Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Outcomes of Radiation Segmentectomy for Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease versus Chronic Viral Hepatitis
Authors De la Garza-Ramos C , Montazeri SA, Musto KR, Kapp MD, Lewis AR, Frey G, Paz-Fumagalli R, Ilyas S, Harnois DM, Majeed U, Patel T , Toskich B
Received 25 April 2023
Accepted for publication 1 June 2023
Published 23 June 2023 Volume 2023:10 Pages 987—996
DOI https://doi.org/10.2147/JHC.S414853
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Cynthia De la Garza-Ramos,1 S Ali Montazeri,1 Kaitlyn R Musto,1 Melissa D Kapp,1 Andrew R Lewis,1 Gregory Frey,1 Ricardo Paz-Fumagalli,1 Sumera Ilyas,2 Denise M Harnois,3 Umair Majeed,4 Tushar Patel,3 Beau Toskich1
1Division of Interventional Radiology, Mayo Clinic Florida, Jacksonville, FL, USA; 2Division of Gastroenterology and Hepatology, Mayo Clinic Rochester, Rochester, MN, USA; 3Department of Transplant, Mayo Clinic, Florida, FL, USA; 4Division of Hematology and Medical Oncology, Mayo Clinic Florida, Jacksonville, FL, USA
Correspondence: Beau Toskich, Division of Interventional Radiology, Mayo Clinic Florida, 4500 San Pablo Road, Jacksonville, FL, 32224, USA, Tel +1 904-953-1496, Email [email protected]
Purpose: To compare the outcomes of radiation segmentectomy for early-stage hepatocellular carcinoma (HCC) in patients with non-alcoholic fatty liver disease (NAFLD) versus hepatitis C virus (HCV).
Materials and Methods: A retrospective analysis of consecutive patients with NAFLD- or HCV-related HCC treated with radiation segmentectomy from 01/2017-06/2022 was performed. Eligibility criteria included solitary tumor ≤ 8 cm or up to 3 HCC ≤ 3 cm, ECOG 0– 1, and absence of vascular invasion or extrahepatic spread. Imaging best response was assessed per modified Response Evaluation Criteria in Solid Tumors. Target tumor and overall progression, time-to-progression (TTP), and overall survival (OS) were calculated. All outcomes were censored for liver transplantation (LT). Complete pathologic response (CPN) was assessed in patients who underwent LT.
Results: Of 142 patients included (NAFLD: 61; HCV: 81), most had cirrhosis (NAFLD: 87%; HCV: 86%) and small tumors (median size NAFLD: 2.3 cm; HCV: 2.5 cm). Patients with NAFLD had higher BMI (p< 0.001) and worse ALBI scores (p=0.003). Patients with HCV were younger (p< 0.001) and had higher AFP levels (p=0.034). Median radiation dose (NAFLD: 508 Gy; HCV: 452 Gy) and specific activity (NAFLD: 700 Bq; HCV: 698 Bq) were similar between cohorts. Objective response was 100% and 97% in the NAFLD and HCV cohorts, respectively. Target tumor progression occurred in 1 (2%) NAFLD and 8 (10%) HCV patients. Target tumor TTP was not met for either cohort. Overall progression occurred in 23 (38%) NAFLD and 39 (48%) HCV patients. Overall TTP was 17.4 months (95% CI 13.5– 22.2) in NAFLD and 13.5 months (95% CI 0.4– 26.6) in HCV patients (p=0.86). LT was performed in 27 (44%) NAFLD and 33 (41%) HCV patients, with a CPN rate of 63% and 54%, respectively. OS was not met in the NAFLD cohort and was 53.9 months (95% CI 32.1– 75.7) in the HCV cohort (p=0.15).
Conclusion: Although NAFLD and HCV are associated with different mechanisms of liver injury, patients with early-stage HCC treated with radiation segmentectomy achieve comparable outcomes.
Keywords: hepatocellular carcinoma, radiation segmentectomy, NAFLD, viral hepatitis
Plain Language Summary
Patients with advanced liver disease or cirrhosis are at increased risk for hepatocellular carcinoma (HCC). Radiation segmentectomy is a locoregional therapy with curative intent for early-stage HCC. Patients with early-stage HCC in the setting of non-alcoholic fatty liver disease and viral hepatitis achieve comparable outcomes with radiation segmentectomy as initial treatment, despite different mechanisms of underlying liver injury.
Introduction
Hepatocellular carcinoma (HCC) has the seventh highest incidence and fourth highest cancer-related mortality worldwide.1 Approximately 90% of cases of HCC develop in patients with chronic liver disease and cirrhosis. While viral hepatitis has historically been the leading causative agent of cirrhosis, the incidence of non-alcoholic fatty liver disease (NAFLD) is rising in both Western and Asian populations, increasing the number of patients at risk for HCC.1,2
Patients with NAFLD and viral hepatitis may share risk factors for HCC such as advanced fibrosis/cirrhosis but may differ considerably in associated co-morbidities and risk factors for other diseases. Potential differences in outcomes between populations with non-viral versus viral-related HCC treated with systemic therapies have been suggested in several studies.3,4 However, it remains unknown whether these cohorts may respond differently to locoregional therapy or if they differ in disease course.
Ablative transarterial radioembolization, also known as radiation segmentectomy, using Yttrium-90 containing glass microspheres is a guideline-endorsed therapy for early-stage, solitary HCC in patients considered ineligible for surgical resection or thermal ablation.5 While the practice of radiation segmentectomy is now a common therapy for HCC, it remains unknown whether etiology of liver disease impacts the outcome of this therapy. This study aimed to investigate if the outcomes of radiation segmentectomy for treatment-naïve, early-stage HCC differ in patients with NAFLD vs viral-related liver disease.
Patients and Methods
Patient Selection
This study received Institutional Review Board approval and the requirement for written informed consent was waived. The study population was comprised of consecutive patients with treatment-naïve HCC and a history of NAFLD- or hepatitis C virus (HCV)-related liver disease who underwent radiation segmentectomy at a single tertiary care medical center from January 2017 through June 2022. Inclusion criteria were HCC diagnosis by radiologic criteria or histopathology, solitary HCC ≤ 8 cm or 2-3 tumors each measuring ≤ 3 cm, absence of vascular invasion or extrahepatic spread, an Eastern Cooperative Oncology Cohort performance status of 0–1, and at least 3-month follow-up imaging available. Exclusion criteria were prior HCC therapy and a single-compartment radiation dose <190 Gy. Patients were assigned to a study cohort based on etiology of liver disease. HCV status was assessed from laboratory reports on HCV antibody, HCV ribonucleic acid, and HCV genotype testing. NAFLD status was determined from reported radiologic, histologic, and clinical diagnoses from board certified hepatologists. Patients with other or co-existing etiologies for liver disease (eg, alcoholic liver disease) were excluded. Fibrosis severity was graded according to the Batts-Ludwig system from available magnetic resonance or ultrasound elastography, biopsy, or transplant specimens.
Treatment
All patients underwent transarterial radioembolization as initial treatment for HCC as recommended by a multidisciplinary tumor board. All treatments were performed by one of seven experienced interventional radiologists using Yttrium-90 containing glass microspheres (Therasphere™, Boston Scientific, Marlborough, MA). Radiation segmentectomy treatments were performed utilizing ablative radioembolization targeted to two hepatic segments or less, spanning both tumor(s) and surrounding expendable liver.6 Dosimetry was calculated using single-compartment Medical Internal Radiation Dose (MIRD) schema.7 Angiosome volumes were obtained from contrast enhanced cone-beam computed tomography (CT) at the time of mapping angiography.
Outcomes
Imaging best response was assessed at 3- and 6-months post-treatment with contrast-enhanced multiphase magnetic resonance imaging (MRI), or CT if MRI was contraindicated, per modified Response Evaluation Criteria in Solid Tumors (mRECIST).8 Objective response was defined as the presence of either complete or partial response.
Overall progression was defined as any new hepatic lesion meeting Liver Imaging Reporting and Data System (LI-RADS) 4 or above criteria; all progressions were subject to additional treatment per multidisciplinary tumor board recommendation. Target tumor progression was defined as a tumor arising within or in continuation with the margin of the treatment angiosome. Out-of-field progression was defined as a tumor arising elsewhere within the liver or extrahepatic tissues. Overall time-to-progression (TTP) was assessed from the date of radiation segmentectomy to date of first progression per imaging studies, or date of last available imaging for patients without the event. OS was assessed until the last recorded follow-up. All outcomes were censored for liver transplantation. Complete pathologic necrosis (CPN) was determined as complete absence of any viable cell within the target tumor on histopathologic analysis of liver explants.
Statistical Analysis
Continuous data were reported as median (interquartile range [IQR] 25, 75) or median (95% confidence interval [CI]) and compared between study cohorts using the Mann–Whitney U-test. Categorical data were reported as frequency (percentage) and compared between study cohorts using the chi-square of Fischer’s Exact Test, as appropriate. Kaplan–Meier curves were used to depict OS, target tumor TTP, and overall TTP. The Log rank test was used to compare the Kaplan–Meier curves between study cohorts. Logistic regression analysis was used to calculate propensity scores with the following variables: age, body mass index (BMI), Child-Pugh class, albumin-bilirubin (ALBI) score, alpha-fetoprotein (AFP) level, and hepatic fibrosis stage. A 1:1 nonreplacement approach was used to match the cohorts with the nearest neighbor estimator (caliper) of 0.02. Propensity score matching analysis was not performed as only 33 matched pairs were found. A propensity-adjusted multivariate analysis was performed to determine the hazard ratio (HR) for OS and overall progression between study cohorts. A p-value of <0.05 was considered statistically significant. All analyses were conducted using IBM SPSS for Windows (Armonk, NY: IBM Corp.) v28.0.
Results
Baseline Patient and Treatment Characteristics
A total of 142 patients with treatment-naïve HCC were included for analysis (NAFLD: 61; HCV: 81). Baseline characteristics are summarized in Table 1. Most patients were male (NAFLD: 72% vs HCV: 74%), had solitary HCC (NAFLD: 77%; HCV: 86%), small tumor size (median size NAFLD: 2.3 cm; HCV: 2.5 cm), and low platelet count (median platelet count NAFLD: 92x109/L; HCV: 105x109/L).
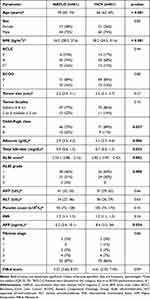 |
Table 1 Baseline Patient and Tumor Characteristics |
Most patients had advanced liver disease. Severity of hepatic fibrosis per the Batts-Ludwig scoring system was similar between cohorts, with stage 3–4 fibrosis in 94% NAFLD- (stage 3: 7%, stage 4/cirrhosis: 87%) and 93% HCV-related HCC patients (stage 3: 7%, stage 4/cirrhosis: 86%). The Fibrosis-4 (FIB-4) score was not significantly different between cohorts (median NAFLD: 5.27; HCV: 4.61).
Patients with NAFLD-related HCC had a higher BMI (median BMI NAFLD: 34.2 kg/m2; HCV: 28.2 kg/m2; p<0.001) and worse ALBI scores (median ALBI score NAFLD: −2.53; HCV: −2.82; p=0.003).
Patients with HCV-related HCC were more likely to have Child-Pugh class A (NAFLD: 75%; HCV: 89%; p=0.037) and ALBI grade 1 liver function (NAFLD: 45%; HCV: 69%; p=0.008), present with HCC at an earlier age (median age NAFLD: 70 years; HCV: 66 years; p<0.001) and have higher AFP levels at diagnosis (median AFP NAFLD: 5.2 ng/dL; HCV: 8.6 ng/dL; p=0.034). Of the 81 patients with a history of HCV, 60 (74%) had received antiviral therapy and achieved sustained virologic response (SVR) prior to HCC diagnosis.
Treatment parameters, including radiation dose (median dose NAFLD: 508 Gy; HCV: 452Gy) and specific activity (median specific activity NAFLD: 700 Bq and HCV: 698 Bq; assuming 2500 Bq at calibration), were similar between cohorts (Table 2). Lung dose was higher in patients with NAFLD-related HCC compared to HCV-related HCC (median lung dose NAFLD: 2.20 Gy; HCV: 1.35 Gy; p=0.04), but neither was clinically significant.
 |
Table 2 Radiation Segmentectomy Treatment Parameters |
Imaging and Pathologic Response
The objective response rate was 100% and 97% in the NAFLD- and HCV-related HCC cohorts per mRECIST, respectively (Table 3). Complete response was observed in 93% NAFLD- and 81% HCV- related HCC patients.
 |
Table 3 Best Imaging Response at 3 and/or 6-Month Follow-Up After Radiation Segmentectomy |
Liver transplantation was performed in 27 (44%) NAFLD- and 33 (41%) HCV-related HCC patients with a total of 32 and 39 target tumors, respectively. The median time to transplantation was 201 days (range: 86–1189 days) in the combined cohort, 390 days (range: 102–890 days) in the NAFLD-related HCC cohort, and 418 days (range: 86–1189 days) in the HCV-related HCC cohort. The combined cohort CPN rate was 58%. CPN was observed in 63% (n=20/32) target tumors in the NAFLD-related HCC cohort and 54% (n=21/39) target tumors in the HCV-related HCC cohort (p=0.46) (Figure 1A).
Histopathologic analysis of the combined cohort performed using previously established response thresholds9 demonstrated a CPN rate of 83% (n=29/35) in target tumors treated with a radiation dose ≥500 Gy and specific activity ≥327 Bq (assuming 2500 Bq at calibration) compared to 40% (n=2/5) in target tumors treated with a radiation dose ≥500 Gy and specific activity <327 Bq (p=0.03) (Figure 1B).
Survival and Progression Outcomes
Survival and progression outcomes are summarized in Table 4. Mean non-censored follow-up was 26.5 months (95% CI 22.6–30.3) in the NAFLD-related HCC cohort and 33.2 months (95% CI 29.5–37.1) in the HCV-related HCC cohort. Mean censored follow-up was 15.6 months (95% CI 12.4–18.8) in the NAFLD-related HCC cohort and 20.5 months (95% CI 16.9–24.0) in the HCV-related HCC cohort.
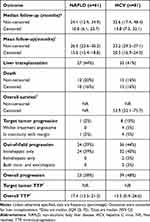 |
Table 4 Survival and Progression Outcomes After Radiation Segmentectomy |
The total number of deaths within the study period in the NAFLD-related HCC cohort was 12 (20%), of which 2 occurred after liver transplantation. Total number of deaths in the HCV-related HCC cohort was 13 (16%). None were related to radiation segmentectomy. Non-censored OS was not met in either cohort (p=0.25) (Figure 2A). Censored OS was not met in the NAFLD-related HCC cohort and 53.9 months (95% CI 32.1–75.7) in the HCV-related HCC cohort (p=0.15) (Figure 2B).
Overall progression occurred in 23 (38%) NAFLD- and 39 (48%) HCV-related HCC patients. Overall TTP was 17.4 months (95% CI 13.5–21.3) in NAFLD- and 13.5 months (0.4–26.6) in HCV-related HCC patients (p=0.86) (Figure 2C). Target tumor progression occurred in 1 (2%) NAFLD- and 8 (10%) HCV-related HCC patients. Target tumor TTP was not met for either cohort (p=0.12) (Figure 2D). Out-of-field progression occurred in 24 (39%) NAFLD- and 35 (43%) HCV-related HCC patients.
Overall progression in the NAFLD cohort was reported in 50% (n=2/4) of patients with fibrosis stage 0–2 and 37% (n=21/57) of patients with fibrosis stage 3–4; in the HCV-related HCC cohort, overall progression was reported in 20% (n=1/5) of patients with fibrosis stage 0–2 and 50% (n=38/76) of patients with fibrosis stage 3–4. The overall progression rate in the HCV-related HCC cohort according to SVR status was 43% (n=26/60) in patients who had achieved SVR at time of HCC diagnosis and 62% (n=13/21) in those without SVR.
Propensity-adjusted multivariate analysis did not show a difference in OS (HR: 0.5 [95% CI 0.2–1.4], p=0.174) and overall progression (HR: 1.0 [95% CI 0.5–1.9], p=0.976) between HCV-related HCC and NAFLD-related HCC.
Discussion
The management of HCC has changed considerably over the past decade, namely with the addition of multiple systemic therapies for which immunotherapy has emerged as a first-line agent. The IMBRAVE150 Phase III trial demonstrated superior OS, progression free survival, and tumor response with atezolizumab/bevacizumab compared to the previous gold-standard systemic agent, sorafenib.10 A subsequent meta-analysis on the results of IMBRAVE150, CheckMATE (nivolumab vs sorafenib), and KEYNOTE240 (pembrolizumab vs placebo) according to etiology of liver disease suggested that viral-related HCC is more responsive to immunotherapy than non-viral related HCC.3 The speculation of altered T-cell response and impaired immune surveillance in NAFLD-related HCC was felt to play a role in this perceived difference.11 A subsequent, large, multicenter, real-world data analysis comparing atezolizumab/bevacizumab versus lenvatinib for unresectable HCC revealed a significantly prolonged TTP and OS in patients with viral-related HCC treated with atezolizumab/bevacizumab, while patients with NAFLD-related HCC had improved OS with lenvatinib.4 While this evidence may be circumstantial and remains to be prospectively validated, it has raised an important question of whether the underlying etiology of liver disease should affect clinical decisions for the treatment of HCC.
Radiation segmentectomy is now a guideline-supported and widely adopted therapy for solitary HCC which has shown comparable efficacy to other curative-intent ablative modalities.12,13 Although historically considered an alternative to chemoembolization, prospective evidence has shown superior tumor control and survival with radioembolization in BCLC A and B disease.14 A large, national-level, retrospective study on liver transplant recipients following locoregional therapy for HCC in the United States recently showed higher target tumor CPN rates with radioembolization than chemoembolization.15 Currently, it is not known whether radiation segmentectomy outcomes differ amongst patients with NAFLD vs viral hepatitis.
The results of this study suggest treatment effects of radiation segmentectomy are similar in NAFLD and HCV-related HCC. In the total cohort, objective response and complete response per mRECIST were achieved in 99% and 87% of patients by 3 and/or 6 months, respectively. Radiation segmentectomy served as a neoadjuvant to liver transplantation in 42% of patients, with a combined CPN rate of 58%. Notably, origin of liver disease did not differently impact CPN, while treatment intensification above previously established response thresholds significantly impacted pathologic response (Figure 1A and B). CPN was observed in 83% of target tumors treated with a radiation dose ≥500 Gy and a specific activity ≥327 Bq (glass microspheres with ≤ 8 days of decay after calibration), further substantiating this treatment as a curative-intent ablation modality when utilizing these treatment parameters.
Although the HCV-related HCC cohort had higher rates of ALBI 1 and Child-Pugh A liver function and 73% had achieved SVR prior to diagnosis, there were non-statistically significant trends in favor of the NAFLD-related HCC cohort for higher complete response rates, lower frequency of target tumor progression and overall progression, and longer overall TTP. It is not feasible to state if this is related to the target tumor or de novo HCC. Despite the efficacy of radiation segmentectomy, similar to other local therapies for HCC, patients remain at risk of recurrence in the absence of liver transplantation.
Several clinical trials are currently investigating the combination of radioembolization and immunotherapy for the treatment of HCC. The results of the current study suggest that the response to radioembolization in early-stage HCC would not be determined by the underlying liver disease. Whether added tumor response and its potential synergy with immunotherapy translates into clinical benefit remains an area of active investigation.
This retrospective study has several limitations. The analysis is limited to a cohort of patients managed by a multidisciplinary team at a single tertiary referral comprehensive cancer center and may not be applicable to a broader population. All patients were treated with Yttrium-90 containing glass microspheres and the results cannot be extrapolated to radioembolization using other microspheres or radioisotopes. Radiation segmentectomy dosimetry recommendations changed during the study and outcomes may not reflect those of current practice. Censoring for liver transplantation limited follow-up time in both study cohorts but remains comparable to other locoregional therapy studies. Using LI-RADS 4 criteria to retrospectively define HCC progression may lead to overdiagnosis, yet in practice it is common for a suspicious lesion to raise attention and warrant treatment. The results of this study may aid in risk assessment for patients on the transplant waitlist and may serve as a foundation for future studies to further delineate the best treatment and surveillance approaches in these disparate populations.
Conclusion
Patients with early-stage HCC in the setting of NAFLD- or HCV-related chronic liver disease achieve comparable outcomes with radiation segmentectomy as initial treatment, despite different mechanisms of underlying liver injury. While origin of liver disease did not differently impact CPN, treatment intensification with a radiation dose over 500 Gy and specific activity up to second week Monday is associated with CPN.
Abbreviations
ALBI, albumin-bilirubin; BMI, body mass index; CPN, complete pathologic necrosis; CT, computed tomography; FIB-4, Fibrosis-4; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LI-RADS - Liver Imaging Reporting and Data System; MRI, magnetic resonance imaging; mRECIST - modified Response Evaluation Criteria in Solid Tumors; NAFLD, non-alcoholic fatty liver disease; OS, overall survival; SVR, sustained virologic response; TTP, time-to-progression.
Ethical Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This retrospective, minimal risk study was approved by the Institutional Review Board (IRB) at Mayo Clinic and the requirement to obtain written informed consent was waived. All collected patient data was maintained with confidentiality. All organs were donated voluntarily with written informed consent, and that this was conducted in accordance with the Declaration of Istanbul.
Consent for Publication
For this type of study consent for publication is not required.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study did not receive financial support.
Disclosure
B.B.T. is an advisor to Boston Scientific, Sirtex Medical, Terumo Medical, ABK, Galvanize Therapeutics, Replimmune, Johnson and Johnson, AstraZeneca, Genentech, VIVOS, HistoSonics, and Delcath. S.I. is a consultant for AstraZeneca. Other authors declare that they have no conflicts of interest in this work.
References
1. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
2. Ogasawara S, Koroki K, Kanzaki H, et al. Changes in therapeutic options for hepatocellular carcinoma in Asia. Liver Int off J Int Assoc Study Liver. 2022;42(9):2055–2066. doi:10.1111/liv.15101
3. Haber PK, Puigvehí M, Castet F, et al. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002–2020). Gastroenterology. 2021;161(3):879–898. doi:10.1053/j.gastro.2021.06.008
4. Casadei-Gardini A, Rimini M, Tada T, et al. Atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: a large real-life worldwide population. Eur J Cancer. 2023;180:9–20. doi:10.1016/j.ejca.2022.11.017
5. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system. The 2022 update. J Hepatol. 2021. doi:10.1016/j.jhep.2021.11.018
6. Riaz A, Gates VL, Atassi B, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79(1):163–171. doi:10.1016/j.ijrobp.2009.10.062
7. Salem R, Padia SA, Lam M, et al. Clinical and dosimetric considerations for Y90: recommendations from an international multidisciplinary working group. Eur J Nucl Med Mol Imaging. 2019;46(8):1695–1704. doi:10.1007/s00259-019-04340-5
8. Lencioni R, Llovet J. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
9. Montazeri SA, De la Garza-Ramos C, Lewis AR, et al. Hepatocellular carcinoma radiation segmentectomy treatment intensification prior to liver transplantation increases rates of complete pathologic necrosis: an explant analysis of 75 tumors. Eur J Nucl Med Mol Imaging. 2022;49(11):3892–3897. doi:10.1007/s00259-022-05776-y
10. Cheng A-L, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
11. Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–456. doi:10.1038/s41586-021-03362-0
12. Lewandowski RJ, Gabr A, Abouchaleh N, et al. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology. 2018;287(3):1050–1058. doi:10.1148/radiol.2018171768
13. la Garza-Ramos C D, Montazeri SA, Croome KP, et al. Radiation segmentectomy for the treatment of solitary hepatocellular carcinoma: can outcomes be compared to surgical resection? J Vasc Interv Radiol. 2022. doi:10.1016/j.jvir.2022.03.021
14. Dhondt E, Lambert B, Hermie L, et al. (90)Y radioembolization versus drug-eluting bead chemoembolization for unresectable hepatocellular carcinoma: results from the TRACE Phase II randomized controlled trial. Radiology. 2022:211806. doi:10.1148/radiol.211806
15. Sarwar A, Bonder A, Hassan L, et al. Factors associated with complete pathologic necrosis of hepatocellular carcinoma on explant evaluation after locoregional therapy: a national analysis using the UNOS database. AJR Am J Roentgenol. 2022. doi:10.2214/AJR.22.28385
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


