Back to Journals » Cancer Management and Research » Volume 12
Overexpression of IGFBP5 Enhances Radiosensitivity Through PI3K-AKT Pathway in Prostate Cancer
Authors Chen X, Yu Q, Pan H , Li P, Wang X, Fu S
Received 10 April 2020
Accepted for publication 29 May 2020
Published 6 July 2020 Volume 2020:12 Pages 5409—5418
DOI https://doi.org/10.2147/CMAR.S257701
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Xue Chen,1,2 Qi Yu,1,2 Hailun Pan,3,4 Ping Li,5 Xufei Wang,3,4 Shen Fu1– 3,6
1Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, People’s Republic of China; 3Key Laboratory of Nuclear Physics and Ion-Beam Application (MOE), Fudan University, Shanghai, People’s Republic of China; 4Institute of Modern Physics, Fudan University, Shanghai, People’s Republic of China; 5Department of Radiation Oncology, Shanghai Proton and Heavy Ion Center, Shanghai, People’s Republic of China; 6Department of Radiation Oncology, Shanghai Concord Cancer Hospital, Shanghai, People’s Republic of China
Correspondence: Shen Fu
Department of Radiation Oncology, Fudan University Shanghai Cancer Center, 270 Dong an Road, Shanghai 200032, People’s Republic of China
Tel/Fax +86-021-64175590
Email [email protected]
Xufei Wang
Key Laboratory of Nuclear Physics and Ion-Beam Application (MOE), Fudan University, 220 Han Dan Road, Shanghai 200433, People’s Republic of China
Tel +86-021-55665471
Fax +86-021-65642787
Email [email protected]
Background: Radiotherapy is the main treatment for localized prostate cancer. The therapeutic effects of radiotherapy are highly dependent on radiosensitivity of target tumors. Here, we investigated the impact of insulin-like growth factor-binding protein 5 (IGFBP5) on irradiation therapy in prostate cancer.
Methods: IGFBP5 gene was overexpressed in human prostate cancer cell lines, PC3 and DU145, with transfection of lentivirus expression vector. Radiosensitivity of the cell lines was assessed with colony formation, cell cycle and cell proliferation assays. The expression of proteins associated with the PI3K-AKT pathway was determined by Western blotting. The effect of IGFBP5 knockdown on PI3K-AKT pathway was tested using PI3K inhibitor.
Results: Higher expression of IGFBP5 improved the efficacy of radiotherapy for prostate cancer patients. The effects of IGFBP5 were linked to the PI3K-AKT signaling pathway. Overexpression of IGFBP5 enhanced radiosensitivity and induced G2/M phase arrest in prostate cancer cells. In contrast, it decreased PI3K, p-AKT expression and cell viability. These effects were reversed by IGFBP5 knockdown.
Conclusion: Our results reveal that IGFBP5 regulates radiosensitivity in prostate cancer via the PI3K-AKT pathway. It is, therefore, a potential biomarker of tumors that influences the therapeutic effect of radiotherapy.
Keywords: IGFBP5, irradiation, prostate cancer, radiosensitivity, PI3K-AKT pathway
Background
Prostate cancer is one of the most common malignancies and the leading cause of deaths in men worldwide.1,2 The incidence of prostate cancer has been on the rise and it is now the sixth leading cause of deaths in China.3 Radiotherapy is the main treatment for localized prostate cancer. External beam radiotherapies such as photon, proton and carbon-ion beam radiation, and brachytherapy have been successful in prostate cancer treatment.4–7 Radiotherapy exerts good local control on the growth of cancer cells and improves of prognosis of cancer patients who show high radiosensitivity. However, radioresistance increases the risk of local recurrence, distant metastasis, and poor prognosis.8 Thus, it is critical to understand the mechanism of radioresistance and radiosensitivity as this will reveal avenues for enhancing radiosensitivity.
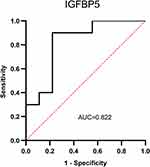
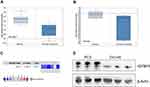
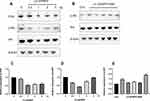
Insulin-like growth factor-binding protein 5 (IGFBP5) regulates proliferation, invasion and metastasis of many cancers.9–11 IGFBP5 belongs to a protein family with six members and is the most conserved among them.12 It has a high affinity for insulin-like growth factors (IGFs) which inhibits or promotes cancer development depending on the cellular context.13 For instance, it suppresses tumor growth or promotes cell proliferation in breast cancer, ovarian cancer, melanoma and osteosarcoma at different contexts.13,14 To date, the function of IGFBP5 in prostate cancer has not been defined. Our previous study revealed that higher expression of IGFBP5 improved the efficacy of radiotherapy for prostate cancer patients through the PI3K-AKT pathway (Figure 1, Supplementary 1). Based on data from the Oncomine database, mRNA expression of IGFBP5 is lower in cancerous tissues compared to normal tissues (Figure 2A and B). A meta-analysis of six databases revealed that IGFBP5 is downregulated in prostate cancer (Figure 2C). Elsewhere, IGFBP5 protein was found to be expressed in prostate cancer cells (Figure 2D). Currently, it is not known whether IGFBP5 influences the sensitivity of prostate cancer cells to radiotherapy. Against this background, we hypothesized that IGFBP5 may regulate radiosensitivity through the PI3K-AKT signaling pathway.
The PI3K is activated by different growth factors. Subsequent to its activation, it phosphorylates and activates AKT, which plays a central role in insulin receptor intracellular signaling.15 Both PI3K and AKT are important intracellular signals regulating various physiological and pathological processes. The PI3K-AKT pathway regulates cell growth, proliferation, survival, cell cycle, metabolism, and inflammation.15 Research indicates that activation of PI3K-AKT pathway contributes to radioresistance in many cancers including prostate cancer.16–18 Mechanistically, PI3K-AKT induces radioresistance by activating tumor-cell proliferation.17,19 Radioresistance is one of the causes of radiotherapy failure in prostate cancer.16,20 Thus, regulating PI3K-AKT may be an effective approach to prevent radioresistance.
In this study, we explored whether IGFBP5 influences radiosensitivity in prostate cancer cells. Specific tests were performed to determine whether IGFBP5 regulates the proliferation of PC3 and DU145 cells via PI3K-AKT pathway. The functions of IGFBP5 were explored using IGFBP5 knockdown or overexpression cell models.
Methods and Materials
Cell Lines and Cell Culture
Prostate cancer PC3 cells and DU145 cells were bought from Cell Bank, Chinese Academy of Sciences. These cells were cultured in F12K medium and MEM medium (Gibco, MD, USA) supplemented with 10% fetal bovine serum (Gemini Foundation, USA), 100 U/mL penicillin and 100 μg/mL streptomycin. The cells were cultured at 37 °C in a humidified atmosphere with 5% CO2.
Data Extraction
The expression data of IGFBP5 in cancer and normal tissues was downloaded from the Oncomine database (https://www.oncomine.org/), based on the selection filtering p<0.01, and fold change > 1.5.
Cell Transfection
Cells were plated in six-well plates and transfected with lentivirus to overexpress or knockdown IGFBP5 expression. The lentivirus expression vector (GV358) for overexpression IGFBP5 (LV-IGFBP5) was constructed by Genechem (Shanghai, China). To choose a suitable concentration of LV-IGFBP5, PC3 cells and DU145 cells were first seeded in 12-well plates and transfected with three different concentrations of the vectors and the resultant fluorescence intensity was measured. Finally, the concentration of 1×10^8 TU/mL was chosen. The cell lines were transfected in six-well plates for a defined time. During transfection, 2μg/mL puromycin was added to PC3 cells and 1.25 μg/mL puromycin to DU145 cells to stabilize the cells. For effective IGFBP5 knockdown with lentivirus expression vector, sh-RNA-IGFBP5 sequences and lentivirus expression sh-IGFBP5 (LV-IGFBP5-RNAi) were obtained from Genechem (Shanghai, China). The target sequence gcAAGTCAAGATCGAGAGAGA and the vector GV493 were used. The negative control group was a lentivirus vector lacking the gene of interest.
Colony Formation
Cells were plated in 60 mm dishes until they reached 70% confluence. They were irradiated with or without treatment. Each group was irradiated with X-ray dose of 2 Gy, 4 Gy, 6 Gy, and 8 Gy. Cells of control group were not irradiated. After irradiation, cells were dissociated by treatment with trypsin (Gibco, Grand Island, NY, USA). They were subsequently re-plated in appropriate dilutions to form colonies for 10–14 days. Colonies were fixed in 4% paraformaldehyde (Beyotime Biotechnology, Shanghai, China), stained with 0.1% crystal violet (Beijing Solarbio Science technology, Beijing, China) and counted using a stereomicroscope. More than 50 cells were counted as a colony. Radiation dose–survival curves were plotted according to plating efficiency and results were normalized to the control group.
Western Blotting
Proteins were extracted from cells using the total protein extraction kit (Invent Biotechnologies, Minnesota, USA). The extracted proteins were mixed with loading buffer (TaKaRa, Tokyo, Japan) and heated at 100 °C for 8 min. Proteins were separated by TGX stain-free SDS-PAGE (Bio-Rad, California, USA) under 150 V for 50 min. Next, proteins were electrotransferred to a PVDF membrane at 250 mA for 60 min. The membrane was blocked in 5% non-fat milk for 1 h at room temperature. Next, it was incubated overnight at 4 °C with primary antibodies against IGFBP5 (1:500, Santa Cruz, Texas, USA), PI3K (1:1000, CST, MA, USA), AKT (1:1000, CST, MA, USA), p-AKT (1:1000, CST, MA, USA), beta-Actin and α-tubulin (1:5000, Proteintech, Wuhan, China). Next, the membrane was washed thrice by TBST for 10 min and incubated with secondary goat-anti-mouse antibody (1:5000, Proteintech, Wuhan, China) or goat-anti-rabbit (1:5000, Proteintech, Wuhan, China). Finally, the immunoblot bands were exposed to the enhanced chemiluminescence solution (Thermo Fisher Scientific, Massachusetts, USA) to detect protein bands.
Cell Cycle Assay
Cells were seeded in a six-well plate with or without irradiation at a density of 5 × 104 cells per well. The irradiation group irradiated with 5 Gy. After 48 h of treatment, samples were collected and diluted to a concentration of 1 × 106 cells/mL. Cells were fixed in 70% cold ethanol at −20 °C overnight. Next, the samples were washed with PBS and centrifuged twice at 800 g, 5 min; followed by staining with Propidium Iodide (PI)/RNase solution (Dojindo, Kumamoto, Japan) according to the manufacturer’s protocol. Finally, flow cytometry (BD Biosciences, USA) was conducted to analyze the cell cycle. The data was analyzed by FlowJo software (Version X; TreeStar, Ashland, OR, USA).
Cell Proliferation Assay
Briefly, cells were plated in 96-well plates at a density of 4 × 103 cells per well, and treated with or without irradiation. Each group was prepared as three repeats. To investigate the proliferation of LV-IGFBP5 PC3 and DU145 cells, cells cultured 48 h after irradiation and then detected by microplate reader. The PI3K inhibitor, LY294002 (25 μM, Selleck, USA) was added to LV-IGFBP5-RNAi cells and cultured for 24 h. After irradiation, all samples were further cultured for 24 h in the same conditions. Then, 10 μL of the CCK-8 solution (Dojindo, Kumamoto, Japan) was added to each well. Three control wells were set up without any cells and treatment. The plates were incubated for 1–4 h in an incubator. Finally, absorbance of the cells was measured at 450 nm using a microplate reader. The cell viability of all groups was normalized to that of the control group.
Statistical Analysis
Data were analyzed with SPSS software version 23.0 (SPSS, Chicago, IL, USA) and GraphPad Prism Software version 8.01 (GraphPad Software, La Jolla, CA, USA) and are presented as mean ± SD. Student’s t-test and Mann–Whitney U-test were used to compare two group means. One-way ANOVA was performed for more than three groups. A P value less than 0.05 was considered statistically significant.
Results
Higher IGFBP5 Expression Correlates with Better Radiotherapy Efficacy
We previously applied proteomics to explore the efficacy of radiotherapy in prostate cancer patients. Results showed that higher expression of IGFBP5 predicts better efficacy (Figure 1, AUC=0.822, P=0.02). We re-analyzed these data with pathway enrichment analysis. Results showed that IGFBP5 is highly correlated with PI3K-AKT signaling pathway. Details of these results were reported in the 58th conference of particle therapy co-operative group (PTC58-0345, unpublished data, Supplementary 1). This study was performed to investigate the function of IGFBP5 on irradiation efficacy in prostate cancer.
Expression of IGFBP5 in Prostate Cancer
The expression level of IGFBP5 in prostate cancer was determined by analyzing six independent microarray datasets from Oncomine database.21–25 We found that IGFBP5 is expressed in multiple cancers including prostate cancer (Table 1). Notably, the expression of IGFBP5 in prostate cancer tissues was significantly lower than in normal prostate gland tissues (Table 1). IGFBP5 mRNA expression in prostate carcinoma was lower compared to normal tissues (Figure 2A, P=2.16E-4; Figure 2B, P=0.002).21,23 The median rank of IGFBP5 among other downregulated genes in prostate cancer was 226 as revealed by a meta-analysis performed across the six datasets (Figure 2C, P=1.10E-4). Moreover, Western blotting assay revealed that IGFBP5 protein was expressed in PC3 cells and DU145 cells (Figure 2D).
 |
Table 1 IGFBP5 Expression in 6 Datasets of Oncomine Database About Prostate Cancer |
Overexpression of IGFBP5 Enhances Radiosensitivity in Prostate Cancer
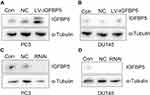
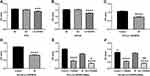
Previous studies found that IGFBP5 affects cancer development and progression. To date, it remains unknown whether IGFBP5 influences radiosensitivity of prostate cancer. We, therefore, explored the association of IGFBP5 expression level with radiosensitivity in prostate cancer cells using colony formation analysis. The effect of overexpression and knockdown IGFBP5 in PC3 cells and DU145 cells was showed in Figure 3. It was found that IGFBP5 overexpression in PC3 and DU145 cells decreased the survival fraction (Figure 4A and B). We preformed three identical experiments. After overexpression of IGFBP5, the mean survival fraction of PC3 cells was 0.6, 0.17, 0.06, 0.01 with defined irradiation, which significantly reduced survival fraction when compared to control group (0.73, 0.32, 0.08, 0.03, respectively). Similarly, the mean survival fraction of DU145 cells was 0.56, 0.29, 0.14, 0.07 with defined irradiation after overexpression IGFBP5, which was lower than control group (0.64, 0.45, 0.18, 0.08, respectively). These results suggest that overexpression of IGFBP5 enhances radiosensitivity.
IGFBP5 Induced G2/M Phase Arrest in Prostate Cancer Cells
In further tests, cell cycle analysis was performed to investigate whether IGFBP5 overexpression enhanced radiosensitivity. Results showed that IGFBP5 overexpression in PC3 cells and DU145 cells induced G2/M phase arrest (P<0.05, Figure 4C and D). We have performed three times experiments to confirmed our results. The mean DNA content of G2/M phase in PC3 cells was 21% (LV-IGFBP5 group) versus 16.14% (control group, *P<0.05). The mean DNA content of G2/M phase in DU145 cells was 29.45% (LV-IGFBP5 group) versus 24.84% (control group, *P<0.05). G2/M phase arrest was reversed by IGFBP5 knockdown in PC3 cells and DU145 cells (*P<0.05, **P<0.01, Figure 4E and F). The mean DNA content of G2/M phase in PC3 cells was 18.62% (LV-IGFBP5-RNAi group) versus 28.35% (control group, **P<0.01). The mean DNA content of G2/M phase in DU145 cells was 24.66% (LV-IGFBP5-RNAi group) versus 26.63% (control group, *P<0.05).
IGFBP5 Regulates PI3K-AKT Pathway in Prostate Cancer Cells
Next, we elucidated the mechanism by which IGFBP5 regulated radiosensitivity. For this purpose, we established stable cell lines with IGFBP5 overexpression and IGFBP5 knockdown by lentivirus vector transfection. Results showed that IGFBP5 overexpression deceased the expression of PI3K and p-AKT in cells exposed to defined dose-irradiation (Figure 5A). By contrast, IGFBP5 knockdown enhanced the expression of p-AKT (Figure 5B), indicating PI3K-AKT pathway activation. The quantification of these results was also presented (Figure 5C–E). One-way ANOVA analysis indicated p<0.05.
IGFBP5 Modulates Cell Proliferation in Prostate Cancer Cells
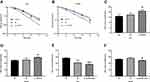
The effect of IGFBP5 on the proliferation of prostate cancer cells lines was determined using the CCK8 assay. Each experiments performed triplicate times. The results demonstrated that the cell growth of cells overexpressing IGFBP5 was lower compared to that of cells from the control group (Figure 6A and B). The mean OD value of LV-IGFBP5 group in PC3 and DU145 cells was 1.06 and 1.56, while the control group was 1.2 (p<0.001) and 1.28 (p<0.0001). The effect of inhibition also observed by combining with irradiation, that is, irradiation inhibited cell proliferation compared to without irradiation in overexpression IGFBP5 cells (Figure 6C and D). The mean OD value of IR group in PC3 and DU145 cells was 0.73 and 0.80, while the control group was 1.07 and 1.28 (p<0.0001).
PI3K Inhibitor Reverses the Effect of IGFBP5 Knockdown on Cell Proliferation
We further tested whether inhibition of PI3K-AKT pathway with PI3K inhibitor, LY294002, could reverse the effect of IGFBP5 knockdown on cell proliferation. We found that LY294002 slowed the growth of PC3 cells and DU145 cells caused by IGFBP5 knockdown (Figure 6E and F). The mean OD value of LY294002 group versus control group was 0.46 versus 1.16 (p<0.0001) in PC3 knockdown cells and 0.58 versus 1.29 (p<0.0001) in DU145 knockdown cells. Interestingly, the inhibition of cell proliferation by LY294002 was more pronounced when combined with irradiation (****P<0.0001, Figure 6E and F) than LY294002 alone group (#P<0.05, ##P<0.01, Figure 6E and F). The mean OD value of IR+ LY294002 group versus LY294002 group was 0.31 versus 0.46 (p<0.05) in PC3 knockdown cells and 0.41 versus 0.58 (p<0.01) in DU145 knockdown cells.
Discussion
This pioneering study demonstrates that IGFBP5 influences radiosensitivity in prostate cancer via PI3K-AKT pathway. The human IGFBP5 is a protein-coding gene, located on chromosome 2q33-q36.26,27 IGFBP5 comprises six family members, all of which bind insulin-like growth factors (IGFs) which modulate the bioavailability and function of IGFs.12,28 Recent studies showed that IGFBPs participate in cancer development and progression. Other studies have reported their roles in predicting the prognosis of pancreatic cancer, ovarian cancer, and nasopharyngeal carcinoma.29–31 IGFBP5 was first recognized to stimulate osteoblast mitogenesis dependent or independent of IGF.32 Several other studies have shown that IGFBP5 is a growth factor that increases bone formation.33 Gyanendra et al reported that IGFBP5 inhibited cell proliferation independent of IGF signaling.34 Accumulating evidence show that IGFBP5 inhibits or stimulates cell proliferation in several types of cancers leading to different outcomes. Some studies reported that IGFBP5 functioned as a growth inhibitor in breast cancer35 and suppressed tumor metastasis in osteosarcoma.14 In contrast, Li et al found that IGFBP5 promoted cancer development and acted as a prognostic factor in breast cancer.36 To date, few studies have explored the role of IGFBP5 in prostate cancer, and to our knowledge, none has reported the association of IGFBP5 with radiosensitivity in prostate cancer.
In the current study, we show that IGFBP5 overexpression combined with irradiation enhances radiosensitivity in prostate cancer cells. Notably, the survival fraction of cells in the LV-IGFBP5 group was significantly lower compared to that of control group. Overexpression of IGFBP5 induced G2/M cell cycle arrest. It has been shown that G2 and M phases are sensitive to radiations.37 Evidence from our proteomic analysis indicated that higher expression of IGFBP5 predicted better radiotherapy efficacy in prostate cancer patients. Pathway analysis suggested that IGFBP5 was largely associated with the PI3K-AKT pathway. Consistently, our results show that overexpression of IGFBP5 decreased PI3K and p-AKT expression. These results demonstrate that IGFBP5 regulates radiosensitivity via the PI3K-AKT signaling pathway. Notably, inhibition of this pathway with PI3K inhibitor partially reversed the effects of IGFBP5 knockdown on cell viability.
Numerous studies indicate that PI3K-AKT pathway contributes to radioresistance in many cancers including prostate cancer.16,17 Radioresistance is the leading cause of radiotherapy-related failure. Thus, our findings imply that regulating PI3K-AKT pathway may be an effective strategy to prevent radioresistance and thus improve the therapeutic efficacy. These findings suggest that targeting IGFBP5 signaling could be a potential therapeutic strategy for enhancing radiosensitivity in prostate cancer. However, some limitations exist in this study. IGFBP5 overexpression induced G2/M phase arrest, which was associated with radiosensitivity. We did not investigate the detail of G2/M phase protein expression. G2/M phase arrest enhanced radiosensitivity in prostate cancer cells, which was consistent with Geldof et al reported.38 Further studies to investigate the function of IGFBP5 will be meaningful. One the one hand, further studies in animal models are needed; on the other, clinical studies to investigate the status of IGFBP5 in a large number of patients with radiotherapy should performed in the future.
Conclusion
In conclusion, this study shows that IGFBP5 overexpression enhances radiosensitivity in prostate cancer via the PI3K-AKT pathway. This suggests that IGFBP5 can be leveraged to develop a novel strategy to improve prostate cancer radiotherapy.
Abbreviations
Con, control; IGFBP5, insulin-like growth factor-binding protein 5; NC, negative control; LV, lentivirus; Wt, wild type.
Ethics Approval and Consent to Participate
This study is an in vitro experiment, Cell lines bought from Cell Bank, Chinese Academy of Sciences.
Acknowledgments
The authors highly thank the members of their laboratory and department for their technical help and excellent support.
Disclosure
The authors declare no conflicts of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77(1):38–52. doi:10.1016/j.eururo.2019.08.005
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Waddle MR, Sio TT, Van Houten HK, et al. Photon and proton radiation therapy utilization in a population of more than 100 million commercially insured patients. Int J Radiat Oncol. 2017;99(5):1078–1082. doi:10.1016/j.ijrobp.2017.07.042
5. Chang AJ, Autio KA, Roach M, Scher HI. High-risk prostate cancer—classification and therapy. Nat Rev Clin Oncol. 2014;11(6):308–323. doi:10.1038/nrclinonc.2014.68
6. Glowa C, Karger CP, Brons S, et al. Carbon ion radiotherapy decreases the impact of tumor heterogeneity on radiation response in experimental prostate tumors. Cancer Lett. 2016;378(2):97–103. doi:10.1016/j.canlet.2016.05.013
7. Pickles T, Tyldesley S, Hamm J, Virani SA, Morris WJ, Keyes M. Brachytherapy for intermediate-risk prostate cancer, androgen deprivation, and the risk of death. Int J Radiat Oncol. 2018;100(1):45–52. doi:10.1016/j.ijrobp.2017.08.042
8. Peitzsch C, Kurth I, Kunz-Schughart L, Baumann M, Dubrovska A. Discovery of the cancer stem cell related determinants of radioresistance. Radiother Oncol. 2013;108(3):378–387. doi:10.1016/j.radonc.2013.06.003
9. Wang J, Luo X, Tang Y, Xu J, Zeng Z. The prognostic values of insulin-like growth factor binding protein in breast cancer. Medicine. 2019;98(19):e15561. doi:10.1097/MD.0000000000015561
10. Ding M, Bruick RK, Yu Y. Secreted IGFBP5 mediates mTORC1-dependent feedback inhibition of IGF-1 signalling. Nat Cell Biol. 2016;18(3):319–327. doi:10.1038/ncb3311
11. Wang J, Ding N, Li Y, et al. Insulin-like growth factor binding protein 5 (IGFBP5) functions as a tumor suppressor in human melanoma cells. Oncotarget. 2015;6(24):20636–20649. doi:10.18632/oncotarget.4114
12. Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20(6):761–787. doi:10.1210/edrv.20.6.0382
13. Gullu G, Karabulut S, Akkiprik M. Functional roles and clinical values of insulin-like growth factor-binding protein-5 in different types of cancers. Chin J Cancer. 2012;31(6):266–280. doi:10.5732/cjc.011.10405
14. Su Y, Wagner ER, Luo Q, et al. Insulin-like growth factor binding protein 5 suppresses tumor growth and metastasis of human osteosarcoma. Oncogene. 2011;30(37):3907–3917. doi:10.1038/onc.2011.97
15. Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46(6):372–383. doi:10.3109/07853890.2014.912836
16. Chang L, Graham PH, Hao J, et al. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014;5(10):e1437. doi:10.1038/cddis.2014.415
17. Chang L, Graham PH, Ni J, et al. Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit Rev Oncol Hemat. 2015;96(3):507–517. doi:10.1016/j.critrevonc.2015.07.005
18. Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9(3):288–296. doi:10.1016/S1470-2045(08)70073-1
19. Skvortsova I, Skvortsov S, Stasyk T, et al. Intracellular signaling pathways regulating radioresistance of human prostate carcinoma cells. Proteomics. 2008;8(21):4521–4533. doi:10.1002/pmic.200800113
20. Chang L, Ni J, Beretov J, et al. Identification of protein biomarkers and signaling pathways associated with prostate cancer radioresistance using label-free LC-MS/MS proteomic approach. Sci Rep-Uk. 2017;7(1):1–15.
21. Arredouani MS, Lu B, Bhasin M, et al. Identification of the transcription factor single-minded homologue 2 as a potential biomarker and immunotherapy target in prostate cancer. Clin Cancer Res. 2009;15(18):5794–5802. doi:10.1158/1078-0432.CCR-09-0911
22. Luo J, Duggan DJ, Chen Y, et al. Human prostate cancer and benign prostatic hyperplasia: molecular dissection by gene expression profiling. Cancer Res. 2001;61(12):4683.
23. Luo J, Yu YP, Cieply K, et al. Gene expression analysis of prostate cancers. Mol Carcinogen. 2002;33(1):25–35. doi:10.1002/mc.10018
24. Tomlins SA, Mehra R, Rhodes DR, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39(1):41–51. doi:10.1038/ng1935
25. Welsh JB, Sapinoso LM, Su AI, et al. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61(16):5974.
26. Ghoussaini M, Edwards SL, Michailidou K, et al. Evidence that breast cancer risk at the 2q35 locus is mediated through IGFBP5 regulation. Nat Commun. 2014;4(1):4999. doi:10.1038/ncomms5999
27. Beattie J, Allan GJ, Lochrie JD, Flint DJ. Insulin-like growth factor-binding protein-5 (IGFBP-5): a critical member of the IGF axis. Biochem J. 2006;395(1):1–19. doi:10.1042/BJ20060086
28. Ding H, Wu T. Insulin-Like Growth Factor Binding Proteins in Autoimmune Diseases. Front Endocrinol (Lausanne). 2018;9:499. doi:10.3389/fendo.2018.00499
29. Yoneyama T, Ohtsuki S, Honda K, et al. Identification of IGFBP2 and IGFBP3 as compensatory biomarkers for CA19-9 in early-stage pancreatic cancer using a combination of antibody-based and LC-MS/MS-based proteomics. PLoS One. 2016;11(8):e161009. doi:10.1371/journal.pone.0161009
30. Gershtein ES, Isaeva ER, Kushlinsky DN, et al. Insulin-like growth factors (IGF) and IGF-binding proteins (IGFBP) in the serum of patients with ovarian tumors. B Exp Biol Med+. 2016;160(6):814–816. doi:10.1007/s10517-016-3317-2
31. Bao L, Liu H, You B, et al. Overexpression of IGFBP3 is associated with poor prognosis and tumor metastasis in nasopharyngeal carcinoma. Tumor Biol. 2016;37(11):15043–15052. doi:10.1007/s13277-016-5400-8
32. Andress DL, Birnbaum RS. Human osteoblast-derived insulin-like growth factor (IGF) binding protein-5 stimulates osteoblast mitogenesis and potentiates IGF action. J Biol Chem. 1992;267(31):22467.
33. Miyakoshi N, Richman C, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that IGF-binding protein-5 functions as a growth factor. J Clin Invest. 2001;107(1):73–81. doi:10.1172/JCI10459
34. Tripathi G, Salih DAM, Drozd AC, Cosgrove RA, Cobb LJ, Pell JM. IGF-independent effects of insulin-like growth factor binding protein-5 (Igfbp5) in vivo. FASEB J. 2009;23(8):2616–2626. doi:10.1096/fj.08-114124
35. Butt AJ, Dickson KA, McDougall F, Baxter RC. Insulin-like growth factor-binding protein-5 inhibits the growth of human breast cancer cells in vitro and in vivo. J Biol Chem. 2003;278(32):29676–29685. doi:10.1074/jbc.M301965200
36. Li X, Cao X, Li X, Zhang W, Feng Y. Expression level of insulin-like growth factor binding protein 5 mRNA is a prognostic factor for breast cancer. Cancer Sci. 2007;98(10):1592–1596. doi:10.1111/j.1349-7006.2007.00565.x
37. Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol. 2004;59(4):928–942. doi:10.1016/j.ijrobp.2004.03.005
38. Geldof AA, Plaizier MABD, Duivenvoorden I, et al. Cell cycle perturbations and radiosensitization effects in a human prostate cancer cell line. J Cancer Res Clin. 2003;129(3):175–182. doi:10.1007/s00432-002-0412-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.