Back to Journals » Cancer Management and Research » Volume 11
Overexpression of KRT17 promotes proliferation and invasion of non-small cell lung cancer and indicates poor prognosis
Authors Wang Z, Yang MQ, Lei L, Fei LR, Zheng YW, Huang WJ, Li ZH, Liu CC, Xu HT
Received 10 June 2019
Accepted for publication 22 July 2019
Published 7 August 2019 Volume 2019:11 Pages 7485—7497
DOI https://doi.org/10.2147/CMAR.S218926
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Zhao Wang,1,2 Mai-Qing Yang,1,3 Lei Lei,1 Liang-Ru Fei,1 Yi-Wen Zheng,1 Wen-Jing Huang,1 Zhi-Han Li,1 Chen-Chen Liu,1 Hong-Tao Xu1
1Department of Pathology, The First Hospital and College of Basic Medical Sciences of China Medical University, Shenyang 110001, People’s Republic of China; 2Department of Pathology, General Hospital of Heilongjiang Land Reclamation Bureau, Harbin 150088, People’s Republic of China; 3Department of Pathology, Changyi People’s Hospital, Changyi, People’s Republic of China
Purpose: Keratin 17 (KRT17) is a 48 KDa type I intermediate filament, which is mainly expressed in epithelial basal cells. KRT17 has been shown to be overexpressed in many malignant tumors and play an important role in the occurrence and development of tumors. Therefore, this study explored the role and underlying mechanism of KRT17 in non-small cell lung cancers (NSCLC).
Methods: KRT17 expression and its correlations with clinicopathological factors were examined in lung cancer tissues by immunohistochemistry. The prognosis value of KRT17 in NSCLCs was retrieved from The Cancer Genome Atlas (TCGA) online databases. The expression level of KRT17 was increased or decreased by KRT17 gene transfection or small RNA interference in lung cancer cells, respectively. Further, proliferation and invasiveness of lung cancer cells were determined by cell proliferation and invasion assays, respectively. Finally, expression levels of proteins related to Wnt signaling pathways and epithelial mesenchymal transition (EMT) were detected by Western blot.
Results: The expression level of KRT17 in NSCLCs was significantly higher than normal lung tissues. High expression of KRT17 predicted poor prognosis of patients with NSCLCs, especially lung adenocarcinomas, and was correlated with poor differentiation and lymphatic metastasis. Overexpression of KRT17 enhanced, while KRT17 knockdown inhibited, the proliferation and invasiveness of lung cancer cells. Overexpression of KRT17 up-regulated β-catenin activity and levels of Wnt target genes, such as cyclin D1, c-Myc, and MMP7. Moreover, KRT17 promoted EMT by up-regulating Vimentin, MMP-9, and Snail expression and down-regulating E-cadherin expression.
Conclusion: Overexpression of KRT17 is common in NSCLCs and indicates poor prognosis. Overexpression of KRT17 enhances the proliferation and invasiveness of NSCLC cells by activating the Wnt signaling pathway and EMT process. KRT17 is a potential indicator of NSCLC progression and poor survival.
Keywords: keratin 17, non-small cell lung cancer, Wnt signaling pathway, epithelial mesenchymal transition, proliferation, invasion
Introduction
Lung cancer is the most common cause of death in men in both developed and developing countries, and it has overtaken breast cancer as the main cause of cancer-related deaths among women in developing countries.1,2 The etiology of lung cancer is very complex. Most lung cancer is first detected when the volume of the mass reaches very large. Due to extensive local spread and distant metastasis, about 60% of lung cancer patients are incurable.3 Therefore, there is an urgent clinical need to find novel biomarkers as new targets for lung cancer therapy.
Keratins are intermediate fibers in epithelial cells, about 10 nm long, which are a component of the cytoskeleton and play a major role in cell protection and structural support.4,5 In humans, there are 54 functional keratins. Keratin 17 (KRT17, CK17) belong to type I keratin with a molecular weight of 48 KDa.6 Through extensive tissue screening, it was found that KRT17 was poorly expressed in mature epithelial tissues, selectively expressed in hyperplasia active cells such as stem cells or reserve cells in the epithelium, and also expressed in glandular myoepithelium. Surprisingly, KRT17 was regenerated and highly expressed in cancer tissues.7 KRT17 has been used as a diagnostic marker in various tumors, such as breast cancer,8,9 skin squamous cell carcinoma,10 and cervical cancer.11
It was found that some keratins have regulatory functions, including cell movement, cell size, proliferation, apoptosis, and cell signal transduction.12–15 In the early stage of human skin injury, expression levels of KRT17, KRT6, and KRT16 increased under the induction of inflammatory factors, and the time of wound healing was significantly shortened.16 In a mouse model of skin basaloid tumors with Gli2 gene transfection, KRT17 gene knockout significantly inhibited tumor development and growth.17 KRT17 was also found to be overexpressed in gastric cancer,12 cervical malignancy,11 breast carcinoma,8 oral squamous cell carcinoma,18 and pancreatic cancer,19 and is associated with poor prognosis of cervical cancer, gastric cancer, and oral squamous cell carcinoma.11,12,18 However, the function of KRT17 in the pathogenesis and progression of lung cancers remains unclear.
In this study, we found that KRT17 was overexpressed in non-small cell lung cancer (NSCLC) tissues and associated with poor prognosis. KRT17 promoted the proliferation and invasiveness of NSCLC by activating the Wnt signaling pathway and epithelial mesenchymal transition (EMT) process. Our findings suggested that KRT17 could be a valuable indicator of NSCLC progression and poor survival.
Materials and methods
Tissue specimens
Tissue specimens from 158 NSCLC patients who underwent surgical resection at the First Hospital of China Medical University between 2012 and 2015 were selected from the archival files of the Department of Pathology. Some lung cancer samples (32 cases) were accompanied by corresponding normal lung tissue samples. The age of patients ranged from 22 to 76 years, with a mean age of 54 years. There were 118 male and 40 female patients. The histological diagnoses and differentiation grades of the samples were classified according to the World Health Organization classification system as squamous cell carcinoma (n=78) and adenocarcinoma (n=80). The cancers were classified as well-differentiated (25 cases), moderately differentiated (92 cases), and poorly differentiated (41 cases). According to the seventh edition of the International Union against Cancer TNM Staging System for Lung Cancer,20 patients were categorized as stage I (n=44), II (n=61), III (n=51), and IV (n=2). Lymph node metastases were found in 85 cases. All patients had provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki, and under the regulations of the Institutional Review Board of China Medical University.
Immunohistochemistry
An immunohistochemical assay was performed as previously described.21 KRT17 rabbit polyclonal antibody (1:1000; Proteintech, Wuhan, China) was used. The tissue sections were reviewed by two experienced evaluators without prior knowledge of the patient data. Five views were examined per slide, and 100 cells were observed per view at 400× magnification. Immunohistochemical results were scored considering both the proportion of positive cells and intensity of cell staining. The intensity of immunostaining was defined as follows: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The frequency of positive cells was scored as follows: 0 (<5%), 1 (5–25%), 2 (26–50%), 3 (51–75%), and 4 (>75%). The value obtained by multiplying the two scores was the final score of each case. Cases with a score less than 6 were classified as low expression of KRT17, while a score greater than or equal to 6 was classified as high expression of KRT17.
Cell culture and transfection
Human lung cancer cell lines A549, H1299, and SK-MES-1 were purchased from American Type Culture Collection (Manassas, VA, USA) and validated by a DNA profiling assessment (short tandem repeat, STR). A549 and H1299 were cultured in RPMI 1640 (Gibco, Invitrogen, NY, USA), whereas SK-MES-1 was cultured in DMEM (Gibco, Invitrogen, NY, USA), supplemented with 10% fetal bovine serum (FBS; Gibco, Invitrogen, NY, USA) at 37°C and 5% CO2. The cells were grown in sterile culture dishes and passaged every 1 or 3 d using 0.25% trypsin (Gibco, Invitrogen, NY, USA).
For transfection, the cells were seeded in a six-well plate 24 h or 48 h before the experiment. The KRT17 plasmid and empty control vectors were purchased from Genechem (Shanghai, China). Small interfering RNA (siRNA) against KRT17 (KRT17-siRNA) and control siRNA were synthesized by RiboBio (Guangzhou, China). The plasmids or siRNAs were transfected into cells using Lipofectamine® 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Western blot
Total protein of cells was extracted with cell lysis buffer (Pierce, Rockford, IL, USA). Cell lysates were separated by 10% SDS-PAGE and then transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5% non-fat milk for 2 h and incubated with corresponding antibodies overnight at 4°C. The primary antibodies used were as follows: KRT17 (1:1000, Proteintech, Wuhan, China), active-β-catenin (1:1000, Cell Signaling Technology, MA, USA), c-Myc (1:1000, Proteintech, Wuhan, China), cyclin D1 (1:100, Santa Cruz Biotechnology, CA, USA), MMP7 (1:100, Santa Cruz Biotechnology, CA, USA), GAPDH (1:2000, Santa Cruz Biotechnology, CA, USA), E-cadherin (1:1000, Cell Signaling Technology, MA, USA), Snail (1:500, Proteintech, Wuhan, China), MMP-9 (1:1000, Proteintech, Wuhan, China), and Vimentin (1:2000, Cell Signaling Technology, MA, USA). The membranes were incubated with diluted secondary antibody for 2 h. Protein bands were visualized using an ECL kit (Pierce) and detected using a bioimaging system (DNR, Jerusalem, Israel). GAPDH was used as a loading control. Image J software was used to quantify the results.
Cell proliferation assay
Cells were seeded in a 96-well plate at 3000 cells/well. Cell proliferation was evaluated using the Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Tokyo, Japan) for 5 d. The CCK-8 reagent was added into each well at a 1:10 (v/v) dilution per 100 µL and incubated for 2 h at 37°C. The results were analyzed using an automated plate reader at 450 nm.
Colony formation assay
Cells were plated on 6 cm cell culture dishes (800 cells per dish) and incubated for 10–14 d. The culture medium was refreshed every 4–5 d according to the color of the culture medium. The cells were fixed with methanol for 15 min and stained with crystal violet for 5 min. Colonies with more than 50 cells were counted.
Matrigel invasion assay
Transwell chambers (Costar, Cambridge, MA, USA) precoated with Matrigel (BD Biosciences, San Jose, CA, USA) were used to detect the invasiveness of the transfected cells. Briefly, 100 µL of Matrigel (1:7 dilution) was applied to each chamber, and the chambers were placed in a 37°C incubator for 2 h. Subsequently, 12×104 cells, which were cultured in serum-free medium, were added into the upper chamber, and 0.6 mL medium supplemented with 20% FBS was added to the lower chamber as a chemoattractant. After 20–40 h of incubation, the filters were fixed with 4% paraformaldehyde for 20 min and stained with hematoxylin for 10 min. The non-invading cells on the upper surface were removed by scraping with a cotton swab. The number of cells that penetrated the membrane was counted under a microscope from five randomly selected fields.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 software (Chicago, IL, USA) and GraphPad Prism 5.0 software (La Jolla, CA, USA). The Chi–square test was used to examine possible correlations between KRT17 expression and clinicopathological factors. Other results were analyzed using two-tailed Student’s t-test. P-values less than 0.05 were considered statistically significant.
Results
Upregulated KRT17 expression in lung cancer tissues
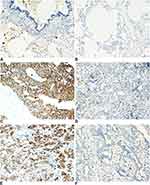
We examined the expression of KRT17 in 158 lung cancers and 32 normal lung tissue samples. The expression of KRT17 was only observed in a few basal cells in bronchial epithelium in normal tissues, but negative in matured bronchial epithelia and alveolar epithelia (Figure 1A and B). High expression of KRT17 was found in 81 cases (51%) of NSCLC, including 56 cases (71.8%) of lung squamous cell carcinomas (LUSC) (Figure 1C) and 25 cases (31.2%) of lung adenocarcinomas (LUAD) (Figure 1E). There were 22 cases (28.2%) of LUSCs (Figure 1D) and 55 cases (68.8%) of LUADs (Figure 1F) with low expression of KRT17. The expression level of KRT17 was significantly higher in both LUAD and LUSC tissues than that in normal lung tissues (P<0.001) (Table 1). To confirm the expression level of KRT17 in lung cancer tissues, we retrieved and analyzed the mRNA expression levels of KRT17 using the online database UALCAN (http://ualcan.path.uab.edu/analysis.html).22 The expression level of KRT17 was significantly elevated in both LUADs (P<0.001) and LUSCs (P<0.001) compared to normal lung tissues (Figure S1).
 |
Table 1 Correlations between KRT17 expression and clinicopathological factors in lung cancers |
KRT17 expression correlates with clinicopathological factors and indicates poor prognosis in non-small cell lung cancer patients
The high expression of KRT17 was associated with the histological type (P<0.001), lymph node metastasis (P=0.040), differentiation (P=0.011), age (P=0.024), and gender (P<0.001), but did not correlate with TNM stage (P=0.121), of NSCLC (Table 1). In LUADs, high KRT17 expression was associated with poor differentiation (P=0.036), advanced TNM stage (P=0.005) and lymph node metastasis (P=0.011). However, KRT17 expression was not correlated with the age (P=0.100) and gender (P=0.183) of LUAD patients (Table 2). In LUSCs, high expression of KRT17 was only associated with gender (P=0.002), but not correlated with age (P=0.990), differentiation (P=0.982), TNM stage (P=0.974), or lymphatic metastasis (P=0.431) (Table 3).
 |
Table 2 Correlations between KRT17 expression and clinicopathological factors in lung adenocarcinomas |
 |
Table 3 Correlations between KRT17 expression and clinicopathological factors in lung squamous cell carcinomas |
To further investigate the prognostic value of KRT17, we retrieved the Kaplan-Meier curves of KRT17 in lung cancers from two online databases, UALCAN and KM-plotter (http://kmplot.com/analysis/).23 In total lung cancers, the survival time was shorter in patients with high KRT17 expression than in patients with low KRT17 expression (P<0.001, Supplementary Figure 1). High expression of KRT17 also indicated poor survival in LUADs (P=0.014, Supplementary Figure 1), which was consistent with total lung cancers. However, KRT17 expression was not correlated with LUSC prognosis (P=0.890, Figure S1).
KRT17 promotes proliferation, colony formation, and invasiveness of non-small lung cancer cells
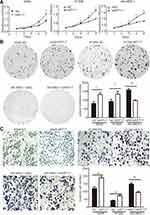
We elevated KRT17 expression by gene transfection in A549 (A549-KRT17) and H1299 (H1299-KRT17) cells. Further, KRT17 expression was knocked down by siRNA interference in SK-MES-1 cells (SK-siKRT17). In A549-KRT17 and H1299-KRT17 cells, overexpression of KRT17 promoted proliferation (P<0.05 and P<0.05, respectively) (Figure 2A) colony formation (P=0.010 and P=0.032, respectively) (Figure 2B) and invasiveness (P=0.002 and P=0.001, respectively) (Figure 2C). In contrast, down-regulation of KRT17 inhibited proliferation (P<0.05), colony formation (P=0.003), and invasiveness (P<0.001) of SK-siKRT17 cells (Figure 2A–C).
KRT17 enhances β-catenin activity and increases expression levels of Wnt target genes
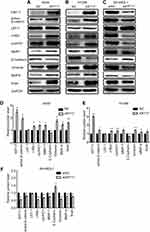
We investigated the effects of KRT17 on β-catenin and LFF-1 expression, key proteins of the Wnt signaling pathway, and the target proteins of the Wnt pathway, such as cyclin D1, c-Myc, and MMP7. KRT17 was highly expressed in A549-KRT17 and H1299-KRT17 cells. During KRT17 overexpression, expression levels of active-β-catenin, cyclinD1, c-Myc, and MMP7 were upregulated in A549-KRT17 cells and H1299-KRT17 cells compared to their control cells (P<0.05) (Figure 3A, B, D and E). In contrast, compared to SK-MES-1 control cells, KRT17 expression was down-regulated in SK-siKRT17 cells. The levels of active-β-catenin, cyclinD1, c-Myc, and MMP7 were also reduced in SK-siKRT17 cells compared to control cells (P<0.05) (Figure 3C and F). However, the expression level of LEF-1 was not significantly changed in A549-KRT17, H1299-KRT17, and SK-siKRT17 cells compared to their respective control cells (P>0.05).
KRT17 promotes EMT process of non-small cell lung cancer cells
We investigated the effect of KRT17 on expression of EMT-related proteins in lung cancer cells. As shown in Figure 3A, B, D and E, the expression levels of Vimentin, MMP9, and Snail were increased, yet E-cadherin expression was reduced, in A549-KRT17 and H1299-KRT17 cells compared to their control cells (P<0.05). Further, with KRT17 knockdown, the opposite results were obtained in SK-siKRT17 cells (P<0.05) (Figure 3C and F).
Discussion
KRT17 promotes carcinogenesis of a variety of cancer types, including gastric cancer,12 cervical cancer,11 breast cancer9 and oral squamous cell carcinoma.24 However, few studies were conducted on KRT17 in lung cancers. Its role and underlying mechanism remain unclear. In this study, our immunohistochemical results and The Cancer Genome Atlas (TCGA) data both confirmed that KRT17 was highly expressed in NSCLC tissues. High expression of KRT17 was correlated with poor differentiation and lymphatic metastasis, and it indicated poor prognosis in NSCLC, especially in lung adenocarcinomas. All these results indicate that KRT17 can be a marker of lung cancer progression and a predictor of poor survival. The high expression and cancer-promotion role of KRT17 was also confirmed in other cancers. Luisa et al demonstrated that KRT17 expression was increased in atypical metaplasia, high-grade intraepithelial neoplasia, and squamous cell carcinoma of the cervix and was related to patient mortality.11 KRT17 was also found significantly increased in gastric cancer12 and oral squamous cell carcinoma,18 which was correlated with poor survival. In addition, the expression level of KRT17 was significantly higher in LUSCs than that in LUADs, suggesting KRT17 as a possible differential diagnostic marker of these two cancer types. As a member of keratins and displaying characteristic expression patterns in tumors, KRT17 has been reported as a diagnostic marker in pathological diagnosis of cancers, such as breast cancer,8,9 skin squamous cell carcinoma,10 and cervical cancer.11 Consistent with our opinion, Yuan et al considered that the immunohistochemical staining of cytokeratin (CK) 5/6, CK7, CK14, CK17, and CK18 could be useful for differentiated diagnosis of LUAD and LUSC.25
Our in vitro studies showed that KRT17 overexpression promoted the proliferative and invasive abilities of lung cancer cells, which confirmed the cancer-promotion role of KRT17 and were consistent with previous reports in other cancers.14,26 It was reported that KRT17 facilitates cell proliferation in gastric cancer.14 Knock-out of the KRT17 gene significantly inhibited the migration and invasion process in areca nut-induced oral squamous cell carcinoma.26 The underlying mechanism of KRT17 promotion of cancer progression has been investigated in oral squamous cell carcinoma. Mikami et al found that phosphorylated KRT17 interacts with 14-3-3 sigma, which then activates the AKT/mTOR signaling pathway to promote cell proliferation in oral squamous cell carcinoma.27 It was also shown that KRT17 was a downstream signaling molecule of GLI1/2 in oral squamous carcinoma, which was overexpressed under the regulation of GLIs. KRT17 affects cell proliferation through an anti-apoptotic effect.18 However, the underlying mechanism of KRT17 in lung cancer has not been addressed previously.
Abnormal activation of the Wnt signaling pathway plays a key role in the promotion of various malignant tumors, including lung cancer.21,28,29 Wnt signaling pathway was first reported by Nusse and Varmus in 1982.30,31 Activation of the Wnt pathway leads to malignant transformation, and eventually tumor formation.32 To date, there have been no reports regarding KRT17 involvement in the Wnt signaling pathway. In this study, our results indicated that KRT17 upregulated activation of β-catenin, the core effector of the Wnt signaling pathway, and enhanced expression levels of Wnt target genes, such as cyclin D1, MMP7 and c-Myc. Moreover, overexpression of KRT17 markedly enhanced expression of MMP9, Snail, and Vimentin, and reduced expression of E-cadherin, which are EMT markers. Similarly, other keratins, such as KRT19, also can promote cell invasion and metastasis in human hepatocellular carcinoma.33 Taken together, KRT17 is not only an epithelial marker, but also a regulator of signaling pathways, Such as Wnt and AKT/mTOR signaling pathways. So, KRT17 can promote EMT process and the proliferative and invasive abilities of NSCLC cells via enhancing activity of the Wnt signaling pathway.
Conclusion
We identified KRT17 as a commonly upregulated protein in NSCLC, and its expression corresponded to poor prognosis of NSCLC, especially adenocarcinomas. KRT17 promotes proliferation and invasion of NSCLC cells via activation of the Wnt signaling pathway and EMT. Therefore, KRT17 may be an indicator of poor survival and a potential therapeutic target of NSCLC.
Abbreviations
KRT17, keratin 17; EMT, epithelial mesenchymal transition; NSCLC, non-small cell lung cancer; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MMP7, matrix metalloproteinase 7; MMP9, matrix metalloproteinase 9; TCGA, The Cancer Genome Atlas; CK, cytokeratin; NC, negative control; siNC, small interfering RNA negative control; siKRT17, small interfering RNA against keratin 17.
Consent for publication
Consents for publication were obtained for experimentation with all participants.
Ethics approval
The study was approved by the China Medical University Institutional Review Board for human studies. The ethical board approval number is LS[2018]016.
Availability of data and materials
All datasets supporting the conclusions of this work are included in the article.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 81372497 to HT Xu).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017;109(9). doi:10.1093/jnci/djx007.
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortettieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
3. Wang LY, Cui JJ, Guo AX, Yin JY. Therapy. Clinical efficacy and safety of afatinib in the treatment of non-small-cell lung cancer in Chinese patients. Onco Targets Ther. 2018;11:529–538. doi:10.2147/OTT.S136579
4. Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu Rev Biochem. 2004;73:749–789. doi:10.1146/annurev.biochem.73.011303.073823
5. Jacob JT, Coulombe PA, Kwan R, Omary MB. Types I and II keratin intermediate filaments. Cold Spring Harb Perspect Biol. 2018;10(4):a018275. doi:10.1101/cshperspect.a018275
6. Karantza V. Keratins in health and cancer: more than mere epithelial cell markers. Oncogene. 2011;30(2):127–138. doi:10.1038/onc.2010.456
7. Troyanovsky SM, Leube RE, Franke WW. Characterization of the human gene encoding cytokeratin 17 and its expression pattern. Eur J Cell Biol. 1992;59(1):127.
8. Matt VDR, Perou CM, Rob T, et al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol. 2002;161(6):1991–1996.
9. SoRlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi:10.1073/pnas.191367098
10. Moll R, Divo M, Langbein LJH. The human keratins: biology and pathology. Cell Biol. 2008;129(6):705–733.
11. Escobar-Hoyos LF, Yang J, Zhu J, et al. Keratin 17 in premalignant and malignant squamous lesions of the cervix: proteomic discovery and immunohistochemical validation as a diagnostic and prognostic biomarker. Mod Pathol. 2014;27(4):621–630. doi:10.1038/modpathol.2013.166
12. Hu H, Xu DH, Huang XX, et al. Keratin 17 promotes tumor growth and is associated with poor prognosis in gastric cancer. J Cancer. 2018;9(2):346–357. doi:10.7150/jca.19838
13. Yang L, Zhang S, Wang G. Keratin 17 in disease pathogenesis: from cancer to dermatoses. J Pathol. 2018;247(2):158–165. doi:10.1002/path.5178
14. Chivu-Economescu M, Dragu DL, Necula LG, et al. Knockdown of KRT17 by siRNA induces antitumoral effects on gastric cancer cells. Gastric Cancer. 2017;20(6):948–959. doi:10.1007/s10120-017-0712-y
15. Savita S, Tanner JM, Russell B, et al. A novel role for keratin 17 in coordinating oncogenic transformation and cellular adhesion in Ewing sarcoma. Mol Cell Biol. 2013;33(22):4448–4460. doi:10.1128/MCB.00241-13
16. Rydlander L, Ziegler E, Bergman T, et al. Molecular characterization of a tissue-polypeptide-specific-antigen epitope and its relationship to human cytokeratin 18. Eur J Biochem. 2010;241(2):309–314. doi:10.1111/j.1432-1033.1996.00309.x
17. Stacy M, Pauline W, Paul M, Coulombe PA. Role for keratins 6 and 17 during wound closure in embryonic mouse skin. Dev Dyn. 2010;226(2):356–365.
18. Mikami Y, Fujii S, Nagata K, et al. GLI-mediated Keratin 17 expression promotes tumor cell growth through the anti-apoptotic function in oral squamous cell carcinomas. J Cancer Res Clin Oncol. 2017;143(8):1381–1393. doi:10.1007/s00432-017-2398-2
19. Moll R. Cytokeratins as markers of differentiation in the diagnosis of epithelial tumors. Subcell Biochem. 1998;31:205–262.
20. Goldstraw P. Updated staging system for lung cancer. Surg Oncol Clin N Am. 2011;20(4):655–666. doi:10.1016/j.soc.2011.07.005
21. Wang Y, Lei L, Zheng YW, et al. Odd-skipped related 1 inhibits lung cancer proliferation and invasion by reducing Wnt signaling through the suppression of SOX9 and beta-catenin. Cancer Sci. 2018;109(6):1799–1810. doi:10.1111/cas.13614
22. Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–658. doi:10.1016/j.neo.2017.05.002
23. Nagy A, Lanczky A, Menyhart O, Gyorffy B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep. 2018;8(1):9227. doi:10.1038/s41598-018-27521-y
24. Regenbogen E, Mo M, Romeiser J, et al. Elevated expression of keratin 17 in oropharyngeal squamous cell carcinoma is associated with decreased survival. Head Neck. 2018;40(8):1788–1798.
25. Chen Y, Cui T, Yang L, et al. The diagnostic value of cytokeratin 5/6, 14, 17, and 18 expression in human non-small cell lung cancer. Oncology. 2011;80(5–6):333–340. doi:10.1159/000329098
26. Chiang CH, Wu CC, Lee LY, et al. Proteomics analysis reveals involvement of Krt17 in areca nut-induced oral carcinogenesis. J Proteome Res. 2016;15(9):2981–2997. doi:10.1021/acs.jproteome.6b00138
27. Mikami T, Maruyama S, Abe T, et al. Keratin 17 is co-expressed with 14-3-3 sigma in oral carcinoma in situ and squamous cell carcinoma and modulates cell proliferation and size but not cell migration. Virchows Archiv. 2015;466(5):559–569. doi:10.1007/s00428-015-1735-6
28. Cadigan KM, Peifer M. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol. 2009;1(2):a002881.
29. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi:10.1016/j.devcel.2009.06.016
30. Niehrs C, Acebron SP. Mitotic and mitogenic Wnt signalling. Embo J. 2012;31(12):2705–2713. doi:10.1038/emboj.2012.124
31. Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. Embo J. 2012;31(12):2670–2684. doi:10.1038/emboj.2012.146
32. Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4(5):a008052. doi:10.1101/cshperspect.a008052
33. Govaere O, Komuta M, Berkers J, et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut. 2014;63(4):674–685. doi:10.1136/gutjnl-2012-304351
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.