Back to Journals » OncoTargets and Therapy » Volume 12
Overexpression Of ERβ Participates In The Progression Of Liver Cancer Via Inhibiting The Notch Signaling Pathway
Authors Zhang Y, Yi B, Zhou X, Wu Y, Wang L
Received 3 June 2019
Accepted for publication 11 September 2019
Published 22 October 2019 Volume 2019:12 Pages 8715—8724
DOI https://doi.org/10.2147/OTT.S218158
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
This paper has been retracted.
Yiping Zhang, Benyi Yi, Xufeng Zhou, Yahua Wu, Lili Wang
Department of Biochemistry and Molecular Biology, Basic Medical College of Jiujiang University, Jiujiang City, Jiangxi Province 332000, People’s Republic of China
Correspondence: Yiping Zhang
Department of Biochemistry and Molecular Biology, Basic Medical College of Jiujiang University, No. 320, Xunyang East Road, Xunyang District, Jiujiang City, Jiangxi Province 332000, People’s Republic of China
Tel +86-13767275087
Email [email protected]
Purpose: This study aimed to explore the role of Estrogen Receptor-β (ERβ)-mediated Notch signaling pathway in the regulation of proliferation and apoptosis in liver cancer cells.
Methods: HepG2 cells (Pbi-EGFP-ER) were transfected with ERβ that mediated by liposome, and normal HepG2 cells (Blank) and empty plasmid-transfected HepG2 cells (Pbi-EGFP-C) were used as controls. Then, Huh7 cells were transfected with shERβ lentivirus to knock down ERβ expression. The Huh7 cells were divided into three groups including Blank, experimental group (shERβ) and negative group (shLuc). Then, qRT-PCR, Western blot, CCK-8 assay, cell scratch assay, Transwell assay, Annexin V-FITC and PI double staining were performed based on these groups. Finally, a mouse xenograft model was constructed to verify the regulation of ERβ on Notch signaling pathway in liver cancer.
Results: In HepG2 cells, the ERβ expression in Pbi-EGFP-E group was higher than that in Blank and Bi-EGFP-C group. Overexpression of ERβ inhibited HepG2 cell proliferation, migration, invasion and Ki67 protein expression, as well as promoted apoptosis, Bcl-2 and Bax expression. Overexpression of ERβ decreased Notch1, Notch2 and Hes1 expression. In Huh7 cells, the effect of low ERβ expression was contrary to that of high ERβ expression. The shERβ + DAPT group reversed the effect of shERβ on the volume and weight of transplanted tumors.
Conclusion: ERβ may inhibit the development of liver cancer and promote apoptosis via inhibiting the Notch pathway.
Keywords: liver cancer, ERβ, Notch signaling pathway, HepG2 and Huh7, proliferation and apoptosis
Introduction
Liver cancer is the second leading cause of cancer death worldwide, causing more than 700,000 deaths each year.1,2 Treatment for liver cancer includes surgery, radiofrequency and microwave ablation, chemotherapy, radiation therapy and liver transplantation.3 Actually, the effects of drug treatment vary from person to person, and surgical treatment is prone to recurrence.4,5 Although previous study shows that liver disease is associated with imbalance between serum estradiol and testosterone,6 the molecular mechanism of liver cancer cell metastasis has not been fully elucidated and needs further clarification.
Importantly, the liver is a hormone-sensitive organ, and the hepatic estrogen receptor subtypes α (ERα) and ERβ have been characterized.7 ERβ, a member of the nuclear receptor superfamily, has important effects on cell proliferation, development and progression in many diseases.8,9 Michele et al10 have indicated that the low expression of ERβ is directly related to apoptosis and negatively correlated with cell proliferation. Overexpression of ERβ can attenuate the role of apoptotic proteins and inhibit the level of pro-apoptotic proteins.11 Moreover, the Notch signaling pathway, one of the most frequently activated signaling pathways in cancer, is proved to be involved in the regulation of hepatic metabolism, inflammation and cancer.12,13 As an evolutionarily conserved pathway, Notch is critical for the development and homeostasis of many organs, including the liver.14 A previous study has shown that ERβ-dependent Notch1 activation regulates apoptosis in vascular endothelial cells.15 Although sporadic researches have proved the relations among ERβ, Notch pathway and liver cancer, whether ERβ takes part in the liver cancer progression via Notch signaling pathway is still not fully revealed.
In this study, the human hepatoma HepG2 cells and Huh7 cell lines were transfected with ERβ gene. Based on this, the quantitative real-time polymerase chain reaction (qRT-PCR) analysis, CCK-8 detection, cell scratch assay, Transwell assay, Annexin V-FITC and PI double staining and Western blot analysis were investigated. Finally, a mouse xenograft model of liver cancer was constructed to verify the regulation of ERβ on Notch signaling pathway. This study hoped to reveal the biological function of ERβ in the progression of liver cancer, and provided new insights of ERβ in liver cancer treatment.
Materials And Methods
Cell Grouping And Transfection
Human hepatocellular carcinoma (HCC) cell lines HepG2 (American Type Culture Collection, ATCC) and Huh7 (Cell Bank of the Chinese Academy of Sciences, Shanghai, China) were cultured by our laboratory (37°C, 5% CO2, antibody-free DMEM medium containing 10% fetal bovine serum). The plasmids of Pbi-EGFP-ERβ and Pbi-EGFP-C were purchased from Beijing Huada Gene Technology Co., Ltd. (Beijing, China) followed by transduction of Huh7 cells with shERβ lentivirus to knock down ERβ. Then, HepG2 cells were divided into blank control (Blank) group, experimental (Pbi-EGFP-ERβ) group and negative control (Pbi-EGFP-C) group. Meanwhile, Huh7 cells were divided into blank control (Blank) group, experimental (shERβ) group and negative control (shLuc) group. The cells with a good growth state were transfected by Lipofectamine Fisher 2000 transfection reagent (Invitrogen, USA). After aspirating the original medium, a total of 200 pmol of Pbi-EGFP-ERβ/empty plasmid and 5 μL of LipofectamineTM 2000 were diluted with 250 μL Opti-MEM. Then, diluted shERβ, shLuc, Pbi-EGFP-ERβ, empty plasmid and LipofectamineTM 2000 were mixed and incubated at room temperature for 20 mins. When transfected for 24 hrs, the number of green fluorescent cells was observed under an inverted fluorescence microscope, and five fields were randomly selected to measure the cell transfection rate.
After transfection for 72 hrs, cells in Pbi-EGFP-ERβ group and shERβ group were cultured in DMEM medium containing 5 μmmol/L DAPT (Notch inhibitor, Sigma, Missouri, USA), which was named as Pbi-EGFP-ERβ + DAPT group and shERβ + DAPT group, respectively. After 48 hrs of culture, the expression levels of Notch1, Notch2 and Hes1 proteins were detected.
qRT-PCR
Total RNA was extracted by TRIZOL kit (Invitrogen, Carlsbad, California, USA). For gene expression detection, 10 μL of ABI system was used, including 1 μL of single-stranded cDNA, 5 μL of SYBR Green Real-time PCR Master Mix and 0.5 μL (1 μmol/L) of upstream primers and downstream primers. The reaction conditions were 95°C for 5 mins, then 40 cycles of 95°C for 60 s, 60°C for 15 s and 72°C for 34 s. β-actin was used as an internal reference, and expression of the target gene was analyzed by 2−ΔΔCt method.16 All primer sequences were synthesized by Invitrogen and are shown in Table 1.
 |
Table 1 PCR Primer Sequences |
Western Blot Assay
After transfection of HepG2 cells for 72 hrs, the transmembrane protein was extracted according to the member protein extraction kit (Beyotime, Shanghai, China). SDS-PAGE was performed to separate proteins, then the proteins were transferred to PVDF membranes, followed by blocking with 5% BSA for 1 hr. Primary antibodies (Hesl, Notchl, Notch2, 1:1000, Santa Cruz, USA, Bax, BCL-2, Ki67, 1:1000, Abcam, UK) and HRP-labeled goat anti-rabbit (1:3000, Boaosen Biotechnology, China) were used, respectively. The image was scanned using a gel imaging system (Chemilu-mines-cenceimaging system, USA). Image J analysis software was used to analyze the expression levels of Hes1, Notch1 and Notch2 proteins.
CCK-8 Detection
Cell proliferation assay was performed strictly according to the instructions of the CCK-8 kit (Beyotime, China). After transfection for 0 hr, 24 hrs, 48 hrs and 72 hrs, 90 μL serum-free medium containing 10 μL of CCK-8 reagent was added to each sample. After incubation for 2 hrs, the supernatant was transferred to a new 96-well colorimetric plate. The Anthos microplate reader (Biochrom Anthos 2010, Britain) was used to measure absorbance (A450) and plot the in vitro growth curve of the cells.
Scratch Assay
Cell scratch assay was used to detect cell migration in the current study. Simply, after adjusting the cell density of each group, the cells were inoculated into the 6-well plate. After drew a line across the surface of culture medium, washed by PBS and added fresh culture medium, cells were continuous cultured for 24–48 hrs. Then, these cells were observed and photographed under inverted microscope (Olympus Ckx53) to calculate the cell migration rate.
Transwell Assay
Cells were placed in the gelatin-coated Transwell upper chamber, a culture medium containing 10% fetal bovine serum (FBS) was added to the lower chamber. After 12 hrs of culture, the upper chamber was removed, and the unmigrated cells in the upper chamber were wiped off with a cotton swab. The cells were fixed with 4% paraformaldehyde at room temperature for 10 mins, stained with 0.25% Coomassie brilliant blue for 15 mins and randomly taken 5 visual fields under a 400× field microscope (Olympus Ckx53) to count the number of invading cells.
Annexin V-FITC And PI Double Staining
After transfection for 72 hrs, cells were digested with 0.25% trypsin, and then PBS was used to prepare a cell suspension. Then, cells were centrifuged (1500 r/min, 5 mins, 4°C), washed and re-suspended. A total of 100 μL cell suspension, 5 μL of FITC-labeled Annexin V and 10 μL of propidium iodide (20 g/L) were added and incubated at room temperature for 15 mins in the dark. Finally, 400 μL binding buffer was added to each tube, and the flow cytometry was quantitatively detected by FACScan.
Mouse Xenograft Model Experiment
A total of 20 BALB/c-nu mice (6 weeks old, purchased from Shanghai Institute of Materia Medica) were randomly divided into 4 groups (5 in each group). Then, Huh7 cells (3 × 106) from Blank, shLuc, shERβ and shERβ + DAPT groups were subcutaneously inoculated into the right axillary region of the mice to establish a xenograft tumor model. The growth of the transplanted tumor was examined every 5 days after continuous modeling (30 days of continuous measurement). The tumor diameter was measured with a vernier caliper, followed by the tumor volume was calculated. After the last measurement, the mice were sacrificed by neck dislocation, and the tumor weight was weighed after the tumor was removed. The tumor growth curve was drawn by taking the tumor volume as the ordinate and the time as the abscissa. All the above experiments were approved by the Animal Ethics Committee of our hospital, and all experiments were in accordance with the local guide for the care and use of laboratory animals.
Statistical Analysis
GaphPad Prism 5.0 software (GaphPad software, Inc., La Jolla, USA) was used for all statistical analyses. All results were expressed as the mean ± SD. One-Way ANOVA was used for the current study. Tukey’s multiple comparison test was used for the pairwise comparison after ANOVA. P < 0.05 was considered to be statistically significant.
Results
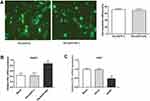
Successful Transfection Of HepG2 And Huh7 Cells
After transfection, cells were investigated by green fluorescent protein (GFP) under an inverted fluorescence microscope. The percentage of fluorescent cells was counted by the same field of view of multiple people, and the transfection efficiency was determined to be about 70% (Figure 1A). The results of qRT-PCR showed that compared with Blank and Pbi-EGFP-C, ER expression in Pbi-EGFP-ER group was significantly increased (P < 0.05, Figure 1B). Moreover, compared with Blank and shLuc groups, ER expression in shERβ group was decreased significantly (P < 0.05, Figure 1C).
Overexpression Of ERβ Inhibits Cell Proliferation
After cultivation for 24 hrs, the effect of overexpression and low-expression of ERβ on HepG2 cell proliferation was measured by the CCK-8. Compared with the Blank group and Pbi-EGFP-C group, cell proliferation of Pbi-EGFP-ERβ group was significantly decreased (P < 0.05, Figure 2A). Conversely, compared with Blank and shLuc group, the proliferation of cells in shERβ group was increased (P < 0.05, Figure 2B).
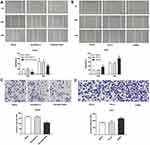
Overexpression Of ERβ Inhibits Cell Migration And Invasion
The effect of overexpressed and low-expressed ERβ on the migration of HepG2 cells was examined by cell scratch assay. Compared with Blank and PBI-EGFP-C groups, the cell migration rate in P bi-EGFP-ER group was significantly decreased (P < 0.05), but there was no significant difference between Blank and PBI-EGFP-C groups (P > 0.05, Figure 3A). Meanwhile, compared with the Blank and shLuc group, the cell migration in the shERβ group was significantly increased (P < 0.05, Figure 3B). Furthermore, Transwell assay showed that the invasive ability of Huh7 cells in P bi-EGFP-ER group was decreased than that in Blank and Bi-EGFP-C groups (P < 0.05, Figure 3C). On the contrary, the low expression of ERβ resulted in the enhancement of the invasive ability of Huh7 cells (P < 0.05, Figure 3D).
Overexpression Of ERβ Promotes Cells Apoptosis
The results of Annexin V-PI double staining showed that the apoptosis rate of Pbi-EGFP-ERβ group was evidently higher in Pbi-EGFP-ERβ group than that in the Blank and Pbi-EGFP-C group (P < 0.05, Figure 4A). Low ERβ expression resulted in a decrease in the apoptotic rate of Huh7 cells (P < 0.05). Furthermore, Western blot showed that in HepG2 cells, compared with Blank and Bi-EGFP-C groups, the expression of Ki67 and Bcl-2 protein in Pbi-EGFP-ER group were decreased significantly (all P < 0.05), while the expression of ERβ and Bax protein were increased significantly (all P < 0.05, Figure 4B). In Huh7 cells, the expression of Ki67 and Bcl-2 protein in ERβ group was increased significantly (all P < 0.05), and the expression of ERβ and Bax protein was significantly decreased (all P < 0.05, Figure 4C).
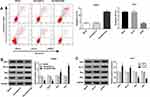
Overexpression Of ERβ Inhibits Notch Signaling Pathway
Compared with the Blank group and Pbi-EGFP-C group, the levels of Notch1, Notch2 and Hes1 mRNA were significantly decreased in the Pbi-EGFP-ERβ group (all P < 0.05). However, there was no distinct difference between the Blank group and Pbi-EGFP-C group (P > 0.05) (Figure 5A). The expression of Notch1, Notch2 and Hes1 protein detected by Western blot was consistent with that by qRT-PCR (Figure 5B). After treatment with Notch inhibitor, the expression levels of Notch1, Notch2 and Hes1 were lower than those of Pbi-EGFP-ER group (all P < 0.05). In Huh7 cells, qRT-PCR results showed that the expression levels of Notch1, Notch2 and Hes1 in shERβ group were significantly higher than those in Blank and shLuc groups (all P < 0.05), while the expression levels of Notch1, Notch2 and Hes1 in Blank and shLuc groups were not significantly different (P > 0.05, Figure 5C). The expression of Notch1, Notch2 and Hes1 protein was consistent with the result of qRT-PCR (Figure 5D). After treatment with Notch inhibitor, the expression of Notch 1, Notch 2 and Hes1 in shERβ was reversed.
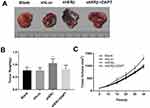
ERβ Affects The Growth Of Transplanted Tumor In Mice By Regulating Notch Signaling Pathway
In order to detect the role of ERβ in vivo, we constructed the mice xenograft model. We found that compared with the Blank and shLuc group, the volume and weight of the transplanted tumors in the shERβ group were significantly increased (all P<0.05, Figure 6A–C). However, the shERβ + DAPT group reversed the effect of shERβ on tumor volume and weight. All these results suggested that ERβ could inhibit the growth of transplanted tumor in mice by regulating Notch signaling pathway.
Discussion
ERβ plays a key role in the development and progression of a variety of human tumors.17 It is expressed in 80% of epithelial cells including the matrix.18 Zhao et al have indicated that ERβ is a “tumor suppressor” in breast cancer, and ERβ stabilization could promote targeted therapy for breast cancer.19 A previous study shows that overexpression of ERβ and treatment with ERβ agonists could enhance tumor suppressor function, resulting in decreased tumor cell survival.20 Moreover, ERβ has growth inhibition and chemical potentiation effect on ovarian cancer cells.21 In addition, up-regulation of ERβ could inhibit the growth of prostate cancer cells in situ and promote tumor cell apoptosis.22 In this study, ERβ was successfully transfected into HepG2 and Huh7. Overexpression of ERβ inhibited the proliferation, migration and promoted apoptosis of HepG2 and Huh7 cell. Therefore, we speculate that the overexpression of ERβ may inhibit the development of liver cancer via inhibiting the proliferation, migration and promoted apoptosis in liver cancer.
Ki67 is a widely used marker of cancer cell proliferation with significant prognostic value.23,24 A previous study shows that Ki67 expression was prominently increased during the proliferative phase of cancer cells.25 Interestingly, ER status is negatively correlated with Ki67 expression, indicating that ER-positive rates are highest when tumors have the lowest proliferative activity.26,27 A previous study indicates that ERβ inhibits prostate cancer cell proliferation and promotes apoptosis by down-regulating the expression of the proliferative marker Ki67.28 Here, overexpression of ERβ significantly reduced the expression of Ki67, resulting in inhibition of tumor proliferation. Bax, a central cell death regulator, is a major pro-apoptotic protein that controls cancer cell apoptosis.29 A previous study shows that the content of Bax protein in cancer tissues is significantly lower than that in adjacent normal tissues.30 Stimulation of Bax protein expression by ERβ has also found to induce tumor cell apoptosis.31 In this study, overexpression of ERβ significantly increased Bax content and promoted apoptosis in HepG2 cells. On the contrary, low expression of ERβ leads to the decrease of apoptotic rate of Huh7 cells. Based on these results, we speculated that overexpression of ERβ might promote apoptosis of liver cancer by down-regulating the expression of Ki67 and up-regulating the expression of Bax.
The activation of ERβ-dependent Notch1 is proved to take part in the process of apoptosis.15 Importantly, an aberrant activation of the Notch signaling pathway in ERβ morphants has been reported.32 A previous study indicates that Notch pathway is involved in cell proliferation and differentiation in cancer.13 Moreover, abnormal activation of Notch signaling often occurs in liver cancer, and liver cancer can be treated by blocking this pathway.33 Wang et al have showed that inhibition of Notch pathway and decreased expression of Notch receptor could regulate liver regeneration and liver cancer development.34 Another study of HCC has also shown that inhibition of the Notch pathway reduces tumorigenicity, cell invasion and migration.35 Similarly, in the present study, we found that overexpression of ERβ could inhibit Notch signaling pathway and suppress the expression of Notch 1, Notch 2 and Hes1, which further inhibit the proliferation of human liver cancer cells and promot apoptosis. Importantly, the xenograft model of mice study showed that ERβ could affect the growth of transplanted tumor in mice by regulating Notch signaling pathway. Thus, we speculate that overexpression of ERβ might participate in the progression of liver cancer by inhibiting the Notch pathway.
Conclusion
In conclusion, overexpression of ERβ may inhibit the development of liver cancer via inhibiting the proliferation, migration and promoted apoptosis in liver cancer. Furthermore, overexpression of ERβ might participate in the progression of liver cancer by inhibiting the Notch pathway.
Acknowledgments
Funding was provided by the National Nature Science Foundation of China (No. 81360364): Antitumor role and mechanisms of liquiritigenin-mediated ERβ isoform in the hypoxia microenvironment of hepatocellular carcinoma.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Affo S, Yu L, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol. 2017;12(1):153–186. doi:10.1146/annurev-pathol-052016-100322
2. Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, Mcglynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978–2007. Int J Cancer. 2016;139(7):1534–1545. doi:10.1002/ijc.30211
3. Kou P, Zhang Y, Shao W, et al. Significant efficacy and well safety of apatinib in an advanced liver cancer patient: a case report and literature review. Oncotarget. 2017;8(12). doi:10.18632/oncotarget.14724.
4. Li B, Gao Ping M, Chuan Ping C. Effects of inflammatory cytokines on the recurrence of liver cancer after an apparently curative operation. J Dig Dis. 2010;8(3):154–159.
5. Ting CT, Kuo CJ, Hu HY, Lee YL, Tsai TH. Prescription frequency and patterns of Chinese herbal medicine for liver cancer patients in Taiwan: a cross-sectional analysis of the National Health Insurance Research Database. BMC Complement Altern Med. 2017;17(1):118. doi:10.1186/s12906-017-1628-0
6. Kuper H, Mantzoros C, Lagiou P, et al. Estrogens, testosterone and sex hormone binding globulin in relation to liver cancer in men. Oncology. 2001;60(4):355–360. doi:10.1159/000058532
7. Assefa S, Kalra M, Kaul A, et al. Altered expression of estrogen receptor subtypes in hepatitis C cirrhosis and hepatitis C related hepatocellular carcinoma. Cancer Res. 2008;68:3152. doi:10.1158/0008-5472.CAN-07-5348
8. Lombardi AP, Pisolato R, Vicente CM, Lazari MF, Lucas TF, Porto CS. Estrogen receptor beta (ERbeta) mediates expression of beta-catenin and proliferation in prostate cancer cell line PC-3. Mol Cell Endocrinol. 2016;430:12–24. doi:10.1016/j.mce.2016.04.012
9. Hiramitsu S, Ishikawa T, Lee WR, et al. Estrogen receptor beta-mediated modulation of lung cancer cell proliferation by 27-hydroxycholesterol. Front Endocrinol. 2018;9(470). doi:10.3389/fendo.2018.00470.
10. Michele B, Maria Principia S, Samanta P, et al. ERβ expression in normal, adenomatous and carcinomatous tissues of patients with familial adenomatous polyposis. Scand J Gastroenterol. 2010;41(11):S27–S27.
11. Chang-Lee SN, Hsu HH, Shibu MA, et al. E 2/ERβ inhibits PPARα to regulate cell-proliferation and enhance apoptosis in Hep3b-hepatocellular carcinoma. Pathol Oncol Res. 2016;23(3):1–9.
12. Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology. 2015;61(1):382–392. doi:10.1002/hep.27268
13. Yuan X, Wu H, Xu H, et al. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett. 2015;369(1):20–27. doi:10.1016/j.canlet.2015.07.048
14. Wu G. Modulation of Notch signaling as a therapeutic approach for liver cancer. Curr Gene Ther. 2015;15(2). doi:10.2174/1566523214666141224100319
15. Fortini F, Vieceli DSF, Caliceti C, et al. Estrogen receptor β-dependent Notch1 activation protects vascular endothelium against tumor necrosis factor α (TNFα)-induced apoptosis. J Biol Chem. 2017;292(44):18178. doi:10.1074/jbc.M117.790121
16. Livak KJST. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
17. Chang J, Liu J, Li H, Li J, Mu Y, Feng B. Expression of ERβ gene in breast carcinoma and the relevance in neoadjuvant therapy. Oncol Lett. 2017;13(3):1641–1646. doi:10.3892/ol.2017.5659
18. Gustafsson JÅ, Cheng G, Warner M. ERβ in normal and malignant breast. Breast Cancer Res. 2005;7(S2):
19. Zhao Z, Wang L, James T, et al. Reciprocal regulation of ERα and ERβ stability and activity by diptoindonesin G. Chem Biol. 2015;22(12):1608–1621. doi:10.1016/j.chembiol.2015.10.011
20. Bado I, Pham E, Soibam B, Nikolos F, Gustafsson JÅ, Thomas C. ERβ alters the chemosensitivity of luminal breast cancer cells by regulating p53 function. Oncotarget. 2018;9(32):22509. doi:10.18632/oncotarget.25147
21. Pinton G, Nilsson S, Moro L. Targeting estrogen receptor beta (ERβ) for treatment of ovarian cancer: importance of KDM6B and SIRT1 for ERβ expression and functionality. Oncogenesis. 2018;7(2). doi:10.1038/s41389-018-0027-9
22. Zhou C, Yu C, Guo L, et al. In vivo study of the effects of ERβ on apoptosis and proliferation of hormone-independent prostate cancer cell lines PC-3M. Biomed Res Int. 2018;2018(15):1439712. doi:10.1155/2018/1439712
23. Thakur SS, Li H, Chan A, et al. The use of automated Ki67 analysis to predict Oncotype DX risk-of-recurrence categories in early-stage breast cancer. PLoS One. 2018;13(1):e0188983. doi:10.1371/journal.pone.0188983
24. Lindsay CR, Moulec SL, Billiot F, et al. Vimentin and Ki67 expression in circulating tumour cells derived from castrate-resistant prostate cancer. BMC Cancer. 2016;16(1):1–11. doi:10.1186/s12885-016-2192-6
25. Morsi HM, Leers MP, J?Ger W. et al. The patterns of expression of an apoptosis-related CK18 neoepitope, the bcl-2 proto-oncogene, and the Ki67 proliferation marker in normal, hyperplastic, and malignant endometrium. Int J Gynecol Pathol. 2000;19(2):118–126.
26. Helen T, Susan M, Karen P, et al. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors – a surrogate marker? Cancer. 2003;97(5):1321–1331. doi:10.1002/cncr.11188
27. Inwald CE. Ortmann, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a;large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139(2):539–552. doi:10.1007/s10549-013-2560-8
28. Rochel-Maia SS, Taboga SR, Warner M, Gustafsson JA. Abstract A53: effect of an ER agonist in the gerbil senile prostate: novel treatment for age related disorders? Cancer Res. 2012;72(4 Supplement):A53–A53. doi:10.1158/1538-7445.PRCA2012-A53
29. Liu Z, Ding Y, Ye N, Wild C, Chen H, Zhou J. Direct activation of bax protein for cancer therapy. Med Res Rev. 2016;36(2):313–341. doi:10.1002/med.21379
30. Yang Y, Shao ZQ, Guo JX. Detection of Bcl-2 and Bax protein contents in bladder cancer tissue and their relationship with postoperative recurrence. J Hainan Med Univ. 2016;22(7):105–108.
31. Cheng J, Lee EJ, Madison LD, Lazennec G. Expression of estrogen receptor β in prostate carcinoma cells inhibits invasion and proliferation and triggers apoptosis. FEBS Lett. 2004;566(1):169–172. doi:10.1016/j.febslet.2004.04.025
32. Froehlicher M, Liedtke A, Groh K, et al. Estrogen receptor subtype β2 is involved in neuromast development in zebrafish (Danio rerio) larvae. Dev Biol. 2009;330(1):32–43. doi:10.1016/j.ydbio.2009.03.005
33. Ma L, Dong P, Liu L, et al. Overexpression of protein O-fucosyltransferase 1 accelerates hepatocellular carcinoma progression via the Notch signaling pathway. Biochem Biophys Res Commun. 2016;473(2):503–510. doi:10.1016/j.bbrc.2016.03.062
34. Wang XP, Zhou J, Han M, et al. MicroRNA-34a regulates liver regeneration and the development of liver cancer in rats by targeting Notch signaling pathway. Oncotarget. 2017;8(8):13264.
35. Jing L, Peng W, Wang R, et al. The Notch pathway promotes the cancer stem cell characteristics of CD90+cells in hepatocellular carcinoma. Oncotarget. 2016;7(8):9525–9537. doi:10.18632/oncotarget.6672
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.