Back to Journals » Journal of Pain Research » Volume 12
Overexpression Of miR138 Ameliorates Spared Sciatic Nerve Injury-Induced Neuropathic Pain Through The Anti-Inflammatory Response In Mice
Authors Zhu B, Gao J, Ouyang Y, Hu Z, Chen X
Received 15 June 2019
Accepted for publication 3 October 2019
Published 18 November 2019 Volume 2019:12 Pages 3135—3145
DOI https://doi.org/10.2147/JPR.S219462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Benfan Zhu,1,* Jie Gao,1–4,* Yeling Ouyang,3,4 Zhiqiang Hu,3,4 Xiangdong Chen3,4
1Department of Pain, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, People’s Republic of China; 2Department of Anesthesiology, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, People’s Republic of China; 3Department of Anesthesiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, People’s Republic of China; 4Institute of Anesthesiology and Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiangdong Chen
Department of Anesthesiology, Union Hospital, No.1277 Jiefang Avenue, Wuhan, Hubei province 43022, People’s Republic of China
Tel +86-15071096621
Fax +86-27-85351633
Email [email protected]
Background: The emerging role of inflammation in the initiation and maintenance of neuropathic pain has been confirmed. Previous studies have reported that miR138 has neuroprotective and anti-inflammatory effects in animal models of spinal cord injury and in human coronary artery endothelial cell injury, while its effect on neuropathic pain is still not known. As the mechanism of neuropathic pain remains unclear, we investigated whether miR138 is involved in the development of neuropathic pain and the role of miR138 in the modulation of inflammation in the spinal cord in a mouse model of neuropathic pain induced by spared sciatic nerve injury (SNI).
Materials and methods: Firstly, the expression of miR138 in spinal cord was evaluated on days 1, 3, 5, 7, 9 and 14 after SNI. And then, LV-miR-control and LV-miR138 were intrathecally injected 1 week before the surgery followed by investigation of the expression of miR138, mechanical allodynia and thermal hyperalgesia on day 1, 3, 5, 7, 9, 14 after SNI. Ipsilateral L4-L6 spinal cord tissue was harvested on day 14 post-SNI and detected by Western blotting, enzyme-linked immunosorbent assay or immunohischemistry.
Results: We observed decreased expression of miR138 and increased expression of proinflammatory cytokines, along with activated microglia, astrocytes and nuclear factor-κВ (NF-κВ), in the spinal cord dorsal horn after SNI. Moreover, the intrathecal upregulation of miR138 significantly alleviated SNI-induced mechanical allodynia and thermal hyperalgesia, downregulated the production of proinflammatory cytokines, and deactivated microglia, astrocytes and NF-κВ.
Conclusion: The results indicate that miR138 contributes to the development of neuropathic pain and that the overexpression of miR138 alleviates pain hypersensitivity by inhibiting proinflammatory cytokine production and glial activation, which suggests a novel target for reducing neuropathic pain.
Keywords: neuropathic pain, miR138, spared sciatic nerve injury, NF-κВ
Introduction
Peripheral nerve injury-induced neuropathic pain remains a challenging topic in clinical practice because of the complicated mechanism of its initiation and maintenance.1,2 In addition, the poor curative effects and severe side effects of traditional therapeutic methods hinder their wide and long-term application.2,3 It is very important to search for optimal strategies for the management of neuropathic pain. Inflammation and the activation of glial cells have long been demonstrated to play a pivotal role in the mechanism of neuropathic pain.4,5 Therefore, blocking inflammatory cascades by different methods has been identified as an important management strategy for ameliorating neuropathic pain.
MicroRNAs (miRNAs) are small noncoding RNA molecules (containing approximately 22 nucleotides) that are found in plants, animals and some viruses and function in RNA silencing and the posttranscriptional regulation of gene expression.6,7 In recent years, miRNAs have been found to be involved in a large number of diseases.8–10 Studies have found that some miRNAs are downregulated in pathological processes, which indicates the protective role of miRNAs in some diseases.11,12
Among these miRNAs, miRNA 138 (miR138) has been demonstrated to be decreased in the initiation and maintenance stages of multiple diseases, suggesting that the upregulation of miR138 might be beneficial.12 Previous studies have verified the role of miR138 in ameliorating traumatic brain injury (TBI)-induced cerebral damage and inhibiting the metastasis of breast cancer and non-small cell lung cancer.13–15 Recent studies have also reported the neuroprotective effect of miR138 in transected spinal cord animals.10 Additionally, the downregulation of miR138 contributes to the sustained activation of NF-κB and the inflammatory response by increasing the release of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6,12,16 which indicates the potential role of miR138 in neuropathic pain. In our pilot study, we found that the expression of miR138 decreased significantly with the development of neuropathic pain in the L4-L6 spinal dorsal horn in a mouse model of spared sciatic nerve injury (SNI). However, whether miR138 can attenuate pain hypersensitivity in SNI mice and the underlying molecular mechanisms remain unclear.
Thus, the present study was designed to investigate the role of miR138 in pain behavior and the inflammatory response and revealed the underlying mechanism in SNI-induced neuropathic pain in a mouse model.
Materials And Methods
Animals
All protocols were approved by the Institutional Animal Experimental Ethics Committee of Huazhong University of Science and Technology (LLSC 20180221) and performed in accordance with the guide for the Care and Use of Laboratory Animals of Tongji Medical College and the Declaration of Helsinki. The experiments were performed on male wild-type C57BL/6 mice (22 ± 2 g). All mice (10 mice per cage) were maintained under standard housing conditions with a 12-h/12-h light-dark cycle, and food and water were provided ad libitum.
Animal Model Of Neuropathic Pain Induced By Spared Sciatic Nerve Injury (SNI)
The mice were anesthetized with ketamine hydrochloride plus xylazine (ketamine hydrochloride: 90 mg/kg body weight; xylazine: 5 mg/kg). The skin on the lateral surface of the left thigh was incised, and an incision was made directly through the biceps femoris muscle to expose the sciatic nerve and its three terminal branches, the sural, common peroneal and tibial nerves. The tibial and common peroneal nerves were ligated, and the sural nerve was left intact. The common peroneal and tibial nerves were tightly ligated with a 5.0 silk suture and severed distal to the ligation to removed 2 ± 4 mm of the distal nerve stump. Great care was taken to avoid any contact with or stretching of the intact sural nerve. The muscle and skin were closed in two layers.17,18
RNA Extraction And Real-Time PCR
Total RNA from the ipsilateral L4-L6 spinal dorsal horn was extracted using TRIzol (Invitrogen) according to the manufacturer’s instructions on days 1, 3, 5, 7, 9, and 14 after SNI. The level of miR138 (F primer: 5ʹ-GGTGTCCGTGGAGTCGGCAA-3ʹ, R primer: 5ʹ-AACTTCACAACACCAGCTTA-3ʹ) was quantitatively analyzed using an miRNA qRT-PCR primer (Invitrogen) and Platinum SYBR Green qPCR SuperMix-UDG with ROX (Invitrogen) on an ABI 7000H instrument (Applied Biosystems) according to the manufacturer’s instructions. β-actin (F primer: 5ʹ-CACCACACCTTCTACAATGAGC-3ʹ, R primer: 5ʹ-GTGATCTCCTTCTGCATCCTGT-3ʹ) was used as an internal control. Relative quantification was calculated based on fold changes determined by the 2-ΔΔCt method.11,12
Oligonucleotide Transfection And Plasmid Construction
A miR138 mimic (5ʹ-CCUGCUUGCU-CAAAUCAAUTT-3ʹ) and miR-control (5ʹ-CAGUACUUUU-GUGUAGUACAA-3ʹ) were purchased from Invitrogen. The miRNAs at a working concentration of 50 nmol/L were transfected using RNAiMAX reagent (Invitrogen, Carlsbad, CA), and oligonucleotide transfection was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The miR-mimic and miR-control were cloned into the pLVTHM lentiviral vector (Invitrogen, Carlsbad, CA), and the recombinant plasmid was called LV-miR138.11,16
Intrathecal Injection
LV-miR-control and LV-miR138 were intrathecally injected using a modified version of a previously described method.19 In brief, a mouse was held firmly in one hand by the pelvic girdle at an angle of approximately 20 degrees above the vertebral column. A microsyringe (10 μL) was inserted into the space between the L5 and L6 spinous processes so that it entered the groove between the spinous and transverse processes. A sudden slight lateral flick of the tail or paw movement indicated successful entry into the dorsal subarachnoid space. Then, LV-miR-control or LV-miR138 (5 μL, 1 × 105 U) were slowly injected over a 1-min period, and the needle was left in place for a further 5 s. No abnormal behavioral consequences of the injection were observed.
Treatment And Groups
The day of surgery was considered day 0 (Figure 1). Forty mice were randomly divided into 4 groups: (1) the control (ctrl) group; (2) the sham group; (3) the SNI+LV-miR-control (ctrl) group; (4) and the SNI+LV-miR138 (miR138) group. There were 10 mice in each group. The mice in the sham group only underwent surgery to separate the sciatic nerve, and the mice in the SNI group underwent sciatic nerve injury surgery. LV-miR-control and LV-miR138 were intrathecally injected 1 week before the surgery. To control measurement bias, all behavioral tests and other evaluated were performed in a double-blind manner. Additionally, after all tests, we found that there were no differences between the sham mice and the control mice; thus, we included only the sham mice in the results.
 |
Figure 1 The timeline of the experiment. |
Behavioral Tests
The behavioral tests were performed on days 1, 3, 5, 7, 9, 11, and 14 after SNI (Figure 1). To measure the mechanical withdrawal threshold (MWT; in grams) and paw withdrawal latency (PWL; in seconds), mice were habituated to plastic cages with a metal mesh floor (for the MWT test) or a glass floor (for the PWL test) for at least 30 min.20,21 An electronic von Frey device (IITC Life Science Inc., USA) was used to stimulate the midplantar surface of the hind paws, and the corresponding readout was recorded until a withdrawal reflex was observed. The measurement was repeated for the same paw three times with a 5 min interval. The Hargreaves Test (Type 7370, Planter Test Instrument, UgoBasile, Italy) was employed to produce a beam of radiant heat to stimulate the mid-plantar surface of the hind paws. and the readout was recorded until the mouse lifted its paw away. The cut-off time was set at 20 s to prevent damage to the paws of the mice. The measurement was repeated for the same paw three times with a 5 min interval, and the mean average of three measures was calculated. After the behavioral tests on day 14, the mice were decapitated under ketamine hydrochloride and xylazine anesthesia for further analysis.
Western Blot Analysis
The ipsilateral L4-L6 spinal dorsal horn was collected on day 14 (Figure 1) and homogenized in RIPA buffer (Keygen, Biotech, China) supplemented with a protease inhibitor cocktail and phosphatase inhibitors. Proteins were separated by 10% SDS-PAGE and electrophoretically transferred to PVDF membranes. The membranes were blocked with 5% nonfat milk for 2 h at room temperature and then incubated with primary antibodies against GFAP (1:1000, Abcam, Cambridge), Iba-1 (1:1000, Abcam, Cambridge), NF-κB p65, p-p65 (1:1000, Millipore, Temecula, CA), β-actin and lamin B (1:1000, Cell Signaling Technology, Beverly, MA) overnight at 4°C. Then, the membranes were incubated with a corresponding secondary antibody for 1 h at room temperature. The bands were analyzed using ImageJ Software (version 1.45s; NIH, Bethesda, MD, USA).4
Enzyme-Linked Immunosorbent Assay
For ELISA, ipsilateral L4-L6 spinal dorsal horn tissue samples were collected after MWT and PWL measurement on day 14 (Figure 1). The contents of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and interleukin (IL)-6 (μg/mg) were quantified using enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, San Diego, CA) according to the manufacturer’s instructions. The samples were measured in duplicate. The readings from each sample were normalized to the protein concentration.19,20
Immunofluorescence Assay
After the behavioral tests on day 14 (Figure 1), 5-mm paraffin-embedded L4-L6 ipsilateral spinal cord sections were permeabilized with PBS containing 1% Triton X-100 for 10 min, blocked with 10% normal goat serum for 1 h, and then incubated overnight at 4°C with a rabbit-anti-Iba-1 (1:100, Abcam, Cambridge, MA, USA) or rabbit-anti-GFAP (1:100, Abcam, Cambridge, MA, USA) primary antibody. After 3 washes with PBS, the sections were incubated with donkey anti-rabbit IgG (1:200, Abbkine, California, USA) for 1 h at room temperature followed by DAPI (Sigma, USA). Images were captured using an Olympus fluorescence microscope (Olympus, Tokyo, Japan).9
Statistical Analysis
The data are presented as the mean ± SD. GraphPad Prism 5.0 (San Diego, CA, USA) software was used for all analyses. Differences in behavior, the results of ELISA, and immunofluorescence were analyzed by one-way or two-way repeated-measures analysis of variance (ANOVA) followed by Tukey’s post hoc test. The correlation between the MWT and PWL and the expression of miR138 were evaluated by logistic regression. Statistical significance was defined as P < 0.05.
Results
The Decreased Expression Of miR138 Was Correlated With The Development Of Pain Hypersensitivity
First, the behavioral tests were performed after SNI surgery. The results suggested that the MWT (Figure 2A, from 8.253 ± 0.453 g to 1.205 ± 0.469 g, P<0.05, P<0.01, P<0.001, two-way ANOVA) and PWL (Figure 2B, from 10.8 ± 0.8 s to 4.7 ± 0.9 s, P<0.05, P<0.01, P<0.001, two-way ANOVA) declined sharply after the first 3 days following SNI. They were persistently maintained at a low level for at least 14 days (P<0.001, two-way ANOVA). Then, the expression of miR138 was detected. Interestingly, the expression of miR138 decreased upon the development of mechanical allodynia and thermal hyperalgesia (Figure 2C, P<0.01, P<0.001, two-way ANOVA) and showed a similar trend as that of the MWT and PWL in SNI mice. We also analyzed the relationship between the expression of miR138 and the MWT and PWL. Using logistic regression, we found that the changes in the expression of miR138 were closely correlation with the MWT and PWL (MWT: R2=0.99, 95% CI= −1 to-0.96, P<0.001; PWL: R2=0.95, 95% CI=−0.99 to −0.96, P<0.05, logistic regression), which indicated that the decreased expression of miR138 contributed to SNI-induced pain hypersensitivity.
Overexpression Of miR138 Alleviated SNI-Induced Mechanical Allodynia And Thermal Hyperalgesia
To examine whether the increased expression of miR138 in the spinal cord can alleviate pain hypersensitivity, SNI mice were pretreated with LV-miR138 via intrathecal injection. The results indicated that the expression of miR138 in the spinal cord was significantly increased in SNI mice after pretreatment with miR138 (Figure 3A, P<0.01, one-way ANOVA). It was also shown that there was only a slight decrease in the MWT (Figure 3B, from 8.086 ± 0.497 g to 6.607 ± 0.494 g, P<0.05, P<0.01, P<0.001, two-way ANOVA) and PWL (Figure 3C, 10.5 ± 0.8 s to 8.8 ± 0.6 s, P<0.05, P<0.01, P<0.001, two-way ANOVA) after pretreatment with miR138 and that the MWT and PWL maintained at a relatively high level after SNI, which indicated that, compared with the mice in the SNI+ctrl group, the mice in the SNI were insensitive to the mechanical and thermal stimuli. It was also shown that the expression of miR138 was closely correlated with changes in the MWT (R2= 0.96, 95% CI= −0.99 to-0.95, P<0.001, logistic regression) and PWL (R2=0.99, 95% CI=−0.97 to −0.94, P<0.05, logistic regression). There was no observed effect of LV-miR-control on pain behavior. These results confirmed that the overexpression of miR138 can alleviate pain hypersensitivity.
The Levels Of Proinflammatory Cytokines Were Decreased After miR138 Was Upregulated In The Spinal Cord
As proinflammatory cytokines are important for the initiation and maintenance of neuropathic pain,4 the levels of TNF-α, IL-1β and IL-6 (Figure 4A–C, one-way ANOVA) in the ipsilateral L4-L6 spinal dorsal horn were examined by ELISA on day 14 after SNI. The results showed that the levels of TNF-α, IL-1β and IL-6 were dramatically increased after SNI (Figure 4A–C, P<0.001, one-way ANOVA), while pretreatment with miR138 via intrathecal injection significantly inhibited the production and release of these proinflammatory cytokines (Figure 4A–C, P<0.01, one-way ANOVA). These results confirmed that the inflammation that occurs during SNI induces neuropathic pain and indicated the important role of miR138 in inflammatory responses.
Upregulation Of miR138 Suppressed Glial Activation
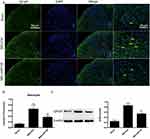
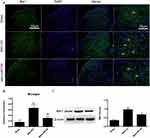
The activation of glial cells is involved in the inflammatory response.22–24 GFAP (Figure 5A and B) and Iba-1 (Figure 6A and B) were used as markers for astrocytes and microglia, respectively. There was a high number of GFAP-positive cells after SNI (Figure 5A and B, P<0.001, one-way ANOVA), and the number of these cells was significantly reduced by pretreatment with miR138 (Figure 5A and B, P<0.01, one-way ANOVA) in the L4-L6 ipsilateral spinal cord dorsal horn. We also found that the level of Iba-1-positive cells detected by immunofluorescence staining was significantly increased after SNI (Figure 6A and B, P<0.001, one-way ANOVA) but decreased sharply after treatment with miR138 (Figure 6A and B, P<0.01, one-way ANOVA). The Western blotting results of the expression of GFAP (Figure 5C, P<0.01, one-way ANOVA) and Iba-1 (Figure 6C, P<0.05, one-way ANOVA) were consistent with the results of immunofluorescence staining. Collectively, this evidence confirmed that the upregulation of miR138 in the spinal cord can significantly decrease the activation of astrocytes and microglia, which are activated by SNI.
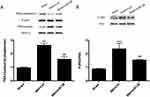
Activation Of Nuclear Factor-κB (NF-κB) Was Suppressed After An Increase In The Expression Of miR138
NF-κB has been implicated in the inflammatory response and neuropathic pain.24 To determine the role of miR138 in the activation of NF-κB, Western blotting was used to detect NF-κB activity in the L4-L6 ipsilateral spinal dorsal horn. The results showed that the SNI-induced increase (Figure 7A, P<0.001, one-way ANOVA) in the nuclear-cytoplasmic ratio of p65 was sharply decreased after pretreatment with miR138 (Figure 7A, P<0.01, one-way ANOVA) on day 14 after SNI. Similarly, p65 phosphorylation at Ser 536 was increased after SNI (Figure 7B, P<0.001, one-way ANOVA) but was dramatically reduced by miR138 (Figure 7B, P<0.01, one-way ANOVA).
Discussion
In our present study, we showed that the upregulation of miR138 in the spinal cord ameliorated neuropathic pain in a mouse model of SNI. We used a gene intervention technique to identify the role of miR138 in neuropathic pain. The results suggested that the therapeutic effect of increased miR138 expression on neuropathic pain probably relied on the anti-inflammatory effects of miR138 because the upregulation of miR138 suppressed the production of proinflammatory factors and the activation of astrocytes and microglia in the spinal cord. Furthermore, the activation of NF-κB was also inhibited after the upregulation of miR138 in the spinal dorsal horn.
The downregulated expression of miR138 has been demonstrated to contribute to the exacerbation of brain damage and spinal injury.10,13 Therefore, facilitating the expression of miR138 during the initiation and maintenance of injury represents a promising therapeutic approach. Similarly, neuropathic pain and subsequent pain hypersensitivity result from nerve injury, which indicates that miR138 might contribute to neuropathic pain. In addition, a previous study also revealed that decreased miR138 activates the NF-κB pathway and the inflammatory response.12,16 To determine the role of miR138 in neuropathic pain and the inflammatory response, specific miR138 mimics were used in our study. The results showed that the upregulation of miR138 alleviated the proinflammatory response in the spinal cord and therefore ameliorated neuropathic pain induced by SNI.
Microglia and astrocytes play key roles in maintaining the hemostasis of the nervous system.22–24 Previous studies have suggested that microglia contribute to protective effects against nerve injury and that astrocytes provide structural and nutritive support for neurons. However, in the early stage of nerve injury, activated microglia and astrocytes produce and release a large number of cytokines and chemokines. It has been reported that the activation of microglia and astrocytes facilitates the development of mechanical allodynia and thermal hyperalgesia after nerve injury.25–27 Thus, inhibiting the activation of microglia and astrocytes may attenuate nerve injury-induced pain hypersensitivity. In our present study, the activation of microglia and astrocytes was significantly decreased by the overexpression of miR138.
A variety of inflammatory cytokines and chemokines, such as TNF-α, IL-1β and IL-6, are sharply increased within a few days after nerve injury.28 Previous studies have reported that the regional administration of exogenous IL-6 can induce significant pain hypersensitivity. TNF-α is one of the most recognized cytokines, and the intrathecal injection of a neutralizing antibody against TNF can also attenuate mechanical allodynia and thermal hyperalgesia in several animal models of neuropathic pain.29 Additionally, the increased levels of these proinflammatory cytokines and chemokines contribute to glial activation. Reciprocally, activated glia then exacerbate the inflammatory response.25 Thus, the downregulation of the production of inflammation and the activation of glia may be beneficial. In the present study, the increased expression of miR138 in the spinal cord significantly decreased the expression of these factors.
It has long been demonstrated that NF-κB is involved in neuropathic pain, and the phosphorylation of NF-κB p65 promotes the degradation of IκB and subsequently activates p65.24 Activated NF-κB facilitates p65 translocation to the nucleus from the cytoplasm and then increases the transcription and expression of its target genes, such as TNF-α, IL-6 and IL-1β.29,30 In addition, activated NF-κB contributes to the activation of astrocytes and microglia. Reciprocally, NF-κB can also be activated by increased levels of proinflammatory cytokines.31 Previous studies have also demonstrated that decreased expression of miR138 accelerates the activation of NF-κB. In our mouse model of neuropathic pain, pretreatment with miR138 significantly decreased the nuclear translocation and phosphorylation of NF-κB, which suggests that the therapeutic effect of miR138 on neuropathic pain might correlate with the inhibition of NF-κB in the spinal cord.
In the present study, a mouse model of spared sciatic nerve injury was used to simulate the pain symptoms of clinical patients. Consistently, the expression of miR138 remains steady for nearly 16 days after injection,9,11 and consistent with earlier studies, and our results showed that the expression of miR138 was maintained at a high level for 14 days after SNI.
There are some limitations to this study. First, as seen in the results, pretreatment with miR138 did not completely alleviate mechanical allodynia and thermal hyperalgesia, indicating that other factors or signaling pathways must be involved in neuropathic pain; this will be determined in future studies. In addition, we only pretreated the mice with miR138 before the establishment of neuropathic pain. The effects of treatment with miR18 after the establishment of neuropathic pain also needs to be investigated. Second, even though the overexpression of miR138 had a beneficial effect on neuropathic pain, the possible side effects or adverse effects should be further evaluated. Third, the effect of the downregulated expression of miR138 on neuropathic pain should also be investigated in the future, even though the expression of miR138 is decreased in SNI mice.
In conclusion, our present study demonstrated that the upregulation of miR138 in the spinal cord significantly ameliorates spared sciatic nerve injury-induced neuropathic pain, probably through an anti-inflammatory effect that is correlated with the suppression of the NF-κB signaling pathway. Additionally, the present results indicate a novel target for clinical research for neuropathic pain.
Abbreviations
miR138, microRNA 138; SNI, spared sciatic nerve injury; MWT, mechanical withdrawal threshold; PWL, paw withdrawal latency; NF-κB, nuclear factor-κB; IκB, inhibitor of kappa B.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain an updated grading system for research and clinical practice. Pain. 2016;157:1599–1606. doi:10.1097/j.pain.0000000000000492
2. Deng Y, Luo L, Hu Y, et al. Clinical practice guidelines for the management of neuropathic pain a systematic review. BMC Anesthesiol. 2016;16:12. doi:10.1186/s12871-015-0150-5
3. Kim HK, Kwon JY, Yoo C, et al. The analgesic effect of rolipram, a phosphodiesterase 4 inhibitor, on chemotherapy-induced neuropathic pain in rats. Anesth Analg. 2015;121:822–828. doi:10.1213/ANE.0000000000000853
4. Zhu A, Shen L, Xu L, et al. Suppression of Wnt5a, but not Wnts, relieves chronic post-thoracotomy pain via anti-inflammatory modulation in rats. Biochem Biophys Res Commun. 2017;493:474–480. doi:10.1016/j.bbrc.2017.08.167
5. Gwak YS, Kang J, Unabia GC, et al. Spatial and temporal activation of spinal glial cells role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol. 2012;234:362–372. doi:10.1016/j.expneurol.2011.10.010
6. Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi:10.1038/nature02871
7. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi:10.1016/S0092-8674(04)00045-5
8. Liu X, Wang C, Chen Z, et al. MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. Biochem J. 2011;440:23–31. doi:10.1042/BJ20111006
9. Yeh YM, Chuang CM, Chao KC, et al. MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis by targeting SOX4 and HIF-1α. I Int J Cancer. 2013;133:867–878. doi:10.1002/ijc.28086
10. Qian BJ, You L, Shang FF, et al. Vimentin regulates neuroplasticity in transected spinal cord rats associated with micRNA138. Mol Neurobiol. 2015;51:437–447. doi:10.1007/s12035-014-8745-2
11. Peng XH, Huang HR, Lu J, et al. MiR-124 suppresses tumor growth and metastasis by targeting Foxq1 in nasopharyngeal carcinoma. Mol Cancer. 2014;13:186. doi:10.1186/1476-4598-13-186
12. Gong H, Song L, Lin C, et al. Downregulation of miR-138 sustains NF-κB activation and promotes lipid raft formation in esophageal squamous cell carcinoma. Clin Cancer Res. 2013;19:1083–1093. doi:10.1158/1078-0432.CCR-12-3169
13. Miao W, Bao TH, Han JH, et al. Voluntary exercise prior to traumatic brain injury alters miRNA expression in the injured mouse cerebral cortex. Braz J Med Biol Res. 2015;48:433–439. doi:10.1590/1414-431x20144012
14. Zhang J, Liu D, Feng Z, et al. MicroRNA-138 modulates metastasis and EMT in breast cancer cells by targeting vimentin. Biomed Pharmacother. 2016;77:135–141. doi:10.1016/j.biopha.2015.12.018
15. Zhang H, Zhang H, Zhao M, et al. MiR-138 inhibits tumor growth through repression of EZH2 in non-small cell lung cancer. Cell Physiol Biochem. 2013;31:56–65. doi:10.1159/000343349
16. Li J, Wang H, Yao Y, et al. Overexpression of microRNA‐138 alleviates human coronary artery endothelial cell injury and inflammatory response by inhibiting the PI3K/Akt/eNOS pathway. J Cell Mol Med. 2017;21:1482–1491. doi:10.1111/jcmm.2017.21.issue-8
17. Wang CL, Yang DJ, Yuan BY, et al. Antiallodynic effects of endomorphin-1 and endomorphin-2 in the spared nerve injury model of neuropathic pain in mice. Anesth Analg. 2017;125:2123–2133. Epub ahead of print. doi:10.1213/ANE.0000000000002318
18. Ohsawa M, Mutoh J, Yamamoto S, et al. Involvement of protein isoprenylation in neuropathic pain induced by sciatic nerve injury in mice. Neurosci Lett. 2014;564:27–31. doi:10.1016/j.neulet.2014.01.039
19. Ren BX, Ji Y, Tang JC, et al. Effect of Tanshinone IIA intrathecal injections on pain and spinal inflammation in mice with bone tumors. Genet Mol Res. 2015;14:2133–2138. doi:10.4238/2015.March.20.24
20. Nascimento FP, Magnussen C, Yousefpour N, et al. Sympathetic fibre sprouting in the skin contributes to pain-related behaviour in spared nerve injury and cuff models of neuropathic pain. Mol Pain. 2015;11:59. doi:10.1186/s12990-015-0062-x
21. Decosterd I, Woolf CJ. Spared nerve injury an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi:10.1016/S0304-3959(00)00276-1
22. Ni HD, Yao M, Huang B, et al. Glial activation in the periaqueductal gray promotes descending facilitation of neuropathic pain through the p38 MAPK signaling pathway. J Neurosci Res. 2015;94:50–61. doi:10.1002/jnr.23672
23. Piotrowska A, Kwiatkowski K, Rojewska E, et al. Maraviroc reduces neuropathic pain through polarization of microglia and astroglia - evidence from in vivo and in vitro studies. Neuropharmacology. 2016;108:207–219. doi:10.1016/j.neuropharm.2016.04.024
24. Lim H, Lee H, Noh K, et al. IKK/NF-κB-dependent satellite glia activation induces spinal cord microglia activation and neuropathic pain after nerve injury. Pain. 2017;158:1666–1677. doi:10.1097/j.pain.0000000000000959
25. Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi:10.1002/(ISSN)1098-1136
26. Liu X, Liu H, Xu S, et al. Spinal translocator protein alleviates chronic neuropathic pain behavior and modulates spinal astrocyte-neuronal function in rats with L5 spinal nerve ligation model. Pain. 2016;157:103–116. doi:10.1097/j.pain.0000000000000339
27. Guan Z, Kuhn JA, Wang X, et al. Injured sensory neuron-derived CSF1 induces microglia proliferation and DAP12-dependent pain. Nat Neurosci. 2016;19:94–101. doi:10.1038/nn.4189
28. Coronel MF, Raggio MC, Adler NS, et al. Progesterone modulates pro-inflammatory cytokine expression profile after spinal cord injury: implications for neuropathic pain. J Neuroimmunol. 2016;292:85–92. doi:10.1016/j.jneuroim.2016.01.011
29. Kawasaki Y, Zhang L, Cheng JK, et al. Cytokine mechanisms of central sensitization distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi:10.1523/JNEUROSCI.3338-07.2008
30. Obuchowicz E, Bielecka-Wajdman AM, Paul-Samojedny M, et al. Different influence of antipsychotics on the balance between pro- and anti-inflammatory cytokines depends on glia activation: an in vitro study. Cytokine. 2017;94:37–44. doi:10.1016/j.cyto.2017.04.004
31. Wu Y, Chen X, Ge X, et al. Isoliquiritigenin prevents the progression of psoriasis-like symptoms by inhibiting NF-κB and proinflammatory cytokines. J Mol Med. 2016;94:195–206. doi:10.1007/s00109-015-1338-3
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.