly differential expressed, including 404 up-regulation and 868 down-regulation (Figure 3B, log2FoldChange ≥1.5 folds, p < 0.05). Whereafter, the possible biological functions and signal pathways of NRP1 were explored based on those differential expressed genes by GO and KEGG enrichment. We also determined genes which were significantly related to NRP1 expression in HNSCC, and the top 20 were exhibited as heatmap in Figure 3C. The visualized GO enrichment result was shown in bubble chart (Figure 3D). We could find that “cell adhesion”, “extracellular matrix organization”, “homophilic cell adhesion via plasma membrane”, “collagen catabolic process”, “muscle filament sliding” were the top 5 counted biological process related to NRP1 in HNSCC. The node diagram visualized the enrichment result of KEGG pathway, which indicated that “Neuroactive ligand-receptor interaction”, “Protein digestion and absorption”, “Calcium signaling pathway”, “Cardiac muscle contraction”, “Hypertrophic cardiomyopathy” were the top 5 pathways associated with NRP1 in HNSCC (Figure 3E).
Relationships of NRP1 and Degree of Immune Infiltration
Considering the adverse impact of NRP1 on the HNSCC prognosis, we wondered the possible means by which it facilitated tumor progression. DNA methylation, an important epigenetic modification of eukaryotic genes, could regulate genome function, and was treated as a significant mechanism in tumorigenesis and development. Thus, we evaluated the methylation level of NRP1 in normal and HNSCC tissue using UALCAN (http://ualcan.path.uab.edu/). Unfortunately, the difference was insignificant and scarcely any influence on the prognosis (Supplementary Figure 1). Cbioportal tool (https://www.cbioportal.org/) has shown that the mutation frequency of NRP1 in HNSCC was less than 2% and the alteration did not show impact on the patients’ survival time (Supplementary Figure 2).
Based on the gene set enrichment analysis (GSEA), immunological memory formation process, cytokine production involved in immune response and negative regulation of immune response were recognized related to NRP1 expression in HNSCC (Figure 4A). Thus, the infiltration level of immune cell in HNSCC was estimated using CIBERSORT algorithm base on TIMER2.0 database (Figure 4B and C). The positive correlations between NRP1 expression and Treg cells as well as macrophage M2. Meanwhile, negative correlations were found to T cell CD8+, B cell plasma and macrophage M1. Besides, NRP1 showed significant positive correlations to multi-immune checkpoint genes (Figure 4D). Various immune cell biomarkers associated with NRP1 are shown in Figure 4E, which have shown that B cell biomarkers (PTPRC, CD5), CD8+ T-cell biomarker (CD4), NK cell biomarker (CD16), Treg cell biomarkers (IL2RA, FOXP3, IL7R, ENTPD1), M1 macrophage biomarkers (CD32, SELP, FCGR1A, CD80 and CD86) and M2 macrophage biomarkers (CD163, MRC1 andCD209) were positively correlated with NRP1 expression. Interestingly, the NRP1 high expression patients showed higher Tumor Immune Dysfunction and Exclusion (TIDE) score (Figure 4F) (P < 0.001), which meant NRP1 played an adverse role in the ICB treatment.
NRP1 Might Be an Effective Predict Marker in ICB Treatment for HNSCC
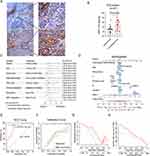
To verify the relationships between NRP1 expression and the ICB treatment outcome, we further enrolled 37 HNSCC patients received ICB therapy with defined therapeutic effects records. The IHC examinations showed NRP1 was upregulated in ICB treatment nonresponse patients (Figure 5A and B, P = 0.009). The univariate and multivariate logistic regression showed NRP1 was an independent predictive biomarker in ICB treatment outcome (Figure 5C). Integrating the clinical features of patients in this cohort, we constructed a nomogram in predicting the immunotherapy response (Figure 5D). This nomogram could predict the ICB response rate effectively with the favorable sensitivity (95.2%) and specificity (75.0%) (Figure 5E-H). By all means, more evidences were urgently needed to explore the usage of NRP1 as a biomarker for HNSCC immunotherapy.
Discussion
Targeted therapy provided a new concept in the treatment of malignancy tumor.21,22 However, the response ratio was still too low to meet treatment need. Such deficiency mainly attributed to the heterogeneity of tumor and lack of effective agent.23 As a result, for the vast majority of tumors, effective targeted agents were urgent to be detected for HNSCC patients. Taking advantage of samples from HNSCC patients who visited Stomatology Hospital Affiliated to Nanjing Medical University and TCGA database, the NRP1 function as a predicated biomarker of metastasis, invasion, and communication with extracellular matrix was investigated. In this study, NRP1 content was significantly elevated in HNSCC tissue compared to normal. The IHC and mRNA enrichment analyses indicated that overexpression of NRP1 significantly accelerated HNSCC tumor proliferation and metastasis via remodeling the tumor associated microenvironment. The NRP1 protein purity could be an independent prognostic index for HNSCC patients.
In this study, the NRP1 mRNA and protein levels elevated simultaneously in HNSCC tissues, which was consistent with other types of cancer reports, including lung, prostate, and gastric cancer. It was also positively associated with T stage, N stage and tumor recurrence, implied its involvement of proliferation and metastasis. According to enrichment analysis of biological process and signal pathway, NRP1 might play an irreplaceable role in the extracellular signal reception and intracellular signal transduction. A recent investigation found that NRP1 sustained EGFR activation upon cisplatin exposure and activated downstream MAPK/AKT pathways in vitro.24 Besides, NRP1 regulated p65-dependent proliferation signaling pathway via activating cAMP responsive element binding protein (CREB).25 Additionally, NRP1 could enhance the migration and invasion ability of gastric cancer by activating Pi3k/Akt signaling pathway and induced epithelial-mesenchymal transformation (EMT).26 Yang et al demonstrated that the tumor immune evasion in cervical cancer was attributed to NRP1 that was regulated by Far upstream element binding protein 1 (FUBP1).27 Furthermore, as a transmembrane glycoprotein, NRP1 was confirmed to be a credible biomarker of tumor-initiating cells in lung cancer in which NRP1+ lung cancer cells possessed tumor-initiating properties.28 Therefore, we speculated that NRP1 might be used as a potential therapy target in HNSCC.
Apart from functions above-mentioned, the ability of remodeling tumor microenvironment by mediating the interaction between tumor cells and immune associated cells, fibroblasts, or stromal cells were determined for NRP1. Our results showed that high level NRP1 expression meant more cancer associated fibroblast cells (CAF), Treg cells and macrophage/monocyte cells within tumor. It suggested that the function of NRP1 might be mediated by the immune system in HNSCC. Chen et al reported that NRP1 could be up-regulated by IL-8 secreted by cancer-associated fibroblast cells, which subsequently promoted gallbladder cancer cell proliferation.29 In T cell-specific NRP1 knockout mice, numbers of tumor-infiltrating Foxp3(+) Treg cells were significantly reduced and CD8(+) T cells activation enhanced, which finally induced tumor growth reduction and tumor-free survival improvement.30 Sohini Roy et al31 proved that tumor cells could drive the physiological roles of NRP1 in dendritic cells and macrophages to promote tumor angiogenesis and tumor-associated immunosuppression. Moreover, the self-renewal of the CD44+/PD1+/TCF1+/TIM3- progenitor exhausted T cells could be restricted by NRP1. That led to the ability of c-Jun/AP-1 expression reduction when T cell receptor restimulation. Blocking of NRP1, a unique “immune memory checkpoint”, may prolong the memory of T cell and reduced the tumor relapse possibility.32 The result of this preclinical study was in accordance to our observation that high level expression of NRP1 patients tended to more likely to relapse (23.7% vs 12.4%, p = 0.036). Hence, our study suggested that NRP1 could be an ideal assisted targeted that increased the sensitivity and prolonged the efficacy of immunotherapy.
Meanwhile, many immune related markers, such as forkhead box P3 (FOXP3), fibroblast activation protein (FAP), THY1, CD163 were also associated with NRP1 abundance as shown in Figure 4G. A retrospectively study33 shown that low content of FOXP3+ cells in tumor stroma was associated with favorable prognosis in triple-negative breast carcinoma (TNBC). Overexpression of miR-30a-5p could inhibited cell proliferation, migration, and invasion by targeted FAP in oral cancerous tissues.34 Elizabeth V Connor et al35 observed that THY1 (CD90) was highly expressed in cancer stem cells (CSCs) compared to non-CSCs in ovarian based on a high-throughput screen. Epithelial ovarian cancer cells with THY1 high expression could increase its proliferation and self-renewal. Meanwhile, high CD163+/CD68+ ratio in the tumor invasive front (TF) than that in tumor center (TC) was closely correlated with the enhancement of lymphovascular invasion, neoplasm invasiveness and TNM stage. Higher CD163+/CD68+ TF ratio could also facilitate the EMT program and increased circulating tumor cells (CTCs) counts.36 Thus, the carcinogenesis of NRP1 was thought to be associated with cancer stem cell properties, epithelial mesenchymal transformation and lymphovascular invasion.
There were still some limitations in our study. Firstly, the IHC staining scores were evaluated by the researchers that the subjective bias may exist. Secondly, the transcriptome data in TCGA database were the total RNA isolation, and different cell types were not separated within samples. Thus, it could hardly avoid the influence of other subgroup cells in tumor tissues. Finally, the calculated bias existed in bioinformatic results, and the conclusion might be interpreted with caution.
Conclusion
In general, our results indicate that NRP1 can be regarded as a significant biomarker to predict the HNSCC patients’ prognosis. It could also be a useful therapy target that can increase the sensitivity and prolong the efficacy of immunotherapy.
Ethical Approval
This study was approved by the Ethical Committee of the Affiliated Stomatology hospital of Nanjing Medical University (No. PJ2018-051-001). All patients signed the informed consent to participate in this study. We obey the principles of the 1983 Declaration of Helsinki. In other words, all experiments in this paper obey this principle.
Funding
This work was supported by the National Natural Science Foundation of China (81772887), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, 2018-87) and Jiangsu Provincial Health Commission Scientific research project (Z2022080).
Disclosure
The authors declared that they have no conflicts of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Deleyiannis FW, Thomas DB, Vaughan TL, Davis S. Alcoholism: independent predictor of survival in patients with head and neck cancer. J Natl Cancer Inst. 1996;88(8):542–549. doi:10.1093/jnci/88.8.542
3. Kashigar A, Habbous S, Eng L, et al. Social environment, secondary smoking exposure, and smoking cessation among head and neck cancer patients. Cancer. 2013;119(15):2701–2709. doi:10.1002/cncr.28088
4. Labarge B, Hennessy M, Zhang L, et al. Human papillomavirus integration strictly correlates with global genome instability in head and neck cancer. Mol Cancer Res. 2022;20(9):1420–1428. doi:10.1158/1541-7786.MCR-21-0831
5. Pinatti LM, Walline HM, Carey TE. Human papillomavirus genome integration and head and neck cancer. J Dent Res. 2018;97(6):691–700. doi:10.1177/0022034517744213
6. Seiwert TY, Zuo Z, Keck MK, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21(3):632–641. doi:10.1158/1078-0432.CCR-13-3310
7. Gan LL, Hii LW, Wong SF, Leong CO, Mai CW. Molecular mechanisms and potential therapeutic reversal of pancreatic cancer-induced immune evasion. Cancers. 2020;12(7). doi:10.3390/cancers12071872
8. Qin A, Coffey DG, Warren EH, Ramnath N. Mechanisms of immune evasion and current status of checkpoint inhibitors in non-small cell lung cancer. Cancer Med. 2016;5(9):2567–2578. doi:10.1002/cam4.819
9. Fantin A, Vieira JM, Plein A, et al. NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood. 2013;121(12):2352–2362. doi:10.1182/blood-2012-05-424713
10. Koch S, van Meeteren LA, Morin E, et al. NRP1 presented in trans to the endothelium arrests VEGFR2 endocytosis, preventing angiogenic signaling and tumor initiation. Dev Cell. 2014;28(6):633–646. doi:10.1016/j.devcel.2014.02.010
11. Chen Z, Gao H, Dong Z, et al. NRP1 regulates radiation-induced EMT via TGF-beta/Smad signaling in lung adenocarcinoma cells. Int J Radiat Biol. 2020;96(10):1281–1295. doi:10.1080/09553002.2020.1793015
12. Ding Z, Du W, Lei Z, et al. Neuropilin 1 modulates TGF‑beta1‑induced epithelial‑mesenchymal transition in non‑small cell lung cancer. Int J Oncol. 2020;56(2):531–543. doi:10.3892/ijo.2019.4938
13. Dong Z, Zhang H, Gong X, et al. The role of the tumor microenvironment in neuropilin 1-induced radiation resistance in lung cancer cells. J Cancer. 2019;10(17):4017–4030. doi:10.7150/jca.28163
14. Stockl S, Reichart J, Zborilova M, Johnstone B, Grassel S. Semaphorin 3A-Neuropilin-1 signaling modulates MMP13 expression in human osteoarthritic chondrocytes. Int J Mol Sci. 2022;23(22). doi:10.3390/ijms232214180
15. Glinka Y, Mohammed N, Subramaniam V, Jothy S, Prud’homme GJ. Neuropilin-1 is expressed by breast cancer stem-like cells and is linked to NF-kappaB activation and tumor sphere formation. Biochem Biophys Res Commun. 2012;425(4):775–780. doi:10.1016/j.bbrc.2012.07.151
16. Luo M, Hou L, Li J, et al. VEGF/NRP-1 axis promotes progression of breast cancer via enhancement of epithelial-mesenchymal transition and activation of NF-kappaB and beta-catenin. Cancer Lett. 2016;373(1):1–11. doi:10.1016/j.canlet.2016.01.010
17. Cantuti-Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856–860. doi:10.1126/science.abd2985
18. Li B, Severson E, Pignon JC, et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17(1):174. doi:10.1186/s13059-016-1028-7
19. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–118. doi:10.1038/cr.2016.151
20. Wang SS, Liu W, Ly D, Xu H, Qu L, Zhang L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol. 2019;16(1):6–18. doi:10.1038/s41423-018-0027-x
21. Huang A, Garraway LA, Ashworth A, Weber B. Synthetic lethality as an engine for cancer drug target discovery. Nat Rev Drug Discov. 2020;19(1):23–38. doi:10.1038/s41573-019-0046-z
22. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi:10.1038/nrclinonc.2016.217
23. Chiorean EG, Coveler AL. Pancreatic cancer: optimizing treatment options, new, and emerging targeted therapies. Drug Des Devel Ther. 2015;9:3529–3545. doi:10.2147/DDDT.S60328
24. Napolitano V, Russo D, Morra F, et al. Neuropilin-1 expression associates with poor prognosis in HNSCC and elicits EGFR activation upon CDDP-induced cytotoxic stress. Cancers. 2021;13(15):56.
25. Shi F, Shang L, Yang LY, et al. Neuropilin-1 contributes to esophageal squamous cancer progression via promoting P65-dependent cell proliferation. Oncogene. 2018;37(7):935–943. doi:10.1038/onc.2017.399
26. Jin Q, Ren Q, Chang X, et al. Neuropilin-1 predicts poor prognosis and promotes tumor metastasis through epithelial-mesenchymal transition in gastric cancer. J Cancer. 2021;12(12):3648–3659. doi:10.7150/jca.52851
27. Yang B, Chen J, Teng Y. TNPO1-mediated nuclear import of FUBP1 contributes to tumor immune evasion by increasing NRP1 expression in cervical cancer. J Immunol Res. 2021;2021:9994004. doi:10.1155/2021/9994004
28. Jimenez-Hernandez LE, Vazquez-Santillan K, Castro-Oropeza R, et al. NRP1-positive lung cancer cells possess tumor-initiating properties. Oncol Rep. 2018;39(1):349–357. doi:10.3892/or.2017.6089
29. Chen C, Zhang R, Ma L, et al. Neuropilin-1 is up-regulated by cancer-associated fibroblast-secreted IL-8 and associated with cell proliferation of gallbladder cancer. J Cell Mol Med. 2020;24(21):12608–12618. doi:10.1111/jcmm.15825
30. Hansen W, Hutzler M, Abel S, et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med. 2012;209(11):2001–2016. doi:10.1084/jem.20111497
31. Roy S, Bag AK, Singh RK, Talmadge JE, Batra SK, Datta K. Multifaceted role of neuropilins in the immune system: potential targets for immunotherapy. Front Immunol. 2017;8:1228. doi:10.3389/fimmu.2017.01228
32. Liu C, Somasundaram A, Manne S, et al. Neuropilin-1 is a T cell memory checkpoint limiting long-term antitumor immunity. Nat Immunol. 2020;21(9):1010–1021. doi:10.1038/s41590-020-0733-2
33. Tavares MC, Sampaio CD, Lima GE, et al. A high CD8 to FOXP3 ratio in the tumor stroma and expression of PTEN in tumor cells are associated with improved survival in non-metastatic triple-negative breast carcinoma. BMC Cancer. 2021;21(1):901. doi:10.1186/s12885-021-08636-4
34. Ruan P, Tao Z, Tan A. Low expression of miR-30a-5p induced the proliferation and invasion of oral cancer via promoting the expression of FAP. Biosci Rep. 2018;38(1). doi:10.1042/BSR20171027
35. Connor EV, Saygin C, Braley C, et al. Thy-1 predicts poor prognosis and is associated with self-renewal in ovarian cancer. J Ovarian Res. 2019;12(1):112. doi:10.1186/s13048-019-0590-5
36. Yang C, Wei C, Wang S, et al. Elevated CD163(+)/CD68(+) ratio at tumor invasive front is closely associated with aggressive phenotype and poor prognosis in colorectal cancer. Int J Biol Sci. 2019;15(5):984–998. doi:10.7150/ijbs.29836
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.